Macro-Mineral Uptake, Relative Water Content, Retention Capability, and Tolerance Index of Sunn Hemp (Crotalaria juncea L.) under Salinity Stress at Early Seedling
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Germination Tests
2.3. Determination of Tolerance Indices
2.4. Chemical Analyses
2.5. Statistical Analyses
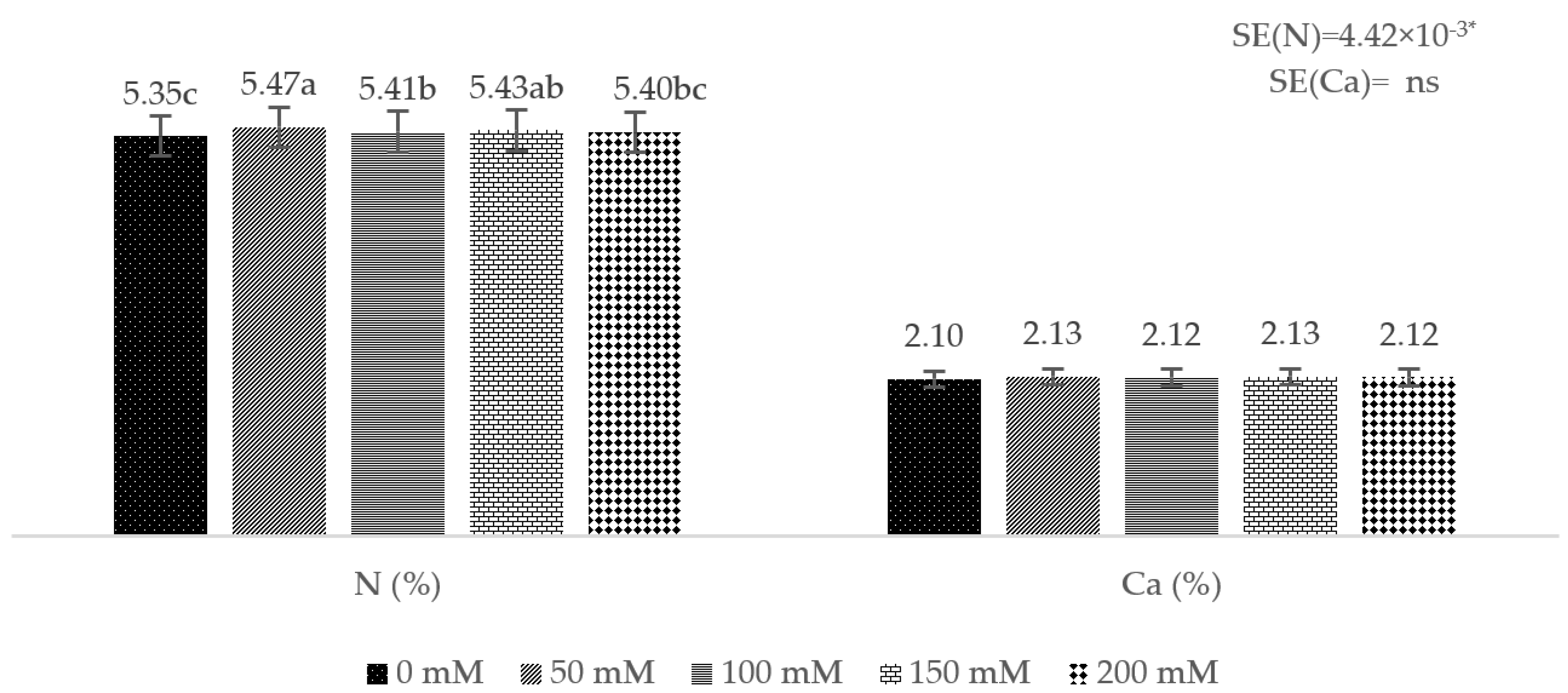
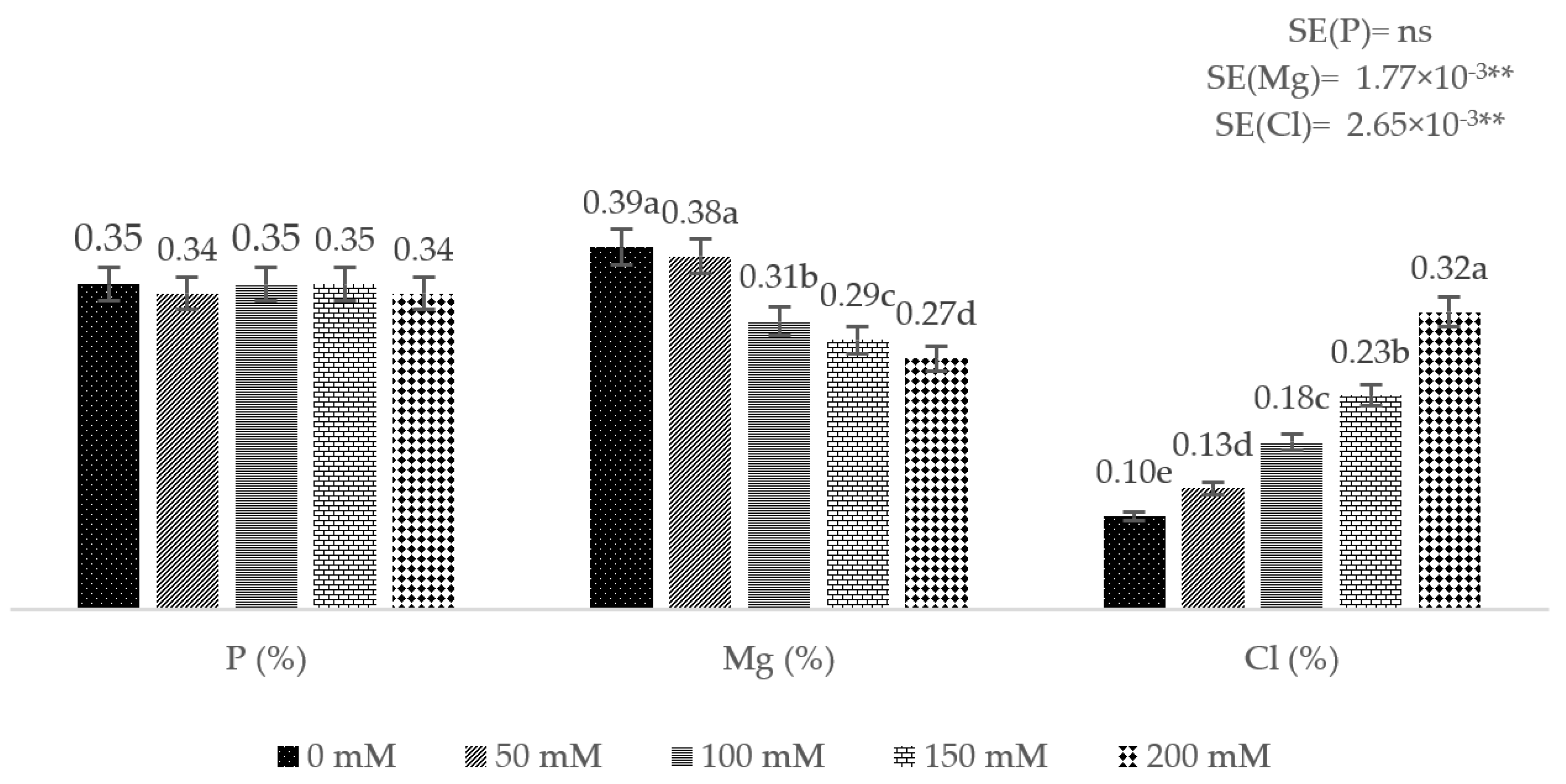
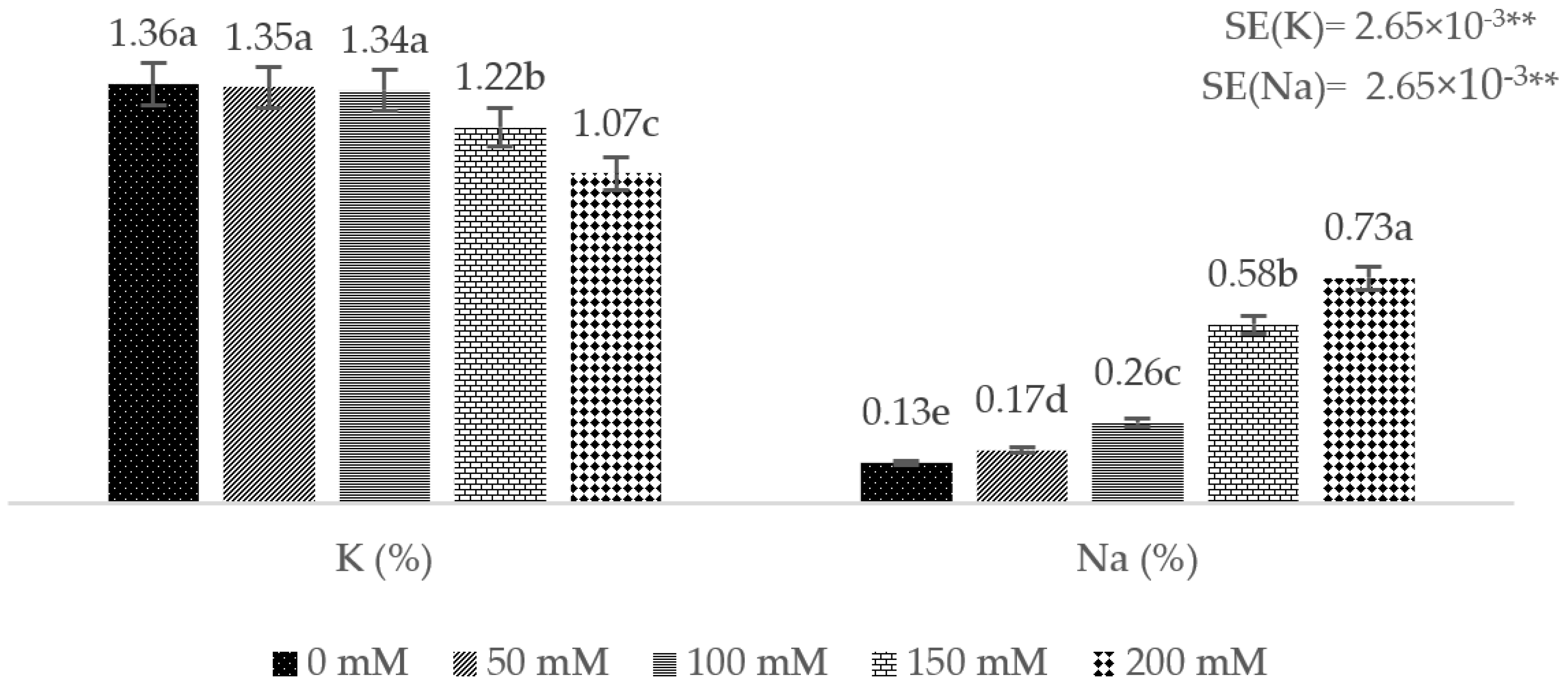
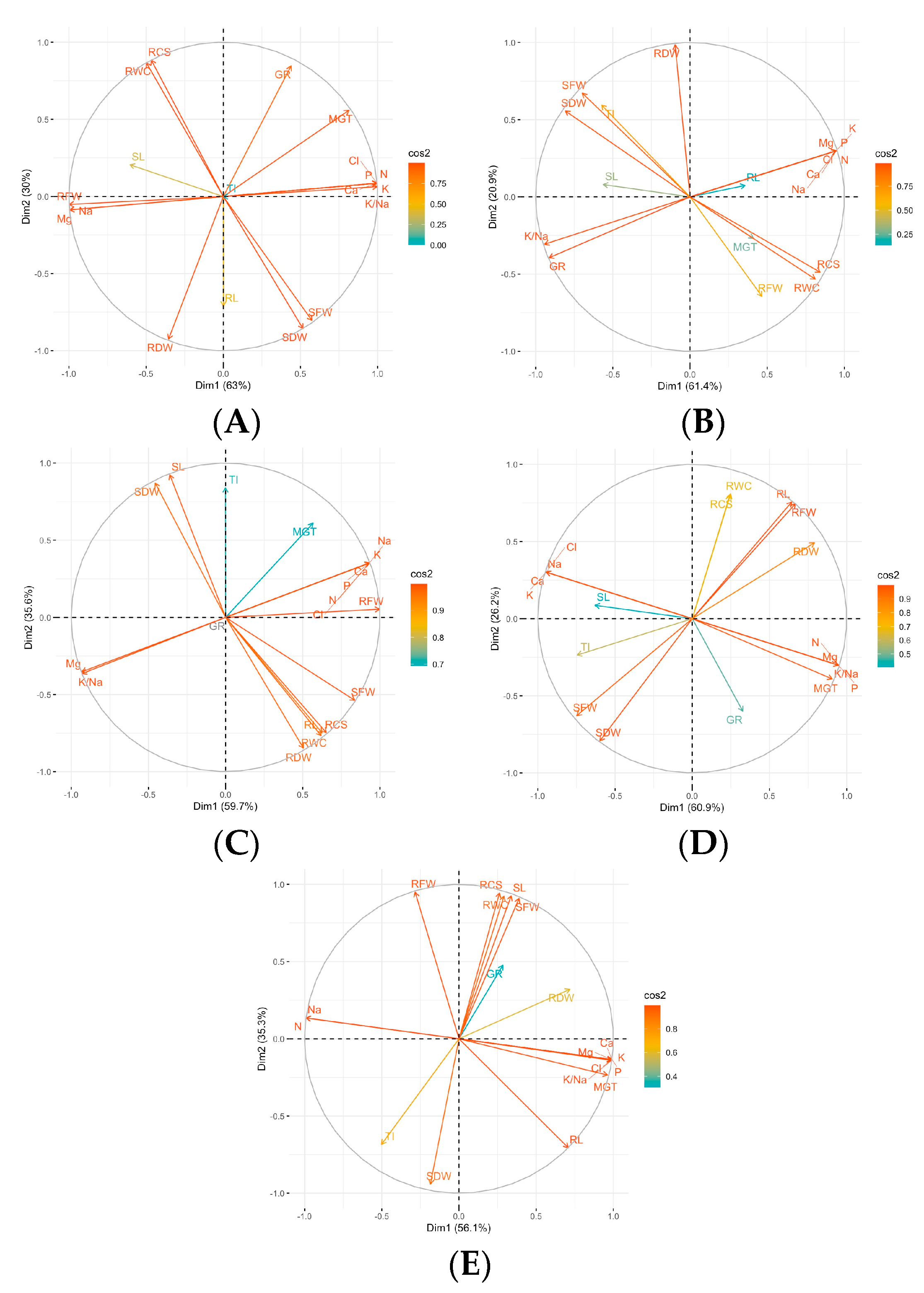
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shokat, S.; Großkinsky, D.K. Tackling salinity in sustainable agriculture-What developing countries may learn from approaches of the developed world. Sustainability 2019, 11, 4558. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.E.; Jensen, C.R.; Liu, F. Improving crop production in the arid Mediterranean climate. Field Crops Res. 2012, 128, 34–47. [Google Scholar] [CrossRef]
- Srivastava, N. Reclamation of saline and sodic soil through phytoremediation. In Environmental Concerns and Sustainable Development; Springer: Singapore, 2020; pp. 279–306. [Google Scholar]
- Dadaşoğlu, E.; Ekinci, M. Effects of different degrees of temperature, salt and salicylic acid applications on seed germination of bean (Phaseolus vulgaris L.). Atatürk Univ. J. Agric. Fac. 2013, 44, 145–150. [Google Scholar]
