Comparison of Juvenile Development of Maize and Sorghum in Six Temperate Soil Types under Extreme Water Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Analysis of Soil Samples
2.2. Experimental Setup and Irrigation Schemes
2.3. Experimental Measurements
2.3.1. Germination and Growth Indices
2.3.2. In Vivo Stress Analytical Method
2.4. Statistical Methods
3. Results
3.1. Germination and Growth Rate
3.1.1. Laboratory Germination Test
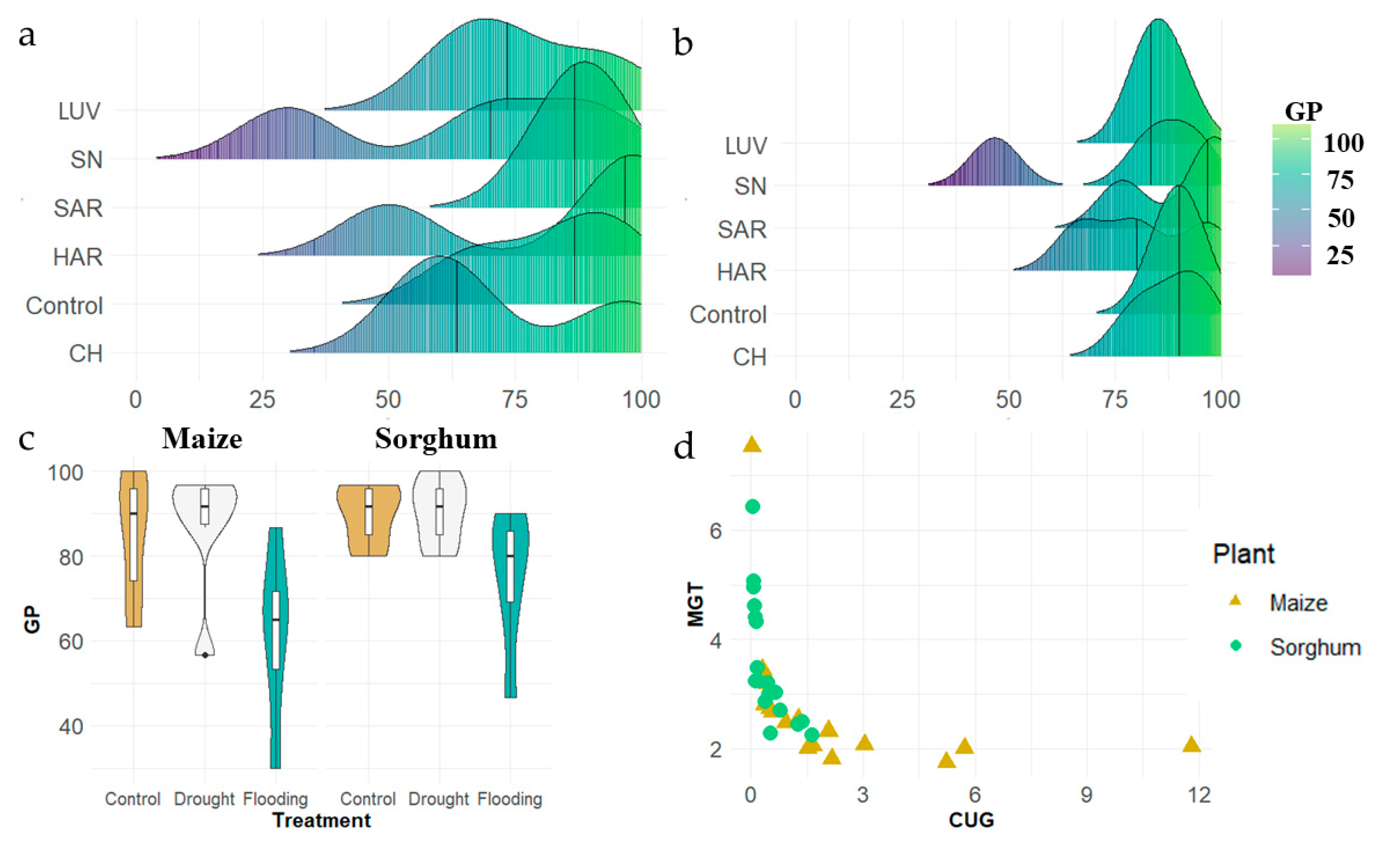
3.1.2. Germination in Different Environmental Conditions
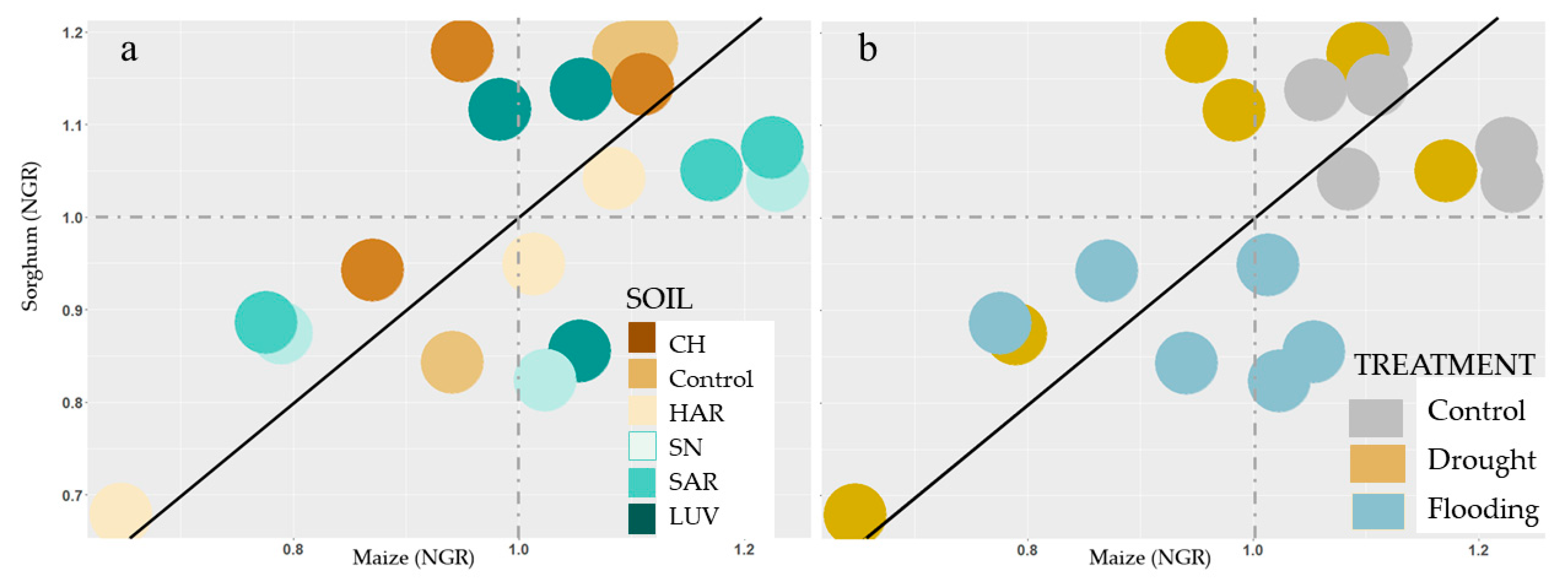
3.1.3. Growth Rate in Different Environmental Conditions
