Deep Soil Water Availability Regulates the Transpiration of Afforested Apple Trees (Malus pumila Mill.) in a Sub-Humid Loess Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Site
2.2. Experimental Design
2.3. Measurements
2.3.1. Meteorological Measurements

2.3.2. Soil Water Content
2.3.3. Vegetation Survey
2.3.4. Probe Design and Sap Flow Measurements
2.3.5. Statistical Analysis
3. Results
3.1. Meteorology and Leaf Area Index
3.2. Soil Water Content (SWC)
3.3. Sap Flow Velocity
3.4. Transpiration of Apple Trees
3.5. Growing Season Water Stress
4. Discussion
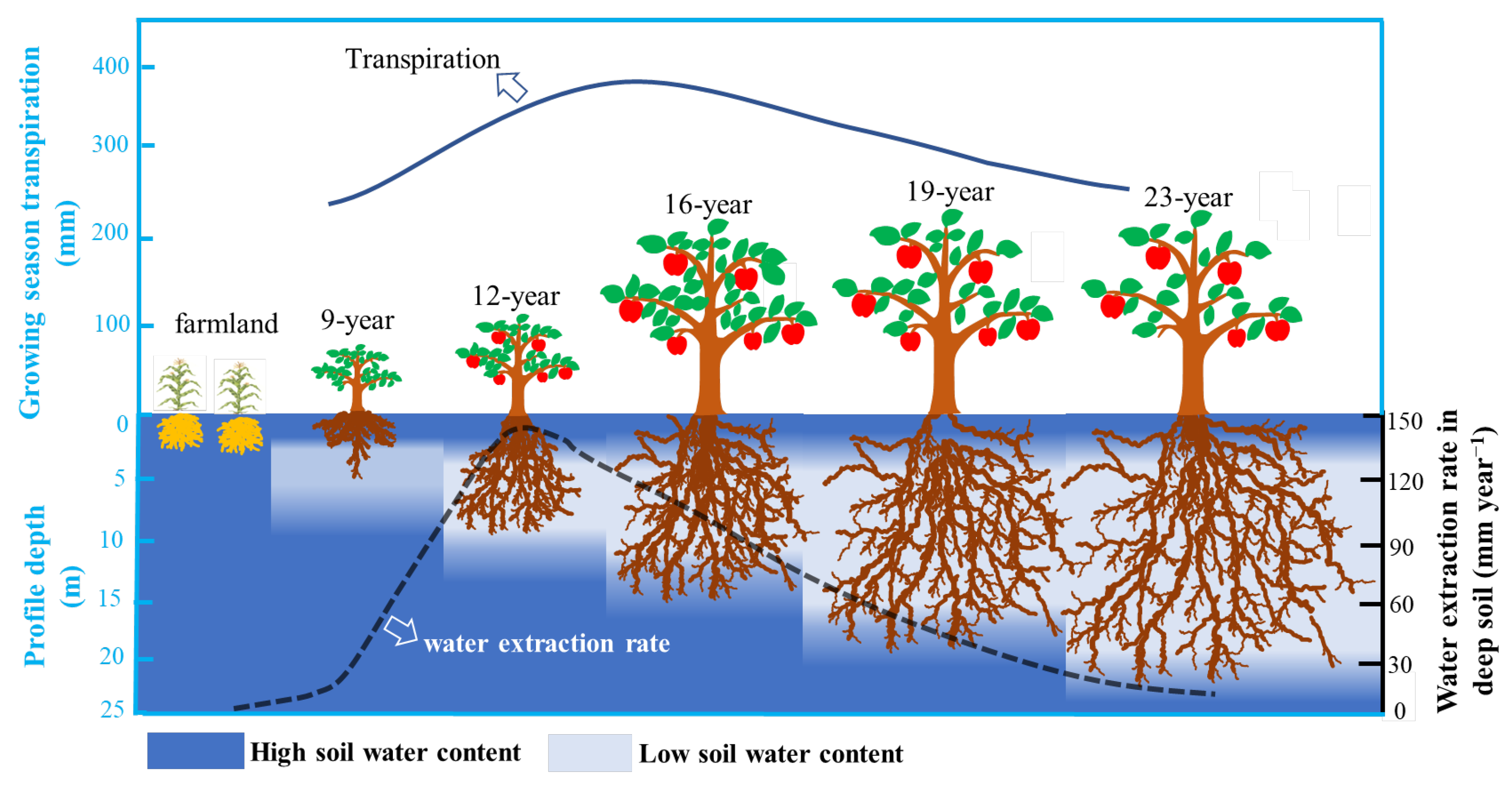
4.1. Deep Soil Cannot Provide Long-Term Stable Water for Apple Trees in the Loess Plateau
4.2. Soil Water Availability in Deep Soil Regulates Transpiration of Afforested Apple Trees
Strengths and Weaknesses of This Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kauppi, P.E.; Ciais, P.; Hogberg, P.; Nordin, A.; Lappi, J.; Lundmark, T.; Wernick, I.K. Carbon benefits from Forest Transitions promoting biomass expansions and thickening. Glob. Chang. Biol. 2020, 26, 5365–5370. [Google Scholar] [CrossRef]
- Di Sacco, A.; Hardwick, K.A.; Blakesley, D.; Brancalion, P.H.S.; Breman, E.; Cecilio Rebola, L.; Chomba, S.; Dixon, K.; Elliott, S.; Ruyonga, G.; et al. Ten golden rules for reforestation to optimize carbon sequestration, biodiversity recovery and livelihood benefits. Glob. Chang. Biol. 2021, 27, 1328–1348. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Kim, D.-G.; Li, M.; Huang, C.; Liu, Q.; Cheng, M.; Shangguan, Z.; Peng, C. Land-use changes driven by ‘Grain for Green’ program reduced carbon loss induced by soil erosion on the Loess Plateau of China. Glob. Planet. Chang. 2019, 177, 101–115. [Google Scholar] [CrossRef]
- Van Leeuwen, C.C.E.; Cammeraat, E.L.H.; de Vente, J.; Boix-Fayos, C. The evolution of soil conservation policies targeting land abandonment and soil erosion in Spain: A review. Land Use Policy 2019, 83, 174–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Han, Y.; Qian, M.; Guo, X.; Chen, R.; Xu, D.; Chen, Y. The contribution of Fintech to sustainable development in the digital age: Ant forest and land restoration in China. Land Use Policy 2021, 103, 105306. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, G.; Fu, B.; Gupta, H.V. Investigation of the relationship between precipitation extremes and sediment discharge production under extensive land cover change in the Chinese Loess Plateau. Geomorphology 2020, 361, 107176. [Google Scholar] [CrossRef]
- Feng, X.; Fu, B.; Piao, S.; Wang, S.; Ciais, P.; Zeng, Z. Revegetation in China’s Loess Plateau is approaching sustainable water resource limits. Nat. Clim. Chang. 2016, 6, 1019–1022. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, F. Ecological stoichiometry of plant leaves, litter and soils in a secondary forest on China’s Loess Plateau. PeerJ 2020, 8, e10084. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Lü, Z. Analysis of spatiotemporal variations in land use on the Loess Plateau of China during 1986–2010. Environ. Earth Sci. 2016, 75, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Lin, Y.; Shi, W.; Song, Y.; He, X. Balancing green and grain trade. Nat. Geosci. 2015, 8, 739–741. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Fu, B.; Feng, X.; Chen, Y. Socio-ecological changes on the Loess Plateau of China after Grain to Green Program. Sci. Total. Environ. 2019, 678, 565–573. [Google Scholar] [CrossRef]