- Yıldız, S.; Karagöz, F.P.; Dursun, A. Germination in salt stress of sweet william (Dianthus barbatus L.) seeds applied pretreatment of gibberellic acid. Atatürk Univ. J. Agric. Fac. 2017, 48, 1–7. [Google Scholar]
- Salisbury, F.B.; Ross, C.W. Plant Physiology; Wadsworth Publication Company Inc.: Belmont, CA, USA, 1992. [Google Scholar]
- Vriezen, J.A.C.; Bruijn, F.J.; Nusslein, K. Responses of rhizobia to desiccation in relation to osmotic stress, oxygen, and temperature. Appl. Environ. Microbiol. 2007, 73, 3451–3459. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, W.; Amombo, E.; Hu, L.; Kjorven, J.O.; Chen, L. Mechanisms of environmental stress tolerance in turfgrass. Agronomy 2020, 10, 522. [Google Scholar] [CrossRef]
- Chavarria, M.R.; Wherley, B.; Jessup, R.; Chandra, A. Leaf anatomical responses and chemical composition of warm-season turfgrasses to increasing salinity. Curr. Plant Biol. 2020, 22, 100147. [Google Scholar] [CrossRef]
- Liu, H.; Todd, J.L.; Luo, H. Turfgrass salinity stress and tolerance—A review. Plants 2023, 12, 925. [Google Scholar] [CrossRef]
- Kadioglu, B. Determination of germination biology some sage (Salvia ssp.) species under salinity stress. J. Tekirdag Agric. Fac. 2021, 18, 359–367. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, U. Screening of salt tolerance potential of some native forage grasses from the eastern part of Terai-Duar grasslands in India. Trop. Grassl.-Forrajes Trop. 2017, 5, 129. [Google Scholar] [CrossRef][Green Version]
