The Use of a New Ionic Derivative of Salicylic Acid in Sugar Beet Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Substance
2.2. Locations of the Experimental Fields and Their Characteristics
2.3. Plant Material
2.4. Experimental Design
2.5. Assessment of C. beticola Infection
2.6. Assessment of Pathogen Infection
2.7. Assessment of Qualitative Parameters of Yield
2.8. Statistical Analysis
3. Results
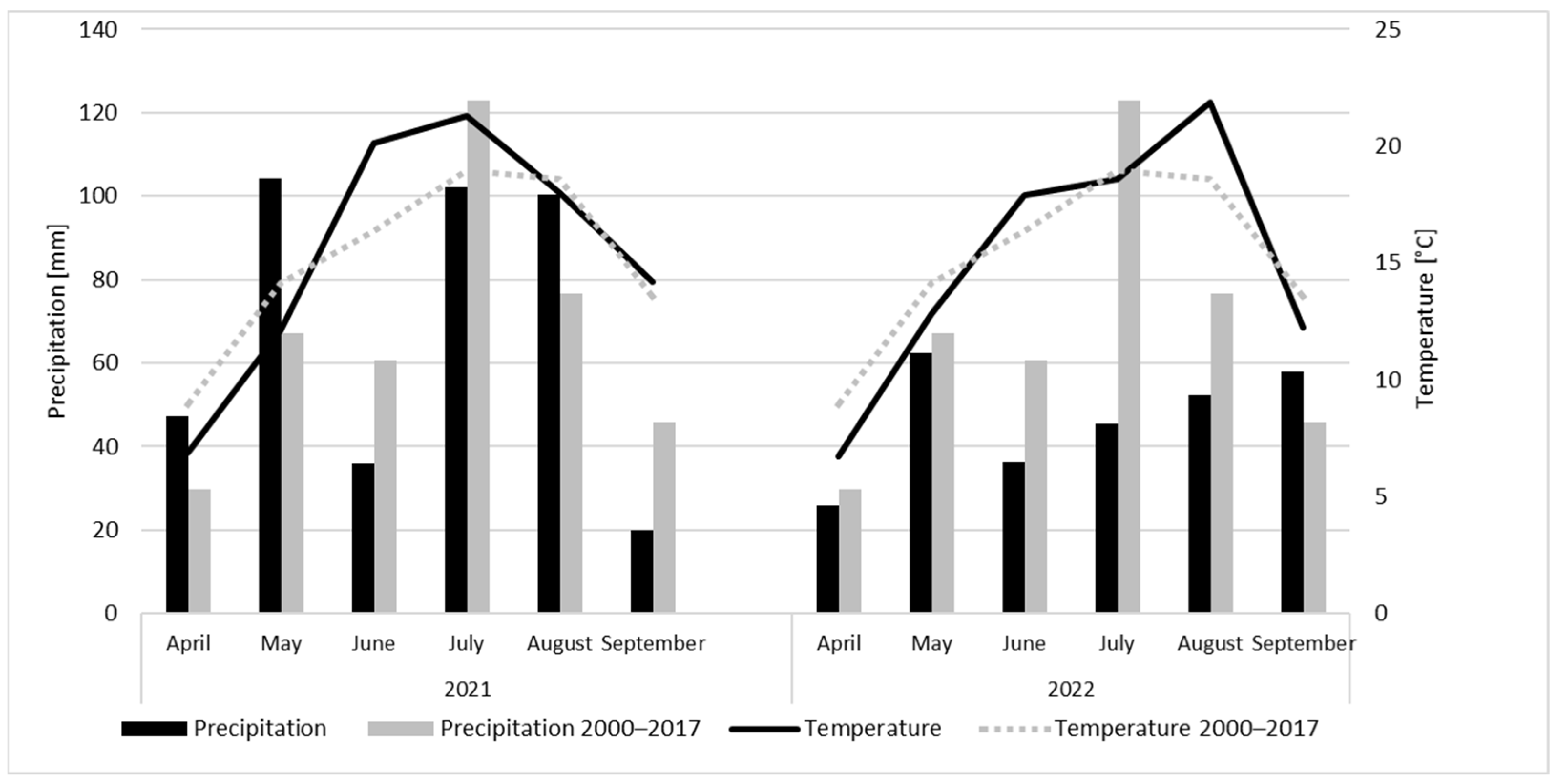
3.1. Weather Conditions
3.2. Assessment of Disease Severity
3.3. Assessment of Qualitative and Quantitative Parameters of Yield
3.3.1. Root Yield
3.3.2. Sugar Polarization
3.3.3. Potassium Content
3.3.4. Sodium Content
3.3.5. α-Amino Nitrogen Content
3.3.6. Technological Sugar Yield
3.4. Summary of Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statistical Yearbook of Agriculture; Główny Urząd Statystyczny: Warsaw, Poland, 2023; pp. 141–144.
