An Optimized Protocol for Comprehensive Evaluations of Salt Tolerance in Crop Germplasm Accessions: A Case Study of Tomato (Solanum lycopersicum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Material Cultivation and Treatment
2.3. Measurement Items and Methods
2.3.1. Morphological Indices and Biomass Measurement
2.3.2. Chlorophyll (Chl) and Chl a Fluorescence (Fv/Fm) Determination
2.3.3. Determination of Index of Salinity Damage (IS) and Salt Tolerance Coefficient (STC)
2.4. Optimization of Comprehensive Evaluation Analysis for Salt Tolerance of Crop Germplasm
2.4.1. Optimization of the Evaluation Index System Construction Process
- (1)
- Identification of Evaluation Indicators. Based on the attributes and range of the STCs for each indicator, evaluation indicators are identified. These indicators are further categorized into positive and negative indicators. Positive indicators directly reflect the plant’s salt tolerance, with higher values signifying stronger tolerance. Conversely, negative indicators inversely reflect salt tolerance. The STCs of evaluation indicators typically range from 0 to 1. Except for evaluation indicators, other indicators are generally classified as descriptive indicators.
- (2)
- Filtering of Irrelevant Variables. The presence of irrelevant variables can affect the accuracy of salt tolerance identification. Thus, identifying and filtering out irrelevant variables is crucial. Using analysis of variance (ANOVA) within K-means clustering analysis, all evaluation indicators are tested, and those with significant value less than 0.05 are utilized for subsequent comprehensive evaluations of salt tolerance.
2.4.2. Data Preprocessing and Selection of SDRM
2.4.3. Optimization of the Weight Calculation Process
2.5. Stability of Comprehensive Evaluations Result
2.6. Concordance of Comprehensive Evaluations Result
2.7. Data Statistics and Analysis
3. Results
3.1. Analysis of Morphological Differences and Salt Tolerance Traits in Tomato Germplasm
3.1.1. Salt-Induced Morphological Differences
3.1.2. Salt Tolerance Trait Analysis
3.2. Optimization of the Comprehensive Evaluation Process
3.2.1. Analysis of the Construction of the Evaluation Index System
3.2.2. Analysis of Data Preprocessing and Dimensionality Reduction Method Selection
3.2.3. Analysis of Index Weight Calculation
3.3. Stability and Accuracy Analysis of Comprehensive Evaluation Results of the Optimized Protocol
3.3.1. Stability and Concordance Analysis of Comprehensive Evaluation Results
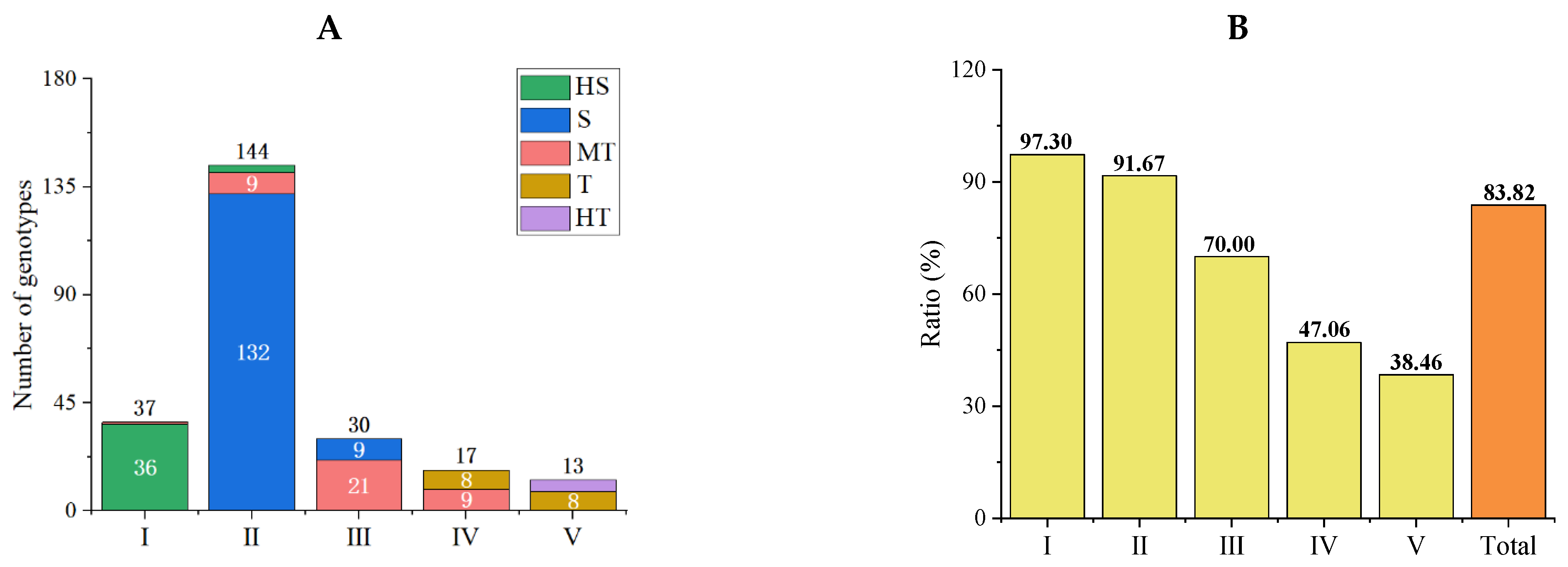
3.3.2. Qualitative Clustering Analysis of Salt Tolerance Traits in Tomato Germplasm
3.3.3. Accuracy Analysis of Comprehensive Evaluation Results of the Optimized Protocol