- Demiroglu Topcu, G.; Ozkan, S.S. Effects of different salt sources and concentrations on germination parameters of barley (Hordeum vulgare L.) seeds. ISPEC J. Agric. Sci. 2020, 4, 456–467. [Google Scholar]
- Camlica, M.; Yaldiz, G. Effect of salt stress on seed germination, shoot and root length in basil (Ocimum basilicum). Int. J. Second. Metab. 2017, 4, 69–76. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Kondetti, P.; Jawali, N.; Apte, S.K.; Shitole, M.G. Salt tolerance in Indian soybean (Glycine max (L.) Merrill) varieties at germination and early seedling growth. Ann. Biol. Res. 2012, 3, 1489–1498. [Google Scholar]
- Dhairyasheel, B.; Patil, B.; Sharad, B. Influence of NaCl-mediated salinity stress on lipid peroxidation in germinating seeds of soybean. Int. J. Pharma Bio Sci. 2015, 6, 549–552. [Google Scholar]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Irshad, M.; Khan, A.L.; Waqas, M.; Shahzad, R.; Iqbal, A.; Ullah, N.; Rehman, G.; et al. Kinetin modulates physio-hormonal attributes and isoflavone contents of soybean grown under salinity stress. Front. Plant Sci. 2015, 6, 377. [Google Scholar] [CrossRef] [PubMed]
- Kandil, A.A.; Sharief, A.E.; Ahmed, K.R. Performance of some soybean (Glycine max (L.) Merrill) cultivars under salinity stress to germination characters. Int. J. Agron. Agric. Res. 2015, 6, 48–56. [Google Scholar]