- Khan, J.; Qi, A.; Khan, M.F.R. Fluctuations in number of Cercospora beticola conidia in relationship to environment and disease severity in sugar beet. Phytopathology 2009, 99, 796–801. [Google Scholar] [CrossRef]
- Pethybridge, S.J.; Sharma, S.; Hansen, Z.; Kikkert, J.R.; Olmstead, D.L.; Hanson, L.E. Optimizing Cercospora Leaf Spot Control in Table Beet Using Action Thresholds and Disease Forecasting. Plant Dis. 2020, 104, 1831–1840. [Google Scholar] [CrossRef]
- Kiniec, A.; Pieczul, K.; Piszczek, J. The First Detection of Multiple Resistant (MBC and QoI) Strains of Cercospora Beticola Sacc. in Poland. Crop Prot. 2022, 158, 106006. [Google Scholar] [CrossRef]
- Piszczek, J.; Pieczul, K.; Kiniec, A. First Report of G143A Strobilurin Resistance in Cercospora Beticola in Sugar Beet (Beta Vulgaris) in Poland. J. Plant Dis. Prot. 2018, 125, 99–101. [Google Scholar] [CrossRef]
- Fontana, D.C.; de Paula, S.; Torres, A.G.; de Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic Fungi: Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef]
- Wang, D.; Liu, B.; Ma, Z.; Feng, J.; Yan, H. Reticine A, a New Potent Natural Elicitor: Isolation from the Fruit Peel of Citrus Reticulate and Induction of Systemic Resistance against Tobacco Mosaic Virus and Other Plant Fungal Diseases. Pest Manag. Sci. 2021, 77, 354–364. [Google Scholar] [CrossRef]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical elicitors of systemic acquired resistance—Salicylic acid and its functional analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic Acquired Resistance and Salicylic Acid: Past, Present, and Future. Mol. Plant Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef]
- Shang, J.; Xi, D.-H.; Xu, F.; Wang, S.-D.; Cao, S.; Xu, M.-Y.; Zhao, P.-P.; Wang, J.-H.; Jia, S.-D.; Zhang, Z.-W. A Broad-Spectrum, Efficient and Nontransgenic Approach to Control Plant Viruses by Application of Salicylic Acid and Jasmonic Acid. Planta 2011, 233, 299–308. [Google Scholar] [CrossRef]
- Kukawka, R.; Czerwoniec, P.; Lewandowski, P.; Pospieszny, H.; Smiglak, M. New Ionic Liquids Based on Systemic Acquired Resistance Inducers Combined with the Phytotoxicity Reducing Cholinium Cation. New J. Chem. 2018, 42, 11984–11990. [Google Scholar] [CrossRef]
- Silverman, F.P.; Petracek, P.D.; Heiman, D.F.; Fledderman, C.M.; Warrior, P. Salicylate Activity. 3. Structure Relationship to Systemic Acquired Resistance. J. Agric. Food Chem. 2005, 53, 9775–9780. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.M.; Mosaad, A.H.I.S.; Sheta, M.H. Promote Sugar Beet Cultivation in Saline Soil by Applying Humic Substances In-Soil and Mineral Nitrogen Fertilization. J. Plant Nutr. 2022, 45, 2447–2464. [Google Scholar] [CrossRef]
- Nassar, M.A.A.; El-Magharby, S.S.E.; Ibrahim, N.S.M.; Kandil, E.E. Response of Sugar Beet Growth to Soil Application of Humic Acid and Foliar Application of Some Biostimulators under Saline Soil Conditions. Egypt. Acad. J. Biol. Sci. H. Bot. 2023, 14, 43–54. [Google Scholar] [CrossRef]
- Rašovský, M.; Pačuta, V.; Ducsay, L.; Lenická, D. Quantity and Quality Changes in Sugar Beet (Beta Vulgaris Provar. Altissima Doel) Induced by Different Sources of Biostimulants. Plants 2022, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