3.4. Secondary Validation of the Optimized Protocol’s Result Stability
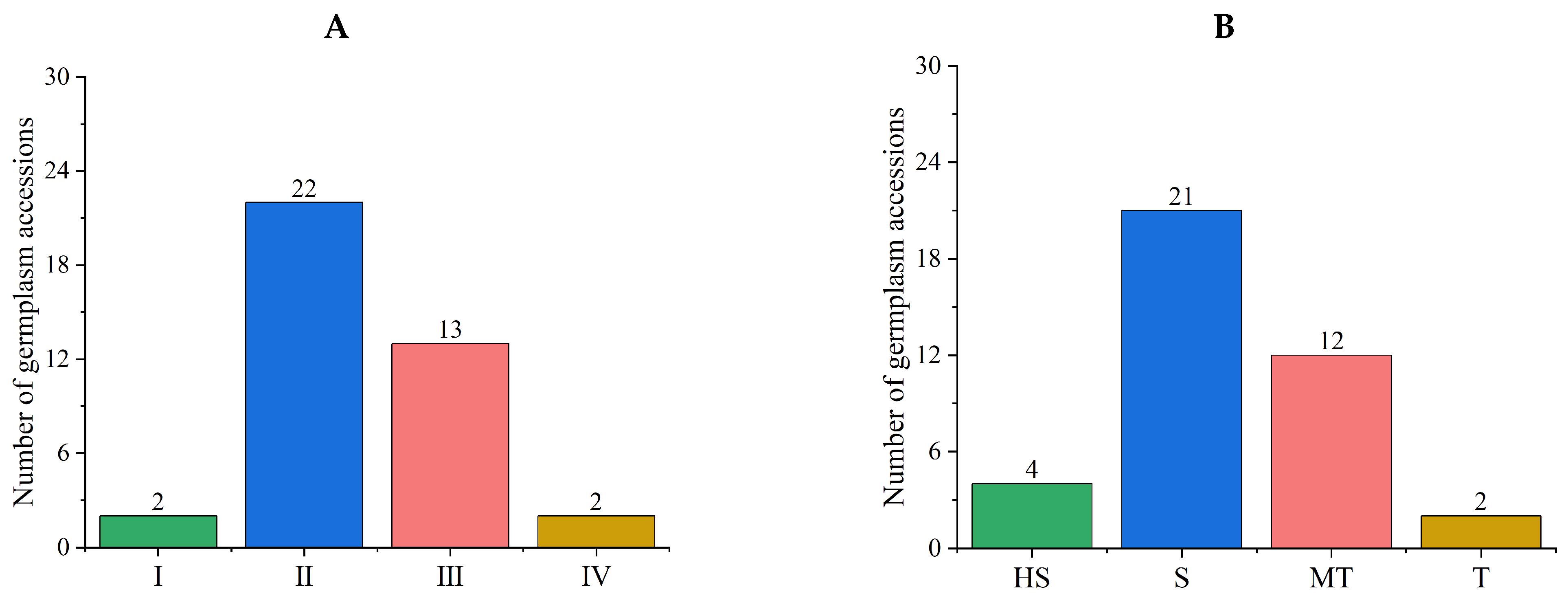
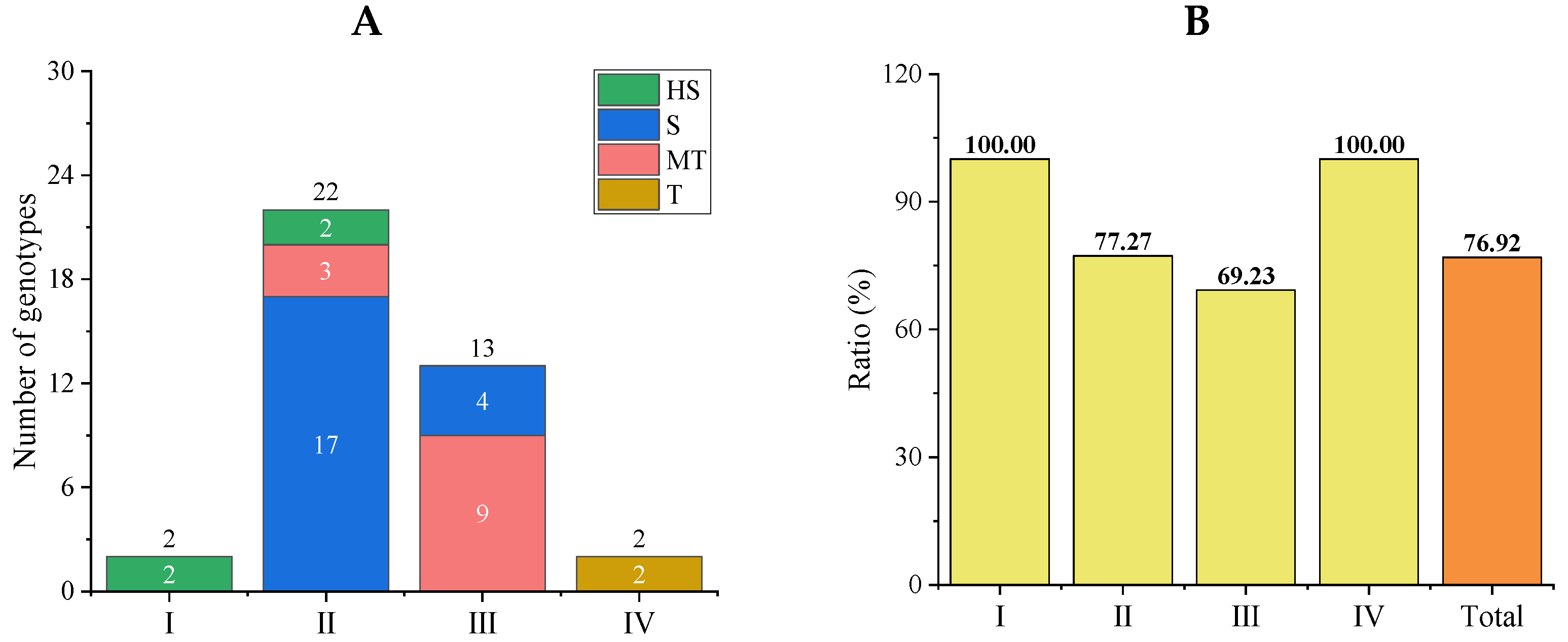
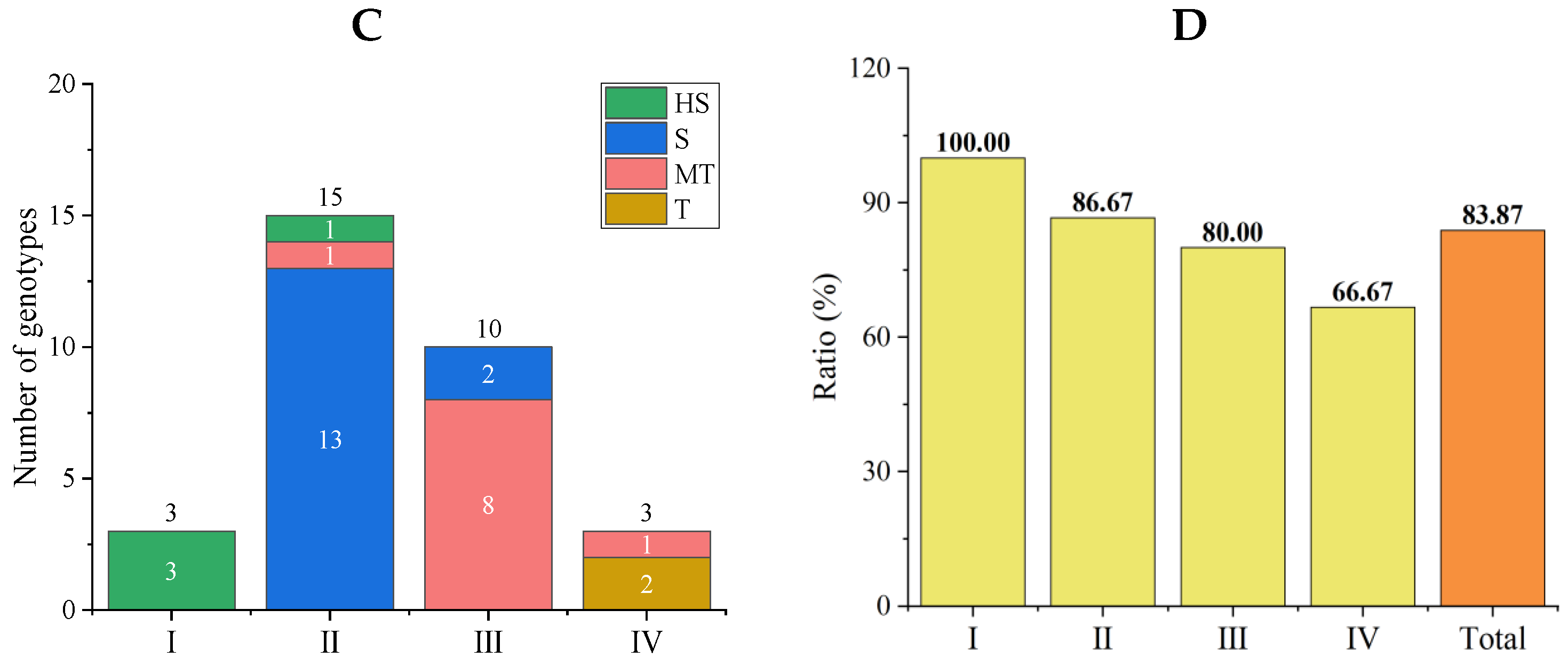
3.4.1. Comprehensive Evaluations of Salt Tolerance in a Tomato Subpopulation
3.4.2. Stability Analysis of Comprehensive Evaluation Results of the New Population
3.5. Salt Tolerance Analysis in Subpopulations of the Initial Population
4. Discussion
4.1. Evaluation of the Salt Tolerance of the Optimized Protocol and Analysis of Its Stability and Accuracy
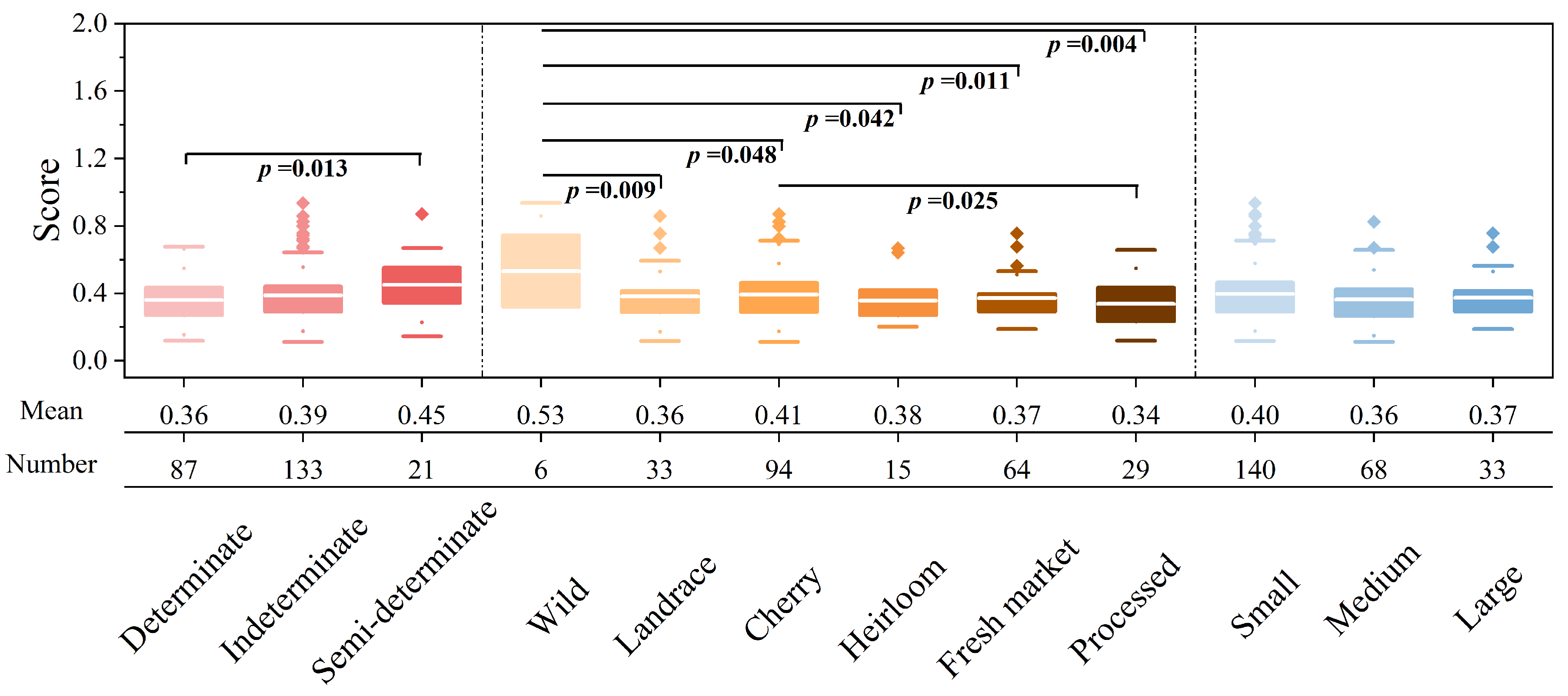
4.2. Salt Tolerance Disparities among Tomato Subgroups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Zelm, E.; Zhang, Y.X.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Bakirov, A.; Zhang, Y.; Zhang, Q.; Seitahmetovna, S.A.; Yu, X.J.; Shi, Y.J.; Xu, Y.; Wang, K.; Qin, M.F.; Xu, A.X.; et al. Screening of salt tolerance traits and the salt tolerance evaluation method in Brassica napus at the seed germination stage. Ital. J. Agron. 2022, 17, 2011. [Google Scholar] [CrossRef]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Ben-Gal, A.; Assouline, S.; Javaux, M.; Minhas, P.S.; et al. Critical knowledge gaps and research priorities in global soil salinity. Adv. Agron. 2021, 169, 1–191. [Google Scholar] [CrossRef]