- Hosseini, H.; Rezvani Moghadam, P. Effect of water and salinity stress in seed germination on isabgol (Plantago ovata). Iran. J. Field Crops Res. 2006, 4, 15–22. [Google Scholar]
- Akbari, G.; Sanavy, S.A.; Yousefzadeh, S. Effect of auxin and salt stress (NaCl) on seed germination of wheat cultivars (Triticum aestivum L.). Pak. J. Biol. Sci. 2007, 10, 2557–2561. [Google Scholar] [CrossRef]
- Mahdavi, B.; Sanavi, S.; Balochi, H.R. The effect of sodium chloride on the germination and seedling growth figures grass pea (Lathyrus sativus L.). Iran. J. Biotechnol. 2007, 20, 363–374. [Google Scholar]
- Hamidi, H.; Safarnejad, A. Effect of drought stress on alfalfa cultivars (Medicago sativa L.) in germination stage. Am.-Eurasian J. Agric. Environ. Sci. 2010, 8, 705–709. [Google Scholar]
- Bernstein, L. Salt Tolerance of Plants (No. 283); US Department of Agriculture: Washington, DC, USA, 1964.
- Yamazaki, K.; Ishimori, M.; Kajiya-Kanegae, H.; Takanashi, H.; Fujimoto, M.; Yoneda, J.I.; Yano, K.; Koshiba, T.; Tanaka, R.; Iwata, H.; et al. Effect of salt tolerance on biomass production in a large population of sorghum accessions. Breed. Sci. 2020, 70, 167. [Google Scholar] [CrossRef] [PubMed]
- Altuner, F.; Oral, E.; Baran, İ. Determination of the effects of salt (NaCl) stress on germination in some barley (Hordeum vulgare L.) varieties. J. Tekirdag Agric. Fac. 2022, 19, 39–50. [Google Scholar]