- Pačuta, V.; Rašovský, M.; Briediková, N.; Lenická, D.; Ducsay, L.; Zapletalová, A. Plant Biostimulants as an Effective Tool for Increasing Physiological Activity and Productivity of Different Sugar Beet Varieties. Agronomy 2024, 14, 62. [Google Scholar] [CrossRef]
- Bertoldo, G.; Chiodi, C.; Della Lucia, M.C.; Borella, M.; Ravi, S.; Baglieri, A.; Lucenti, P.; Ganasula, B.K.; Mulagala, C.; Squartini, A.; et al. Brown Seaweed Extract (BSE) Application Influences Auxin- and ABA-Related Gene Expression, Root Development, and Sugar Yield in Beta vulgaris L. Plants 2023, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- van Butselaar, T.; Van den Ackerveken, G. Salicylic Acid Steers the Growth–Immunity Tradeoff. Trends Plant Sci. 2020, 25, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Macías, J.P.; García, Y.C.; Núñez, M.; Díaz, K.; Olea, A.F.; Espinoza, L. Plant Growth-Defense Trade-Offs: Molecular Processes Leading to Physiological Changes. Int. J. Mol. Sci. 2021, 22, 693. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Hu, H.; Fan, B.; Zhu, C.; Chen, Z. Biosynthesis and Roles of Salicylic Acid in Balancing Stress Response and Growth in Plants. Int. J. Mol. Sci. 2021, 22, 11672. [Google Scholar] [CrossRef]
- Azami-Sardooei, Z.; Seifi, H.S.; de Vleesschauwer, D.; Höfte, M. Benzothiadiazole (BTH)-induced resistance against Botrytis cinerea is inversely correlated with vegetative and generative growth in bean and cucumber, but not in tomato. Australas. Plant Pathol. 2013, 42, 485–490. [Google Scholar] [CrossRef]
- Markiewicz, M.; Lewandowski, P.; Spychalski, M.; Kukawka, R.; Feder-Kubis, J.; Beil, S.; Smiglak, M.; Stolte, S. New Bifunctional Ionic Liquid-Based Plant Systemic Acquired Resistance (SAR) Inducers with an Improved Environmental Hazard Profile. Green Chem. 2021, 23, 5138–5149. [Google Scholar] [CrossRef]
- Kukawka, R.; Spychalski, M.; Stróżyk, E.; Byzia, E.; Zajac, A.; Kaczyński, P.; Łozowicka, B.; Pospieszny, H.; Smiglak, M. Synthesis, Characterization and Biological Activity of Bifunctional Ionic Liquids Based on Dodine Ion. Pest Manag. Sci. 2021, 78, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Czerwoniec, P.; Kukawka, R.; Spychalski, M.; Koczura, R.; Mokracka, J.; Smiglak, M. New Biologically Active Ionic Liquids with Benzethonium Cation-efficient SAR Inducers and Antimicrobial Agents. Pest Manag. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Smiglak, M.; Pospieszny, H.; Kukawka, R.; Spychalski, M. Ionic Derivatives of Aromatic Carboxylic Acid for Use as Plant Stimulants, a Method of Plant Stimulation, and the Application of These Derivatives in the Production of Compositions for Plant Stimulation. PCT/PL2023/050101, 29 December 2023. [Google Scholar]
- Rossi, V.; Battilani, P. Cercopri: A forecasting model for primary infections of Cercospora leaf spot of sugarbeet. EPPO Bull. 1991, 21, 527–531. [Google Scholar] [CrossRef]
- Chaube, H.S.; Singh, U.S. Plant Disease Management: Principles and Practices, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991; p. 335. [Google Scholar]