- Liang, H.; Xue, Y.; Li, Z.; Gao, G.; Liu, G. Afforestation may accelerate the depletion of deep soil moisture on the Loess Plateau: Evidence from a meta-analysis. Land Degrad. Dev. 2022, 33, 3829–3840. [Google Scholar] [CrossRef]
- Chen, L.; Wei, W.; Fu, B.; Lu, Y. Soil and water conservation on the Loess Plateau in China: Review and perspective. Prog. Phys. Geogr. 2007, 31, 389–403. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qiu, K.; López-Vicente, M.; Shen, W.; Wu, G.-L. Soil-water deficit in deep soil layers results from the planted forest in a semi-arid sandy land: Implications for sustainable agroforestry water management. Agric. Water Manag. 2021, 254, 106985. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Wu, Q.; Si, B.; Jobbágy, E.G.; McDonnell, J.J. Afforestation triggers water mining and a single pulse of water for carbon trade-off in deep soil. Agric. Ecosyst. Environ. 2023, 356, 108655. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Yan, M.; Tao, Z.; He, D.; Du, G.; Li, H.; Jobbagy, E.; Li, M.; Si, B. Direct characterization of deep soil water depletion reveals hydraulic adjustment of apple trees to edaphic changes. Agric. For. Meteorol. 2024, 348, 109932. [Google Scholar] [CrossRef]
- Tie, Q.; Hu, H.; Tian, F.; Guan, H.; Lin, H. Environmental and physiological controls on sap flow in a subhumid mountainous catchment in North China. Agric. For. Meteorol. 2017, 240–241, 46–57. [Google Scholar] [CrossRef]
- Chang, X.; Zhao, W.; He, Z. Radial pattern of sap flow and response to microclimate and soil moisture in Qinghai spruce (Picea crassifolia) in the upper Heihe River Basin of arid northwestern China. Agric. For. Meteorol. 2014, 187, 14–21. [Google Scholar] [CrossRef]
- Castagneri, D.; Vacchiano, G.; Hacket-Pain, A.; DeRose, R.J.; Klein, T.; Bottero, A. Meta-analysis Reveals Different Competition Effects on Tree Growth Resistance and Resilience to Drought. Ecosystems 2021, 25, 30–43. [Google Scholar] [CrossRef]
- Page, G.F.M.; Liénard, J.F.; Pruett, M.J.; Moffett, K.B. Spatiotemporal dynamics of leaf transpiration quantified with time-series thermal imaging. Agric. For. Meteorol. 2018, 256–257, 304–314. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Pierret, A.; Maeght, J.L.; Clement, C.; Montoroi, J.P.; Hartmann, C.; Gonkhamdee, S. Understanding deep roots and their functions in ecosystems: An advocacy for more unconventional research. Ann. Bot. 2016, 118, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Germon, A.; Laclau, J.-P.; Robin, A.; Jourdan, C. Tamm Review: Deep fine roots in forest ecosystems: Why dig deeper? For. Ecol. Manag. 2020, 466, 118135. [Google Scholar] [CrossRef]