- Mosjidis, J.A.; Wang, M.L. Crotalaria. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ahlgren, H.G. Forage Crops, 2nd ed.; Department of Farm Crops Rutgers University; Mc. Graw-Hill Book Company Inc.: New York, NY, USA, 1956. [Google Scholar]
- Rotar, P.P.; Joy, R.J. ‘Tropic Sun’ Sunn Hemp, Crotalaria juncea L.; Research Extension Series 036; University of Hawaii: Honolulu, HI, USA, 1983. [Google Scholar]
- Bhandari, H.R.; Tripthi, M.K.; Chaudhary, B.; Sarkar, S.K. Sunn hemp breeding: Challenges and prospects. Indian J. Agric. Sci. 2016, 86, 1391–1398. [Google Scholar]
- Stallings, A. Sunn Hemp (Crotalaria juncea L.) as a Cover Crop for Winter Wheat. Master’s Thesis, Graduate Faculty of Auburn University, Auburn, AL, USA, 2015. [Google Scholar]
- Sadehukhan, S.; Sarkar, U. Production of biodiesel from Crotalaria juncea (Sunn-Hemp) oil using catalytic trans-esterification: Process optimization using a factorial and Box–Behnken Design. Waste Biomass Valorization 2016, 7, 343–355. [Google Scholar] [CrossRef]
- Demiroğlu Topcu, G.; Özkan, Ş.S. An alternative crop for Mediterranean climatic conditions: Crotalaria juncea L. (Sunn hemp). KSU J. Agric. Nat. 2019, 22, 339–345. [Google Scholar]
- Sheahan, C.M. Plant Guide for Sunn Hemp (Crotalaria juncea); USDA-Natural Resources Conservation Service, Cape May Plant Materials Center: Cape May, NJ, USA, 2012.