- Bock, C.H.; Chiang, K.S.; Del Ponte, E.M. Accuracy of plant specimen disease severity estimates: Concepts, history, methods, ramifications and challenges for the future. CAB Rev. Pers. Ag. Vet. Sci. Nutr. Nat. Res. 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D.; Siuda, A. Effect of the Application Date of Fertilizer Containing Silicon and Potassium on the Yield and Technological Quality of Sugar Beet Roots. Plants 2021, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K.; Märländer, B.; Puke, H.; Glattkowski, H.; Thielecke, K. Neubewertung des technischen Wertes von Zuckerrüben. Re-evaluation of technical value of sugar beet. Sugar Ind. 1995, 120, 113–121. [Google Scholar]
- Trzebiński, J. Ocena wydajności cukru z korzeni. In Produkcja Buraka Cukrowego; Gutmański, I., Ed.; PWRiL: Poznań, Poland, 1991; pp. 591–597. [Google Scholar]
- Artyszak, A.; Gozdowski, D. The Effect of Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR) on the Soil Properties, Root Yield, and Technological Quality of Sugar Beet. Agronomy 2020, 10, 1262. [Google Scholar] [CrossRef]
- Turczański, K.; Bełka, M.; Spychalski, M.; Kukawka, R.; Prasad, R.; Smiglak, M. Resistance Inducers for the Protection of Pedunculate Oak (Quercus robur L.) Seedlings against Powdery Mildew Erysiphe alphitoides. Plants 2023, 12, 635. [Google Scholar] [CrossRef]
- Turczański, K.; Bełka, M.; Kukawka, R.; Spychalski, M.; Smiglak, M. A Novel Plant Resistance Inducer for the Protection of European Ash (Fraxinus excelsior L.) against Hymenoscyphus fraxineus—Preliminary Studies. Forests 2021, 12, 1072. [Google Scholar] [CrossRef]
- Spychalski, M.; Kukawka, R.; Krzesiński, W.; Spiżewski, T.; Michalecka, M.; Poniatowska, A.; Puławska, J.; Mieszczakowska-Frąc, M.; Panasiewicz, K.; Kocira, A.; et al. Use of New BTH Derivative as Supplement or Substitute of Standard Fungicidal Program in Strawberry Cultivation. Agronomy 2021, 11, 1031. [Google Scholar] [CrossRef]
- Jarecka-Boncela, A.; Spychalski, M.; Ptaszek, M.; Włodarek, A.; Smiglak, M.; Kukawka, R. The Effect of a New Derivative of Benzothiadiazole on the Reduction of Fusariosis and Increase in Growth and Development of Tulips. Agriculture 2023, 13, 853. [Google Scholar] [CrossRef]
- Spychalski, M.; Kukawka, R.; Prasad, R.; Borodynko-Filas, N.; Stępniewska-Jarosz, S.; Turczański, K.; Smiglak, M. A New Benzothiadiazole Derivative with Systemic Acquired Resistance Activity in the Protection of Zucchini (Cucurbita pepo convar. giromontiina) against Viral and Fungal Pathogens. Plants 2023, 12, 43. [Google Scholar] [CrossRef]
- Gouda, M.I.M.; El-Naggar, A.; Yassin, M.A. Effect of Cercospora Leaf Spot Disease on Sugar Beet Yield. Am. J. Agric. 2022, 10, 138–143. [Google Scholar]
- Rossi, V.; Meriggi, P.; Biancerdi, E.; Rossi, F. Effect of Cercospora leaf spot on sugar beet growth, yield and quality. Adv. Sugar Beet Res. 2000, 2, 49–76. [Google Scholar]
- Kiniec, A.; Piszczek, J.; Miziniak, W.; Sitarski, A. Impact of the Variety and Severity of Cercospora Beticola Infection on the Qualitative and Quantitative Parameters of Sugar Beet Yields. Polish J. Agron. 2020, 41, 29–37. [Google Scholar]
- Artyszak, A.; Gozdowski, D. Influence of Various Forms of Foliar Application on Root Yield and Technological Quality of Sugar Beet. Agriculture 2021, 11, 693. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Official Journal of the European Union, Comission Implementating Regulation (EU) 2015/408 of 11 March 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015R0408 (accessed on 21 February 2024).