- Zorb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.Y.; Awan, A.R.; Mahmood, K. Rehabilitation of saline ecosystems through cultivation of salt tolerant plants. Pak. J. Bot. 2012, 44, 69–75. [Google Scholar] [CrossRef]
- Yu, R.; Wang, G.; Yu, X.; Li, L.; Li, C.; Song, Y.; Xu, Z.; Zhang, J.; Guan, C. Assessing alfalfa (Medicago sativa L.) tolerance to salinity at seedling stage and screening of the salinity tolerance traits. Plant Biol. 2021, 23, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, J.R.; Wang, C.F.; Li, K.L.; Zhang, X.W.; Yang, Z.; Li, M.; Wang, B. An effective screening method and a reliable screening trait for salt tolerance of Brassica napus at the germination stage. Front. Plant Sci. 2019, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.P.; Li, Z.M.; Shen, W.J.; Lev, B. Multi-attribute comprehensive evaluation of individual research output based on published research papers. Knowl. Based Syst. 2013, 43, 135–142. [Google Scholar] [CrossRef]
- Qiu, D.; Liu, T.Y. Multi-indicator comprehensive evaluation: Reflection on methodology. Data Sci. Financ. Econ. 2021, 1, 298–312. [Google Scholar] [CrossRef]
- Liu, C.J.; Gong, X.W.; Wang, H.L.; Dang, K.; Deng, X.P.; Feng, B.L. Low-nitrogen tolerance comprehensive evaluation and physiological response to nitrogen stress in broomcorn millet (Panicum miliaceum L.) seedling. Plant Physiol. Biochem. 2020, 151, 233–242. [Google Scholar] [CrossRef]