- Nedjimi, B.; Zemmiri, H. Salinity effects on germination of Artemisia herba–alba Asso: Important pastoral shrub from North African Rangelands. Rangel. Ecol. Manag. 2019, 72, 189–194. [Google Scholar] [CrossRef]
- Natasha, K.E. Effect of sodium chloride, potassium chloride on germination and growth of Foxtail millet (Setaria italica L.). Pure Appl. Biol. 2019, 8, 1398–1407. [Google Scholar] [CrossRef]
- Ouerghi, K.; Abdi, N.; Maazaoui, H.; Hmissi, I.; Bouraoui, M.; Sifi, B. Physiological and morphological characteristics of pea (Pisum sativum L.) seeds under salt stress. J. New Sci. 2016, 28, 1559–1565. [Google Scholar]
- Naim, A.H. Evaluation of chemical scarification and priming treatments to break physical dormancy of Crotalaria senegalensis seeds. Int. J. Adv. Agric. Environ. Eng. 2015, 2, 67–71. [Google Scholar]
- Dhanda, S.S.; Sethi, G.S.; Behl, R.K. Indices of drought tolerance in wheat genotypes at early stages of plant growth. J. Agron. Crop Sci. 2004, 190, 6–12. [Google Scholar] [CrossRef]
- Ates, E. Determining drought tolerance of new fodder pea and Persian clover genotypes at the germination and early seedling stages. Fresenius Environ. Bull. 2016, 25, 6020–6029. [Google Scholar]
- ISTA. International Rules for Seed Testing; The International Seed Testing Association: Zurich, Switzerland, 1996. [Google Scholar]
- Ellis, R.H.; Roberts, E.H. Towards a rational basis for testing seed quality. In Seed Production; Hebblethwaite, P.D., Ed.; Butterworths: London, UK, 1980; pp. 605–635. [Google Scholar]
- Borawska-Jarmułowicz, B.; Mastalerczuk, G.; Gozdowski, D.; Małuszyńska, E.; Szydłowska, A. The sensitivity of Lolium perenne and Poa pratensis to salinity and drought during the seed germination and under different photoperiod conditions. Zemdirbyste-Agriculture 2017, 104, 71. [Google Scholar] [CrossRef]
- Tenikecier, H.S.; Gençtan, T. A study on germination and seedling growth of seeds with different size created by translocation after fertilization in wheat (Triticum aestivum L. Em Thell). In Proceedings of the 10th Field Crops Congress Turkey, Konya, Türkiye, 10–13 September 2013; pp. 711–717. [Google Scholar]
- Tenikecier, H.S.; Ates, E. Chemical composition of six grass species (Poaceae sp.) from protected forest range in Northern Bulgaria. Asian J. Appl. Sci. 2018, 11, 71–75. [Google Scholar] [CrossRef]
- Clarke, J.M. Effect of leaf rolling on leaf water loss in Triticum ssp. Can. J. Plant Sci. 1986, 66, 885. [Google Scholar] [CrossRef]
- Ates, E.; Tekeli, A.S. Salinity tolerance of Persian clover (Trifolium resupinatum var. Majus Boiss.) lines at germination and seedling stage. J. Agric. Sci. 2007, 3, 71. [Google Scholar]
- Kargbo, S.S.; Showemimo, F.A.; Porbeni, J.B.O.; Akintokun, P.O. Response of rice genotypes to salinity under hydroponic conditions. Agro-Science 2019, 18, 11. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists: Official Methods of Analysis of AOAC International, 21st ed.; AOAC: Washington DC, USA, 2019. [Google Scholar]
- Plank, C.O. Plant Analysis Reference Procedures for the Southern Region of the United States; Sothern Cooperative Services Bulletin 368; University of GA: Athens, GA, USA, 1992. [Google Scholar]
- Isaac, R.A.; Johson, W.C., Jr. Elemental determination by inductively coupled plasma atomic emission spectrometry. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Washington, DC, USA, 1998; pp. 165–170. [Google Scholar]