- Fan, Y.; Miguezmacho, G.; Jobbágy, E.G.; Jackson, R.B.; Oterocasal, C. Hydrologic regulation of plant rooting depth. Proc. Natl. Acad. Sci. USA 2017, 114, 10572–10577. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, D.; Wang, Y.; Wei, X.; Ma, L. Soil water and root distribution under jujube plantations in the semiarid Loess Plateau region, China. Plant Growth Regul. 2015, 77, 21–31. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.; Wu, P.; Zhao, X.; Li, H.; Ling, Q.; Sun, W. Soil Water Content and Root Patterns in a Rain-fed Jujube Plantation across Stand Ages on the Loess Plateau of China. Land Degrad. Dev. 2017, 28, 207–216. [Google Scholar] [CrossRef]
- Li, H.J.; Si, B.C.; Wu, P.T.; McDonnell, J.J. Water mining from the deep critical zone by apple trees growing on loess. Hydrol. Process. 2019, 33, 320–327. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Yang, M.; Zhang, L.; Wang, X.; Wu, P.; Zhao, X. The efficiency of organic C sequestration in deep soils is enhanced by drier climates. Geoderma 2022, 415, 115774. [Google Scholar] [CrossRef]
- Li, H.; Ma, X.; Lu, Y.; Ren, R.; Cui, B.; Si, B. Growing deep roots has opposing impacts on the transpiration of apple trees planted in subhumid loess region. Agric. Water Manag. 2021, 258, 107207. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Evaristo, J.; Li, Z.; Si, B.C.; McDonnell, J.J. Tritium analysis shows apple trees may be transpiring water several decades old. Hydrol. Process. 2017, 31, 1196–1201. [Google Scholar] [CrossRef]
- Rempe, D.M.; Dietrich, W.E. Direct observations of rock moisture, a hidden component of the hydrologic cycle. Proc. Natl. Acad. Sci. USA 2018, 115, 2664–2669. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Vaca, C.; Zotarelli, L.; Beeson Jr, R.C.; Morgan, K.T.; Migliaccio, K.W.; Chaparro, J.X.; Olmstead, M.A. Determining water requirements for young peach trees in a humid subtropical climate. Agric. Water Manag. 2020, 233, 106102. [Google Scholar] [CrossRef]
- Wheeler, W.; Wytsalucy, R.; Black, B.; Cardon, G.; Bugbee, B. Drought Tolerance of Navajo and Lovell Peach Trees: Precision Water Stress Using Automated Weighing Lysimeters. HortScience 2019, 54, 799–803. [Google Scholar] [CrossRef]
- Ford, C.R.; Hubbard, R.M.; Kloeppel, B.D.; Vose, J.M. A comparison of sap flux-based evapotranspiration estimates with catchment-scale water balance. Agric. For. Meteorol. 2007, 145, 176–185. [Google Scholar] [CrossRef]
- Pereira, L.S.; Paredes, P.; Jovanovic, N. Soil water balance models for determining crop water and irrigation requirements and irrigation scheduling focusing on the FAO56 method and the dual Kc approach. Agric. Water Manag. 2020, 241, 106357. [Google Scholar] [CrossRef]
- Evaristo, J.; Kim, M.; Haren, J.; Pangle, L.A.; Harman, C.J.; Troch, P.A.; McDonnell, J.J. Characterizing the Fluxes and Age Distribution of Soil Water, Plant Water, and Deep Percolation in a Model Tropical Ecosystem. Water Resour. Res. 2019, 55, 3307–3327. [Google Scholar] [CrossRef]
- Ding, Y.; Nie, Y.; Chen, H.; Wang, K.; Querejeta, J.I. Water uptake depth is coordinated with leaf water potential, water-use efficiency and drought vulnerability in karst vegetation. New Phytol. 2020, 229, 1339–1353. [Google Scholar] [CrossRef]
- Knauer, J.; Zaehle, S.; Medlyn, B.E.; Reichstein, M.; Williams, C.A.; Migliavacca, M.; De Kauwe, M.G.; Werner, C.; Keitel, C.; Kolari, P.; et al. Towards physiologically meaningful water-use efficiency estimates from eddy covariance data. Glob. Chang. Biol. 2018, 24, 694–710. [Google Scholar] [CrossRef]