- Aslam, M.; Maqbool, M.A.; Zaman, Q.U.; Shahid, M.; Arslan Akhtar, M.; Rana, A.S. Comparison of different tolerance indices and PCA biplot analysis for assessment of salinity tolerance in lentil (Lens culinaris) genotypes. Int. J. Agric. Biol. 2017, 19, 470–478. [Google Scholar] [CrossRef]
- Raza, M.A.; Saeed, A.; Munir, H.; Ziaf, K.; Shakeel, A.; Saeed, N.; Munawar, A.; Rehman, F. Screening of tomato genotypes for salinity tolerance based on early growth attributes and leaf inorganic osmolytes. Arch. Agron. 2017, 63, 501–512. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, C.Y.; Liang, S.H.; Yuan, Y.H.; Liu, C.J.; Liu, J.J.; Feng, B.L. The alkali tolerance of broomcorn millet (Panicum miliaceum L.) at the germination and seedling stage: The case of 296 broomcorn millet genotypes. Front. Plant Sci. 2021, 12, 711429. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Gao, X.H. Research on discriminability and stability of comprehensive evaluation results. Stat. Decis. 2022, 38, 16–21. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J. A system of selecting multi-index comprehensive evaluation method. Acad. J. Appl. Math. Sci. 2018, 4, 95–97. Available online: http://arpgweb.com/?ic=journal&journal=17&info=aims (accessed on 29 March 2024).