- Borrego, A.C.; Abreu, R.; Carreira, F.A.; Caetano, F.; Vasconcelos, A.L. Environmental Taxation on the Agri-Food Sector and the Farm to Fork Strategy: The Portuguese Case. Sustainability 2023, 15, 12124. [Google Scholar] [CrossRef]
| Year of Experiment | Name and Symbol of Location | Geographical Coordinates | Soil Characteristics | pH |
|---|---|---|---|---|
| 2021 | Skąpe (A) | 53°19′20″ N 18°30′17″ E | sandy loam | 7.3 |
| 2021 | Falęcin (B) | 53°13′06″ N 18°36′33″ E | sandy loam | 6.9 |
| 2022 | Skąpe (A) | 53°19′20″ N 18°30′17″ E | sandy loam | 6.9 |
| 2022 | Falęcin (B) | 53°13′06″ N 18°36′33″ E | sandy loam | 6.7 |
| Variant of Treatment | The Treatment Number and Date | ||||
|---|---|---|---|---|---|
| 1 July 2021 | 9 July 2021 | 15 July 2021 | 22 July 2021 | 12 August 2021 | |
| I | II | III | IV | V | |
| UTC | |||||
| SFP | Spyrale 475 EC | Eminent 125 ME | |||
| 50% SFP | Spyrale 475 EC | ||||
| 3,5diClSal | 3,5diClSal | 3,5diClSal | 3,5diClSal | 3,5diClSal | |
| 3,5diClSal (3 times) + 50% Fungicide | 3,5diClSal | 3,5diClSal | Spyrale 475 EC | 3,5diClSal | |
| 3,5diClSal (4 times) + 50% Fungicide | 3,5diClSal | 3,5diClSal | Spyrale 475 EC | 3,5diClSal | 3,5diClSal |
| Variant of Treatment | The Treatment Number and Date | ||||
|---|---|---|---|---|---|
| 5 July 2022 | 12 July 2022 | 18 July 2022 | 27 July 2022 | 22 August 2022 | |
| I | II | III | IV | V | |
| UTC | |||||
| SFP | Spyrale 475 EC | Eminent 125 ME | |||
| 50% SFP | Spyrale 475 EC | ||||
| 3,5diClSal | 3,5diClSal | 3,5diClSal | 3,5diClSal | 3,5diClSal | |
| 3,5diClSal (3 times) + 50% Fungicide | 3,5diClSal | 3,5diClSal | Spyrale 475 EC | 3,5diClSal | |
| 3,5diClSal (4 times) + 50% Fungicide | 3,5diClSal | 3,5diClSal | Spyrale 475 EC | 3,5diClSal | 3,5diClSal |
| Degree of Infection on the EPPO Scale | Description | Infected Leaf Area [%] | Corresponding Value of DSI [%] 1 |
|---|---|---|---|
| 0 | no symptoms | 0 | 0.00 |
| 1 | single spots on the leaf | 0.1 | 11.11 |
| 2 | single spots on the leaf | 1 | 22.22 |
| 3 | moderately numerous spots on the leaf that begin to merge | 2 | 33.33 |
| 4 | numerous spots on the leaf that are merged together | 5 | 44.44 |
| 5 | numerous spots on the leaves | 10 | 55.56 |
| 6 | numerous spots on the leaves | 25 | 66.67 |
| 7 | numerous spots on the leaves | 50 | 77.78 |
| 8 | numerous spots on the leaves | 75 | 88.89 |
| 9 | numerous spots on the leaves, new leaves begin to develop | 95 | 100.00 |
| Variant of Treatment | Location A (2021) | Location B (2021) | Mean (2021) | Location A (2022) | Location B (2021) | Mean (2022) | Mean (2021 and 2022) |