- Kozii, N.; Haahti, K.; Tor-ngern, P.; Chi, J.; Hasselquist, E.M.; Laudon, H.; Launiainen, S.; Oren, R.; Peichl, M.; Wallerman, J.; et al. Partitioning growing season water balance within a forested boreal catchment using sap flux, eddy covariance, and a process-based model. Hydrol. Earth Syst. Sci. 2020, 24, 2999–3014. [Google Scholar] [CrossRef]
- Black, K.L.; Wallace, C.A.; Baltzer, J.L. Seasonal thaw and landscape position determine foliar functional traits and whole-plant water use in tall shrubs on the low arctic tundra. New Phytol. 2021, 231, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.J.; Aranda, X.; Llorens, P.; Galindo, A.; Biel, C. Sap flow of a wild cherry tree plantation growing under Mediterranean conditions: Assessing the role of environmental conditions on canopy conductance and the effect of branch pruning on water productivity. Agric. Water Manag. 2019, 218, 222–233. [Google Scholar] [CrossRef]
- Bodo, A.V.; Arain, M.A. Radial variations in xylem sap flux in a temperate red pine plantation forest. Ecol. Process. 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Xu, Y.; Peng, Y.; Chen, Y.; Zhao, P. Small inaccuracies in estimating narrow sapwood depth produce large error in sap velocity corrections. Ecohydrology 2022, 15, e2409. [Google Scholar] [CrossRef]
- Pappas, C.; Bélanger, N.; Bastien-Beaudet, G.; Couture, C.; D’Orangeville, L.; Duchesne, L.; Gennaretti, F.; Houle, D.; Hurley, A.G.; Klesse, S. Xylem porosity, sapwood characteristics, and uncertainties in temperate and boreal forest water use. Agric. For. Meteorol. 2022, 323, 109092. [Google Scholar] [CrossRef]
- Ford, C.R.; Goranson, C.E.; Mitchell, R.J.; Will, R.E.; Teskey, R.O. Diurnal and seasonal variability in the radial distribution of sap flow: Predicting total stem flow in Pinus taeda trees. Tree Physiol. 2004, 24, 941. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.R.; McGuire, M.A.; Mitchell, R.J.; Teskey, R.O. Assessing variation in the radial profile of sap flux density in Pinus species and its effect on daily water use. Tree Physiol. 2004, 24, 241. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, J.; Han, X.; Zhang, T.; Dong, Y.; Wu, M.; Qin, S.; Wei, Y.; Chen, Z.; Zhou, Y. Radial and seasonal variation of sap flow and its response to meteorological factors in sandy Pinus sylvestris var. mongolica plantations in the Three North Shelterbelt of China. Agric. For. Meteorol. 2023, 328, 109239. [Google Scholar] [CrossRef]
- Wang, N.; Wolf, J.; Zhang, F.-s. Towards sustainable intensification of apple production in China—Yield gaps and nutrient use efficiency in apple farming systems. J. Integr. Agric. 2016, 15, 716–725. [Google Scholar] [CrossRef]
- Allan, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper No. 56; FAO: Rome, Italy, 1998; Volume 56, pp. 147–151. [Google Scholar]
- Ritchie, J.T. Model for predicting evaporation from a row crop with incomplete cover. Water Resour. Res. 1972, 8, 1204–1213. [Google Scholar] [CrossRef]