- Guo, M.; Wang, X.S.; Guo, H.D.; Bai, S.Y.; Khan, A.; Wang, X.M.; Gao, Y.M.; Li, J.S. Tomato salt tolerance mechanisms and their potential applications for fighting salinity: A review. Front. Plant Sci. 2022, 13, 949541. [Google Scholar] [CrossRef] [PubMed]
- FAO. Crop Queries of Production Volume of Data [EB/OL]. Available online: https://www.fao.org/faostat/en/#data/QCLe (accessed on 29 March 2024).
- Su, X.; Wang, B.A.; Geng, X.L.; Du, Y.F.; Yang, Q.Q.; Liang, B.; Meng, G.; Gao, Q.; Yang, W.C.; Zhu, Y.F.; et al. A high-continuity and annotated tomato reference genome. BMC Genom. 2021, 22, 898. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, H. The regulation of nutrient and flavor metabolism in tomato fruit. Veg. Res. 2022, 2, 41–54. [Google Scholar] [CrossRef]
- Fatima, N.; Saeed, A.; Shakeel, A.; Awan, F.S. Evaluation of tomato germplasm for salinity tolerance at the seedling stage. SABRAO J. Breed Genet. 2022, 54, 1159–1170. [Google Scholar] [CrossRef]
- Kumar, P.A.; Reddy, N.N.; Jyothi Lakshmi, N. Screening tomato genotypes for salt tolerance. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1037–1049. [Google Scholar] [CrossRef]
- Du, X.W.; Si, L.Q.; Jin, X.; Li, P.F.; Yun, Z.H.; Gao, K.H. Classification of plug seedling quality by improved convolutional neural network with an attention mechanism. Front. Plant Sci. 2022, 13, 967706. [Google Scholar] [CrossRef] [PubMed]
- Rao, E.S.; Kadirvel, P.; Symonds, R.C.; Ebert, A.W. Relationship between survival and yield related traits in Solanum pimpinellifolium under salt stress. Euphytica 2013, 190, 215–228. [Google Scholar] [CrossRef]
- Rehman, F.; Saeed, A.; Yaseen, M.; Shakeel, A.; Ziaf, K.; Munir, H.; Tariq, S.A.; Raza, M.A.; Riaz, A. Genetic evaluation and characterization using cluster heat map to assess NaCl tolerance in tomato germplasm at the seedling stage. Chil. J. Agric. Res. 2019, 79, 56–65. [Google Scholar] [CrossRef]
- Nasrin, S.; Mannan, M.A.; Islam, M.M.; Khan, S.; Rahman, S.M.M. Evaluation of fifteen tomato germplasm for salt tolerance. Bangladesh Agric. J. 2022, 24, 43–54. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Islam, M.N.; Hossain, M.F.B.; Rashid, M.M.; Ahamed, K.U. Evaluation of tomato germplasm against salinity. Bull. Inst. Trop. Agric. Kyushu Univ. 2013, 36, 9–16. [Google Scholar] [CrossRef]
- Li, Y.K.; Jiang, F.L.; Niu, L.F.; Wang, G.; Yin, J.; Song, X.M.; Ottosen, C.; Rosenqvist, E.; Mittler, R.; Wu, Z.; et al. Synergistic regulation at physiological, transcriptional, and metabolic levels in tomato plants subjected to a combination of salt and heat stress. Plant J. 2024, 117, 1656–1675. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shah Jahan, M.; Mao, P.P.; Wang, M.M.; Liu, X.Y.; Guo, S.R. Functional growth, photosynthesis and nutritional property analyses of lettuce grown under different temperature and light intensity. J. Hortic. Sci. Biotechnol. 2020, 96, 53–61. [Google Scholar] [CrossRef]
- Gao, X.H.; Li, X.Q. Comparative study of linear dimensionless methods in principal component analysis. Stat. Decis. 2020, 36, 35–36. [Google Scholar] [CrossRef]