|---|---|---|---|---|---|---|---|
| UTC | 73.61 d | 68.06 d | 70.84 e | 98.33 d | 98.33 d | 98.33 d | 84.58 b |
| SFP | 36.12 a | 33.06 a | 34.59 a | 77.50 a | 69.44 a | 73.47 a | 54.03 a |
| 50% SFP | 43.05 b | 41.67 b | 42.36 c | 89.16 c | 80.00 b | 84.58 c | 63.47 a |
| 3,5diClSal | 60.84 c | 51.11 c | 55.98 d | 88.33 c | 87.78 c | 88.06 c | 72.01 ab |
| 3,5diClSal (3 times) + 50% Fungicide | 42.23 b | 38.62 ab | 40.42 bc | 84.72 bc | 80.83 b | 82.78 bc | 61.60 a |
| 3,5diClSal (4 times) + 50% Fungicide | 36.39 a | 33.34 a | 34.86 a | 81.94 ab | 73.33 a | 77.64 ab | 56.25 a |
| Root Yield [t ha−1] | Sugar Polarization [%] | Potassium Content [mmol 1000 g−1 of Pulp] | Sodium Content [mmol 1000 g−1 of Pulp] | α-Amino Nitrogen Content [mmol 1000 g−1 of Pulp] | Technological Sugar Yield [t ha−1] | ||
|---|---|---|---|---|---|---|---|
| Year (A) | 2021 | 65.85 • | 19.34 • | 35.12 •• | 5.70 •• | 5.76 •• | 11.61 • |
| 2022 | 63.29 •• | 17.21 •• | 38.47 • | 8.01 • | 16.61 • | 9.60 •• | |
| Localization (B) | Location A | 63.36 B | 18.55 A | 39.91 A | 5.85 B | 12.87 A | 10.55 A |
| Location B | 65.78 A | 18.00 B | 33.67 B | 7.86 A | 9.50 B | 10.67 A | |
| Treatment (C) | UTC | 58.57 d | 18.14 a | 37.09 ab | 7.74 a | 12.13 a | 9.52 c |
| SFP | 69.04 a | 18.29 a | 38.46 a | 6.48 a | 11.85 ab | 11.33 a | |
| 50% SFP | 63.08 c | 18.33 a | 35.92 b | 6.59 a | 9.77 b | 10.41 b | |
| 3,5diClSal | 62.90 c | 18.19 a | 36.44 ab | 7.23 a | 11.58 ab | 10.28 b | |
| 3,5diClSal (3 times) + 50% Fungicide | 66.25 b | 18.36 a | 37.05 ab | 6.42 a | 10.66 ab | 10.95 a | |
| 3,5diClSal (4 times) + 50% Fungicide | 67.58 ab | 18.33 a | 35.80 b | 6.68 a | 11.12 ab | 11.15 a |
| Source of Variance | Df1 | Df2 | Root Yield [t ha−1] | |
|---|---|---|---|---|
| F | η2 | |||
| Year (A) | 1 | 6 | 218.63 *** | 8.0 |
| Localization (B) | 1 | 6 | 71.77 *** | 7.1 |
| Treatment (C) | 5 | 60 | 46.98 *** | 59.3 |
| A × B | 1 | 6 | 4.30 ns | <1 |
| B × C | 5 | 60 | 4.31 ** | 5.4 |
| A × C | 5 | 60 | 2.31 ns | 2.9 |
| Source of Variance | Df1 | Df2 | Sugar Polarization [%] | |
|---|---|---|---|---|
| F | η2 | |||
| Year (A) | 1 | 6 | 506.73 *** | 81.0 |
| Localization (B) | 1 | 6 | 20.98 ** | 5.4 |
| Treatment (C) | 5 | 60 | <1 ns | <1 |
| A × B | 1 | 6 | <1 ns | <1 |
| B × C | 5 | 60 | <1 ns | <1 |
| A × C | 5 | 60 | <1 ns | <1 |
| Source of Variance | Df1 | Df2 | Potassium Content [mmol 1000 g−1 of Pulp] | |
|---|---|---|---|---|
| F | η2 | |||
| Year (A) | 1 | 6 | 14.28 ** | 15.0 |
| Localization (B) | 1 | 6 | 889.25 *** | 52.1 |
| Treatment (C) | 5 | 60 | 3.57 ** | 4.3 |