- Acikgoz, N.; Ilker, E.; Gokçol, A. Assessment of Biological Research on the Computer; EU TOTEM No. 2; Ege University Press: Izmir, Türkiye, 2004. [Google Scholar]
- Düzgüneş, O.; Kesici, T.; Kavuncu, O.; Gürbüz, F. Research and Experimental Methods (Statistical Methods II); No.1021; Faculty of Agriculture Press, Ankara University: Ankara, Türkiye, 1987. (In Turkish) [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Pavli, O.I.; Foti, C.; Skoufogianni, G.; Karastergiou, G.; Panagou, A.; Khah, E.M. Effect of salinity on seed germination and seedling development of soybean genotypes. Int. J. Environ. Sci. Nat. Res. 2021, 27, 556210. [Google Scholar] [CrossRef]
- Okcu, M. Impact of salinity stress on germination and seedling development in feeding cowpea (Vigna unguiculata L. Walp). J. Inst. Sci. Technol. 2020, 10, 669–676. [Google Scholar]
- Nóbrega, J.S.; Silva, L.G.d.; Bezerra, A.C.; Bruno, R.d.L.A.; Souto, A.G.d.L.; Silva, T.I.d. Physiological response of seeds of Crotalaria spectabilis under drought and heat stress. Braz. Arch. Biol. Technol. 2022, 65, e22220145. [Google Scholar] [CrossRef]
- Misra, M.; Das, N.; Misra, A.N. Sodium chloride salt stress induced changes in protein content and protease activity in callus culture of pearl millet (P. galucum L. R. Br.). Acta Physiol. Plant. 1995, 17, 371–374. [Google Scholar]
- Lopez, M.V.; Satti, M.E. Calcium and potassium-enhanced growth and yield of tomato under sodium chloride stress. Plant Sci. 1996, 114, 19–27. [Google Scholar] [CrossRef]
- Evers, D.; Schmit, C.; Maillet, Y.; Hausman, J.F. Growth characteristics and biochemical changes of Poplar shoots in vitro under sodium chloride stress. J. Plant Physiol. 1997, 151, 748–753. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef]
- Kathpalia, R.; Bhatla, S.C. Plant Mineral Nutrition. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2018. [Google Scholar]
- Arzani, A.; Ashraf, M. Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. Crit. Rev. Plant Sci. 2016, 35, 146–189. [Google Scholar] [CrossRef]
- Sharp, R.E.; Hsiao, T.C.; Silk, W.K. Growth of the maize primary root at low water potentials: Role of growth and deposition of hexose and potassium in osmotic adjustment. Plant Physiol. 1990, 93, 1337–1346. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Keutgen, A.; Pawelzik, E. Impacts of NaCl stress on plant growth and mineral nutrient assimilation in two cultivars of strawberry. Environ. Exp. Bot. 2009, 65, 170–176. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Grusak, A.M. Plant Macro-and Micro Nutrient Minerals; Encyclopedia of Life Sciences, Nature Publishing Group: London, UK, 2001. [Google Scholar]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. Effects of salinity on growth, ion content, and osmotic relations in Halopyrum mocoronatum (L.) Stapf. J. Plant Nutr. 1999, 22, 191–204. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. Effects of sodium chloride treatments on growth and ion accumulation of the halophyte Haloxylon recurvum. Commun. Soil Sci. Plant Anal. 2000, 31, 2763–2774. [Google Scholar] [CrossRef]
- Khan, M.A. Experimental assessment of salinity tolerance of Ceriops tagal seedlings and saplings from the Indus delta, Pakistan. Aquat. Bot. 2001, 70, 259–268. [Google Scholar]