- Sun, M.T.; Jiang, F.L.; Zhang, C.; Shen, M.; Wu, Z. A new comprehensive evaluation system for thermo-tolerance in tomato at different growth stage. J. Agric. Sci. Technol. B 2016, 6, 151–167. [Google Scholar] [CrossRef]
- Su, J.S.; Zhang, F.; Li, P.R.; Guan, Z.Y.; Fang, W.M.; Chen, F.D. Genetic variation and association mapping of waterlogging tolerance in chrysanthemum. Planta 2016, 244, 1241–1252. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, X.; Wang, Y.; Chang, W.; Gao, X.Q.; Fu, B.Z. Salt tolerance evaluation and salt tolerance index screening of 59 alfalfa germplasm materials at seedling stage. Acta Agrestia Sin. 2020, 28, 112–121. [Google Scholar] [CrossRef]
- Gao, X.H.; Li, X.Q. Comparison of comprehensive evaluation methods based on information extraction. Stat. Decis. 2020, 36, 27–31. [Google Scholar] [CrossRef]
- Sim, S.C.; Robbins, M.D.; Van Deynze, A.; Michel, A.P.; Francis, D.M. Population structure and genetic differentiation associated with breeding history and selection in tomato (Solanum lycopersicum L.). Heredity 2011, 106, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.C.; Van Deynze, A.; Stoffel, K.; Douches, D.S.; Zarka, D.; Ganal, M.W.; Chetelat, R.T.; Hutton, S.F.; Scott, J.W.; Gardner, R.G.; et al. High-density SNP genotyping of tomato (Solanum lycopersicum L.) reveals patterns of genetic variation due to breeding. PLoS ONE 2012, 7, e45520. [Google Scholar] [CrossRef]
- Li, B. Problems existing in application of multi-index comprehensive evaluation method and countermeasures. J. Shenyang Inst. Eng. 2010, 6, 349–351. [Google Scholar] [CrossRef]
- Zhao, M.H.; Bai, Y.M.; Meng, L.B.; Sun, T.T.; Wang, D.Q.; Jing, C.Y.; Lv, W.H. Evaluation of salt tolerance of diploid potato and screening of salt tolerance indices. Chin. J. Ecol. 2022, 41, 610–617. [Google Scholar] [CrossRef]
- Bauer, C. Multi-method evaluation: Leveraging multiple methods to answer what you were looking for. In Proceedings of the 2020 Conference on Human Information Interaction and Retrieval, Vancouver, BC, Canada, 14–18 March 2020; pp. 472–474. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, Y.C.; Zhu, G.G.; Li, Y.M.; Niu, Q.F.; Yao, J.J.; Hua, K.; Bai, J.J.; Zhu, Y.F.; Shi, H.Z.; et al. Loss of salt tolerance during tomato domestication conferred by variation in a Na+/K+ transporter. EMBO J. 2020, 39, e103256. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.H.; Guan, X.; Wang, X.; Kong, D.L.; Yu, S.J.; Wang, Z.Q.; Yu, Y.D.; Chao, Z.F.; Liu, X.; Huang, S.W.; et al. Natural variation in SlSOS2 promoter hinders salt resistance during tomato domestication. Hortic. Res. 2023, 10, 232–239. [Google Scholar] [CrossRef]