| A × B | 1 | 6 | 2.39 ns | <1 |
| B × C | 5 | 60 | 2.67 * | 3.2 |
| A × C | 5 | 60 | 3.30 ** | 4.0 |
| Source of Variance | Df1 | Df2 | Sodium Content [mmol 1000 g−1 of Pulp] | |
|---|---|---|---|---|
| F | η2 | |||
| Year (A) | 1 | 6 | 50.90 *** | 28.3 |
| Localization (B) | 1 | 6 | 30.71 *** | 21.3 |
| Treatment (C) | 5 | 60 | 2.16 ns | 4.8 |
| A × B | 1 | 6 | <1 ns | <1 |
| B × C | 5 | 60 | 4.08 ** | 9.0 |
| A × C | 5 | 60 | 1.09 ns | 2.4 |
| Source of Variance | Df1 | Df2 | α-Amino Nitrogen Content [mmol 1000 g−1 of Pulp] | |
|---|---|---|---|---|
| F | η2 | |||
| Year (A) | 1 | 6 | 479.24 *** | 75.4 |
| Localization (B) | 1 | 6 | 26.15 ** | 7.2 |
| Treatment (C) | 5 | 60 | 2.37 * | 1.6 |
| A × B | 1 | 6 | 11.23 * | 3.1 |
| B × C | 5 | 60 | 1.28 ns | <1 |
| A × C | 5 | 60 | <1 ns | <1 |
| Source of Variance | Df1 | Df2 | Technological Sugar Yield [t ha−1] | |
|---|---|---|---|---|
| F | η2 | |||
| Year (A) | 1 | 6 | 557.60 *** | 61.1 |
| Localization (B) | 1 | 6 | 2.16 ns | <1 |
| Treatment (C) | 5 | 60 | 29.98 *** | 22.9 |
| A × B | 1 | 6 | 3.77 ns | <1 |
| B × C | 5 | 60 | 3.42 ** | 2.6 |
| A × C | 5 | 60 | 1.84 ns | 1.4 |
| Variant of Treatment | Root Yield | Sugar Polarization | Potassium Content | Sodium Content | α-Amino Nitrogen Content | Technological Sugar Yield | DSI |
|---|---|---|---|---|---|---|---|
| UTC | - | - | - | - | - | - | - |
| SFP | 117.88% | 100.83% | 103.69% | 83.72% | 97.69% | 119.01% | 36.12% |
| 50% SFP | 107.70% | 101.05% | 96.85% | 85.14% | 80.54% | 109.35% | 24.96% |
| 3,5diClSal | 107.39% | 100.28% | 98.25% | 93.41% | 95.47% | 107.98% | 14.86% |
| 3,5diClSal (3 times) + 50% SFP | 113.11% | 101.21% | 99.89% | 82.95% | 87.88% | 115.02% | 27.17% |
| 3,5diClSal (4 times) + 50% SFP | 115.38% | 101.05% | 96.52% | 86.30% | 91.67% | 117.12% | 33.49% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukawka, R.; Spychalski, M.; Grzempa, B.; Smiglak, M.; Górski, D.; Gaj, R.; Kiniec, A. The Use of a New Ionic Derivative of Salicylic Acid in Sugar Beet Cultivation. Agronomy 2024, 14, 827. https://doi.org/10.3390/agronomy14040827
Kukawka R, Spychalski M, Grzempa B, Smiglak M, Górski D, Gaj R, Kiniec A. The Use of a New Ionic Derivative of Salicylic Acid in Sugar Beet Cultivation. Agronomy. 2024; 14(4):827. https://doi.org/10.3390/agronomy14040827
Chicago/Turabian StyleKukawka, Rafal, Maciej Spychalski, Bartosz Grzempa, Marcin Smiglak, Dariusz Górski, Renata Gaj, and Agnieszka Kiniec. 2024. "The Use of a New Ionic Derivative of Salicylic Acid in Sugar Beet Cultivation" Agronomy 14, no. 4: 827. https://doi.org/10.3390/agronomy14040827