- Gadallah, M.A.A. Effects of proline and glycinebetaine on Vicia faba response to salt stress. Biol. Plant 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Tavora, F.J.A.F.; Hernandez, F.F.F. Dry matter partitioning and mineral composition of roots, stems and leaves of guava grown under salt stress conditions. Pesqui. Agropecu. Bras. 2001, 36, 79–88. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770. [Google Scholar] [CrossRef]
- Kadam Pratima, S. Effect of salinity on growth of Crotalaria species. Bioinfolet 2021, 18, 360–363. [Google Scholar]
- Mbarki, S.; Skalicky, M.; Vachova, P.; Hajihashemi, S.; Jouini, L.; Zivcak, M.; Tlustos, P.; Brestic, M.; Hejnak, V.; Zoghlami Khelil, A. Comparing salt tolerance at seedling and germination stages in local populations of Medicago ciliaris L. to Medicago intertexta L. and Medicago scutellata L. Plants 2020, 9, 526. [Google Scholar] [CrossRef]

| Characteristics | Salinity Level (mM NaCl) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | 200 | Mean | LSD0.01 | |
| Germination rate (%) | 95.00 bc | 96.66 b | 100.00 a | 93.33 c | 96.66 b | 96.33 | 2.97 |
| Mean germination time (day) | 2.00 b | 1.92 c | 1.98 bc | 2.04 ab | 2.07 a | 2.00 | 0.07 |
| Shoot length (cm) | 5.56 a | 4.34 b | 4.08 b | 3.26 c | 1.94 d | 3.84 | 0.39 |
| Root length (cm) | 4.24 a | 3.03 b | 1.75 c | 0.81 d | 0.65 d | 2.10 | 0.17 |
| Root fresh weight (mg) | 54.94 a | 44.46 b | 39.93 b | 28.03 c | 14.67 d | 30.34 | 5.39 |
| Root dry weight (mg) | 3.14 a | 2.49 b | 3.03 a | 2.93 a | 1.77 c | 2.67 | 0.31 |
| Shoot fresh weight (mg) | 283.93 a | 217.71 d | 265.07 b | 240.13 c | 177.15 e | 236.80 | 14.03 |
| Shoot dry weight (mg) | 20.47 b | 21.13 b | 25.80 a | 23.67 ab | 25.37 a | 23.29 | 3.48 |
| Retention Capability of Shoot (RCS) (mg) | 12.90 a | 9.42 b | 9.30 b | 9.15 b | 6.02 c | 9.36 | 1.76 |
| Relative Water Content (RWC) (%) | 92.79 a | 90.30 b | 90.25 b | 90.14 b | 85.61 c | 89.82 | 2.02 |
| Tolerance Index (TI) | 100.00 c | 103.65 bc | 126.41 a | 115.91 abc | 124.15 ab | 114.02 | 20.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demiroğlu Topçu, G.; Tenikecier, H.S.; Ateş, E. Macro-Mineral Uptake, Relative Water Content, Retention Capability, and Tolerance Index of Sunn Hemp (Crotalaria juncea L.) under Salinity Stress at Early Seedling. Agronomy 2024, 14, 823. https://doi.org/10.3390/agronomy14040823
Demiroğlu Topçu G, Tenikecier HS, Ateş E. Macro-Mineral Uptake, Relative Water Content, Retention Capability, and Tolerance Index of Sunn Hemp (Crotalaria juncea L.) under Salinity Stress at Early Seedling. Agronomy. 2024; 14(4):823. https://doi.org/10.3390/agronomy14040823
Chicago/Turabian StyleDemiroğlu Topçu, Gülcan, Hazım Serkan Tenikecier, and Ertan Ateş. 2024. "Macro-Mineral Uptake, Relative Water Content, Retention Capability, and Tolerance Index of Sunn Hemp (Crotalaria juncea L.) under Salinity Stress at Early Seedling" Agronomy 14, no. 4: 823. https://doi.org/10.3390/agronomy14040823
APA StyleDemiroğlu Topçu, G., Tenikecier, H. S., & Ateş, E. (2024). Macro-Mineral Uptake, Relative Water Content, Retention Capability, and Tolerance Index of Sunn Hemp (Crotalaria juncea L.) under Salinity Stress at Early Seedling. Agronomy, 14(4), 823. https://doi.org/10.3390/agronomy14040823