- Li, N.; He, Q.; Wang, J.; Wang, B.K.; Zhao, J.T.; Huang, S.Y.; Yang, T.; Tang, Y.P.; Yang, S.B.; Aisimutuola, P.; et al. Super-pangenome analyses highlight genomic diversity and structural variation across wild and cultivated tomato species. Nat. Genet. 2023, 55, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.E.M.; Yokoi, S. A comparative in vitro study of salt tolerance in cultivated tomato and related wild species. Plant Biotechnol. 2016, 33, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhou, R.; Jiang, F.L.; Wen, J.Q.; Liu, S.; Wu, Z. Evaluation of salt tolerance of introgression line population of Solanum pimpinellifolium LA2093 at seedling stage. Jiangsu J. Agric. Sci. 2022, 38, 1620–1626. [Google Scholar] [CrossRef]

| Trait | Range | Mean ± S.D. | CV (%) | Skewness | Kurtosis |
|---|---|---|---|---|---|
| PHR | 0.25–0.87 | 0.41 ± 0.09 | 22.06 | 1.54 | 4.36 |
| FFR | 0.06–0.71 | 0.30 ± 0.12 | 38.18 | 0.99 | 0.95 |
| SFR | 0.05–0.72 | 0.29 ± 0.12 | 39.62 | 1.05 | 1.27 |
| RFR | 0.07–1.00 | 0.38 ± 0.17 | 45.42 | 1.00 | 1.12 |
| FDR | 0.15–1.04 | 0.39 ± 0.15 | 38.90 | 1.38 | 2.50 |
| SDR | 0.15–1.06 | 0.38 ± 0.15 | 39.13 | 1.48 | 3.16 |
| RDR | 0.10–1.08 | 0.46 ± 0.21 | 44.62 | 1.00 | 0.64 |
| IS | 0.24–0.97 | 0.66 ± 0.16 | 25.16 | −0.36 | −0.29 |
| SPADR | 0.55–1.45 | 0.98 ± 0.12 | 12.46 | 0.13 | 1.48 |
| DSRR | 0.36–2.14 | 0.88 ± 0.25 | 28.30 | 1.12 | 3.18 |
| FSRR | 0.30–2.38 | 0.84 ± 0.32 | 38.24 | 1.32 | 2.50 |
| FDWR | 0.21–1.28 | 0.78 ± 0.15 | 18.80 | −0.22 | 1.31 |
| Indicator | Principal Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Eigenvalue | 5.539 | 0.951 | 0.648 |
| Contribution rate % | 69.238 | 11.883 | 8.095 |
| Total contribution rate % | 69.238 | 81.121 | 89.216 |
| FFR | 0.951 | −0.028 | 0.089 |
| FDR | 0.935 | −0.206 | −0.042 |
| SFR | 0.919 | −0.051 | 0.210 |
| SDR | 0.914 | −0.225 | 0.021 |
| RDR | 0.860 | −0.049 | −0.360 |
| RFR | 0.813 | 0.113 | −0.413 |
| PHR | 0.739 | 0.122 | 0.541 |
| IS (reversed) | 0.366 | 0.908 | −0.031 |
| Group | PHR | FFR | SFR | RFR | FDR | SDR | RDR | IS |
|---|---|---|---|---|---|---|---|---|
| I | 034 e | 0.17 e | 0.16 d | 0.22 d | 0.24 e | 0.24 d | 0.27 e | 0.81 a |
| II | 0.40 d | 0.27 d | 0.26 c | 0.35 c | 0.35 d | 0.34 c | 0.42 d | 0.66 b |
| III | 0.47 c | 0.41 c | 0.40 b | 0.43 b | 0.51 c | 0.51 b | 0.56 c | 0.64 b |
| IV | 0.51 b | 0.46 b | 0.43 b | 0.65 a | 0.58 b | 0.55 b | 0.79 b | 0.58 b |
| V | 0.60 a | 0.57 a | 0.56 a | 0.70 a | 0.78 a | 0.77 a | 0.89 a | 0.46 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Li, X.; Zhou, R.; Hu, E.; Peng, X.; Jiang, F.; Wu, Z. An Optimized Protocol for Comprehensive Evaluations of Salt Tolerance in Crop Germplasm Accessions: A Case Study of Tomato (Solanum lycopersicum L.). Agronomy 2024, 14, 842. https://doi.org/10.3390/agronomy14040842
Chen Z, Li X, Zhou R, Hu E, Peng X, Jiang F, Wu Z. An Optimized Protocol for Comprehensive Evaluations of Salt Tolerance in Crop Germplasm Accessions: A Case Study of Tomato (Solanum lycopersicum L.). Agronomy. 2024; 14(4):842. https://doi.org/10.3390/agronomy14040842
Chicago/Turabian StyleChen, Zheng, Xin Li, Rong Zhou, Enmei Hu, Xianghan Peng, Fangling Jiang, and Zhen Wu. 2024. "An Optimized Protocol for Comprehensive Evaluations of Salt Tolerance in Crop Germplasm Accessions: A Case Study of Tomato (Solanum lycopersicum L.)" Agronomy 14, no. 4: 842. https://doi.org/10.3390/agronomy14040842