- Li, H.; Si, B.; Ma, X.; Wu, P. Deep soil water extraction by apple sequesters organic carbon via root biomass rather than altering soil organic carbon content. Sci. Total Environ. 2019, 670, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Burgess, S.S.; Tu, K.P.; Oliveira, R.S.; Santiago, L.S.; Fisher, J.B.; Simonin, K.A.; Ambrose, A.R. Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiol. 2007, 27, 561. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Xu, X. Species with larger vessel area have higher bias for the original Granier equation in calculating sap flux density. J. Hydrol. 2023, 622, 129762. [Google Scholar] [CrossRef]
- Hatton, T.J.; Catchpole, E.A.; Vertessy, R.A. Integration of sapflow velocity to estimate plant water use. Tree Physiol. 1990, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.B.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Christina, M.; Nouvellon, Y.; Laclau, J.-P.; Stape, J.L.; Bouillet, J.-P.; Lambais, G.R.; le Maire, G.; Tjoelker, M. Importance of deep water uptake in tropical eucalypt forest. Funct. Ecol. 2016, 31, 509–519. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Tohver, I.M.; Ray, D.; Moutinho, P.; Cardinot, G. Mortality of Large Trees and Lianas following Experimental Drought in an Amazon Forest. Ecology 2007, 88, 2259. [Google Scholar] [CrossRef] [PubMed]
- Chitra-Tarak, R.; Xu, C.; Aguilar, S.; Anderson-Teixeira, K.J.; Chambers, J.; Detto, M.; Faybishenko, B.; Fisher, R.A.; Knox, R.G.; Koven, C.D.; et al. Hydraulically-vulnerable trees survive on deep-water access during droughts in a tropical forest. New Phytol. 2021, 231, 1798–1813. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Halberg, N.; Nicolaisen, M.; Olesen, J.E.; Crews, T.E.; Hinsinger, P.; Kirkegaard, J.; Pierret, A.; Dresboll, D.B. Digging Deeper for Agricultural Resources, the Value of Deep Rooting. Trends Plant Sci. 2020, 25, 406–417. [Google Scholar] [CrossRef]
- Tao, Z.; Neil, E.; Si, B.C. Determining deep root water uptake patterns with tree age in the Chinese loess area. Agric. Water Manag. 2021, 249, 106810. [Google Scholar] [CrossRef]
- Jia, X.; Shao, M.a.; Zhu, Y.; Luo, Y. Soil moisture decline due to afforestation across the Loess Plateau, China. J. Hydrol. 2017, 546, 113–122. [Google Scholar] [CrossRef]
- Li, H.; Si, B.; Li, M. Rooting depth controls potential groundwater recharge on hillslopes. J. Hydrol. 2018, 564, 164–174. [Google Scholar] [CrossRef]
- Vertessy, R.A.; Benyon, R.G.; O’Sullivan, S.K.; Gribben, P.R. Relationships between stem diameter, sapwood area, leaf area and transpiration in a young mountain ash forest. Tree Physiol. 1995, 15, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Enquist, B.J.; Brown, J.H.; West, G.B. Allometric scaling of plant energetics and population density. Nature 1998, 395, 163–165. [Google Scholar] [CrossRef]
- Otieno, D.; Li, Y.; Liu, X.; Zhou, G.; Cheng, J.; Ou, Y.; Liu, S.; Chen, X.; Zhang, Q.; Tang, X.; et al. Spatial heterogeneity in stand characteristics alters water use patterns of mountain forests. Agric. For. Meteorol. 2017, 236, 78–86. [Google Scholar] [CrossRef]
- Horna, V.; Schuldt, B.; Brix, S.; Leuschner, C. Environment and tree size controlling stem sap flux in a perhumid tropical forest of Central Sulawesi, Indonesia. Ann. For. Sci. 2011, 68, 1027–1038. [Google Scholar] [CrossRef]
- Saleska, S.R.; Didan, K.; Huete, A.R.; da Rocha, H.R. Amazon forests green-up during 2005 drought. Science 2007, 318, 612. [Google Scholar] [CrossRef]
- Peng, X.; Fan, J.; Wang, Q.; Warrington, D. Discrepancy of sap flow in Salix matsudana grown under different soil textures in the water-wind erosion crisscross region on the Loess Plateau. Plant Soil 2014, 390, 383–399. [Google Scholar] [CrossRef]
- Šimůnek, J.; Hopmans, J.W. Modeling compensated root water and nutrient uptake. Ecol. Model. 2009, 220, 505–521. [Google Scholar] [CrossRef]
- Ivanov, V.Y.; Hutyra, L.R.; Wofsy, S.C.; Munger, J.W.; Saleska, S.R.; de Oliveira, R.C.; de Camargo, P.B. Root niche separation can explain avoidance of seasonal drought stress and vulnerability of overstory trees to extended drought in a mature Amazonian forest. Water Resour. Res. 2012, 48, W12507. [Google Scholar] [CrossRef]
- Moser, G.; Schuldt, B.; Hertel, D.; Horna, V.; Coners, H.; Barus, H.; Leuschner, C. Replicated throughfall exclusion experiment in an Indonesian perhumid rainforest: Wood production, litter fall and fine root growth under simulated drought. Glob. Chang. Biol. 2014, 20, 1481–1497. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Dominguez, C.M.; Carins Murphy, M.R.; Lucani, C.; Brodribb, T.J. Mapping xylem failure in disparate organs of whole plants reveals extreme resistance in olive roots. New Phytol. 2018, 218, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Ranathunge, K.; Kim, Y.X.; Wassmann, F.; Kreszies, T.; Zeisler, V.; Schreiber, L. The composite water and solute transport of barley (Hordeum vulgare) roots: Effect of suberized barriers. Ann. Bot. 2017, 119, 629–643. [Google Scholar] [CrossRef]










| Stand Age Years | DBH cm | TDP Measure Depth cm | Crown Area m2 | Cumulative Ta mm |
|---|---|---|---|---|
| 9 | 11.92 ± 0.87 | 1, 3 | 13.57 ± 0.52 | NA |
| 12 | 15.36 ± 1.19 | 1, 3, 5 | 9.97 ± 0.11 | 267 |
| 15 | 18.18 ± 1.39 | 1, 3, 5, 7 | 12.07 ± 0.05 | 289 |
| 19 | 19.98 ± 1.54 | 1, 3, 5, 7 | 12.61 ± 0.80 | 274 |
| 23 | 19.26 ± 1.67 | 1, 3, 5, 7 | 8.57 ± 0.12 | 222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zuo, Y.; Zhang, X.; Wang, Y.; Wu, Z.; Liu, X.; Wu, N.; Lu, Y.; Li, H.; Si, B. Deep Soil Water Availability Regulates the Transpiration of Afforested Apple Trees (Malus pumila Mill.) in a Sub-Humid Loess Region. Agronomy 2024, 14, 841. https://doi.org/10.3390/agronomy14040841
Li P, Zuo Y, Zhang X, Wang Y, Wu Z, Liu X, Wu N, Lu Y, Li H, Si B. Deep Soil Water Availability Regulates the Transpiration of Afforested Apple Trees (Malus pumila Mill.) in a Sub-Humid Loess Region. Agronomy. 2024; 14(4):841. https://doi.org/10.3390/agronomy14040841
Chicago/Turabian StyleLi, Peng, Yuxiao Zuo, Xuemei Zhang, Yinglei Wang, Zhengli Wu, Xiaoyu Liu, Nan Wu, Yanwei Lu, Huijie Li, and Bingcheng Si. 2024. "Deep Soil Water Availability Regulates the Transpiration of Afforested Apple Trees (Malus pumila Mill.) in a Sub-Humid Loess Region" Agronomy 14, no. 4: 841. https://doi.org/10.3390/agronomy14040841
APA StyleLi, P., Zuo, Y., Zhang, X., Wang, Y., Wu, Z., Liu, X., Wu, N., Lu, Y., Li, H., & Si, B. (2024). Deep Soil Water Availability Regulates the Transpiration of Afforested Apple Trees (Malus pumila Mill.) in a Sub-Humid Loess Region. Agronomy, 14(4), 841. https://doi.org/10.3390/agronomy14040841