3.2. Comparison of Stress Tolerance of Maize and Sorghum
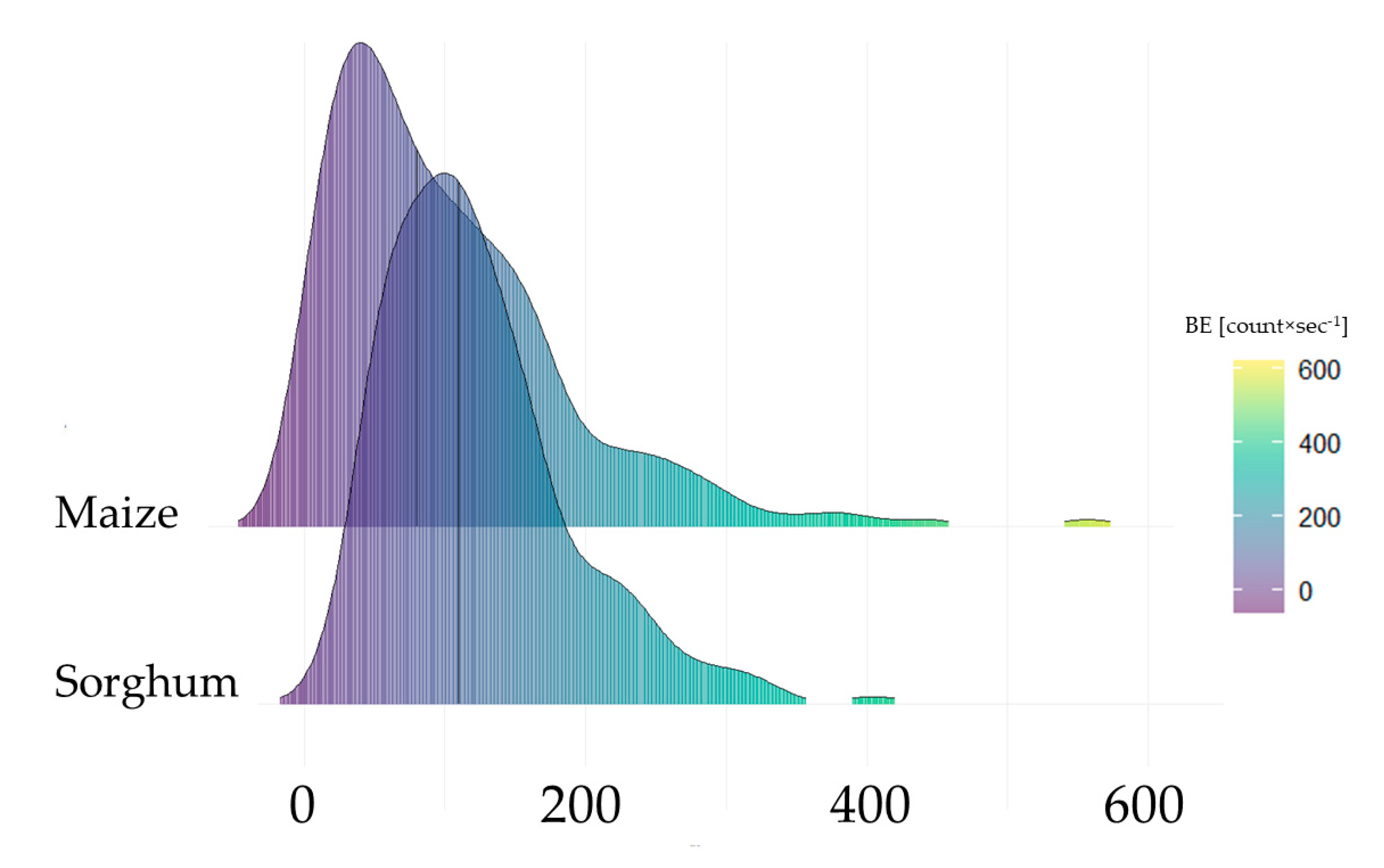
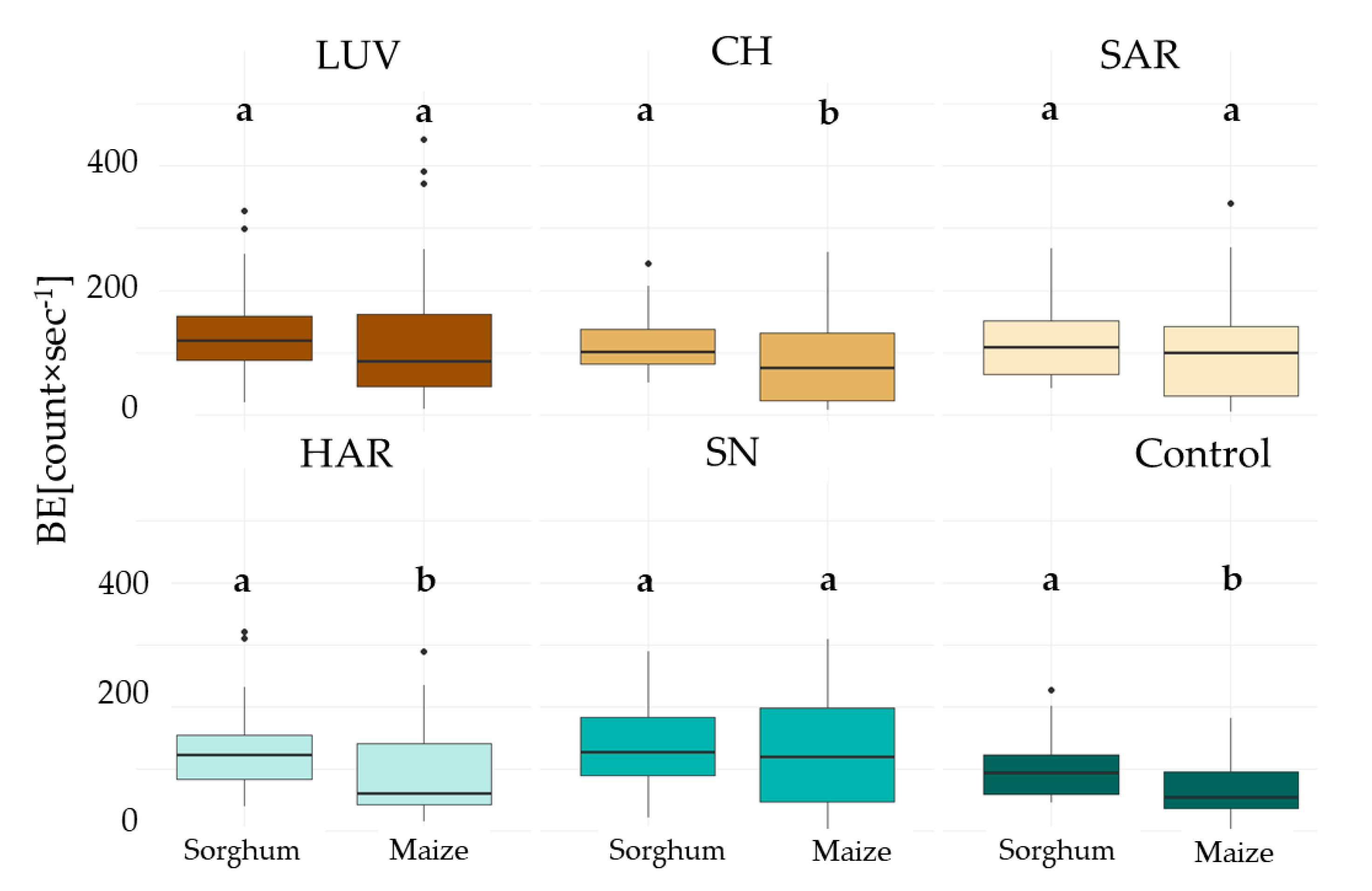
3.2.1. Initial Biophoton Emission Intensity Results
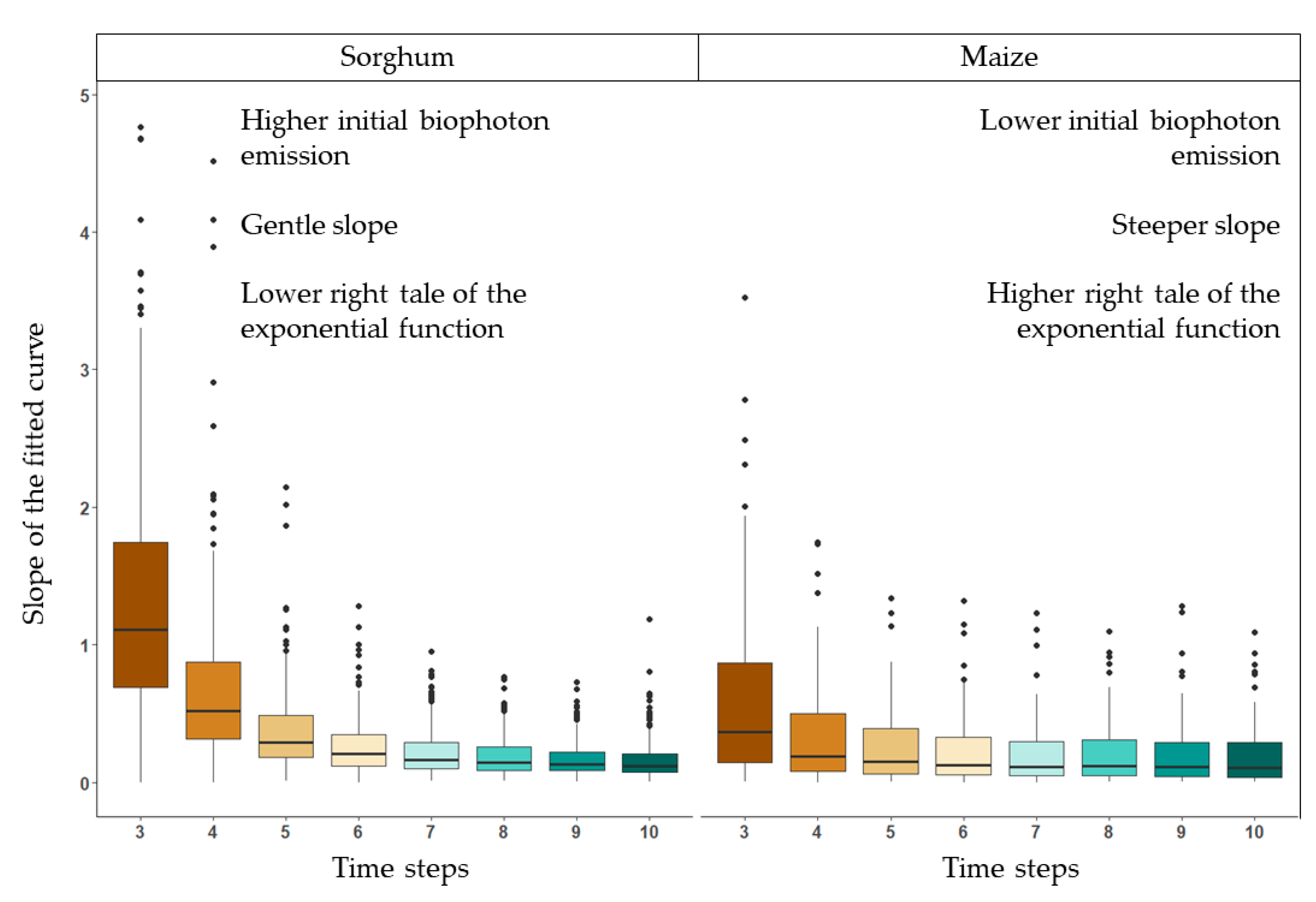
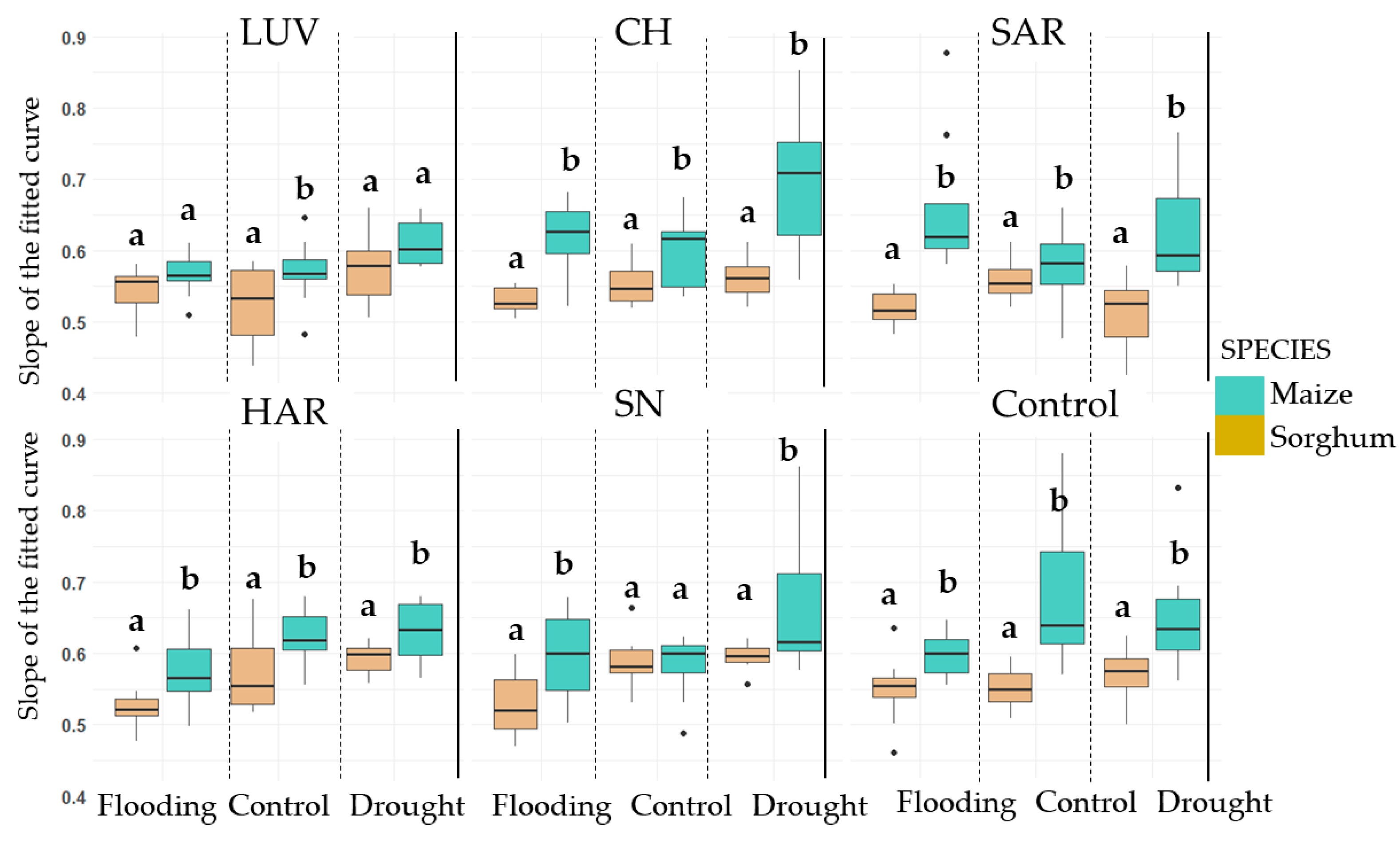
3.2.2. Decay of the Biophoton Emission Intensities
4. Discussion
4.1. Germination
4.2. Growth Rate
4.3. Analysis of Stress Tolerance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khanal, A.R.; Mishra, A.K. Enhancing food security: Food crop portfolio choice in response to climatic risk in India. Glob. Food Sec. 2017, 12, 22–30. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Mustroph, A. Improving Flooding Tolerance of Crop Plants. Agronomy 2018, 8, 160. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 76566. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Chen, H.Y.; Ruan, H. Response of plants to water stress: A meta-analysis. Front. Plant Sci. 2020, 11, 541682. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. J. Hortic. 2021, 7, 50. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wang, H.; Liu, S.; Li, Z.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.; Qin, F.A. Transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 2015, 6, 8326. [Google Scholar] [CrossRef] [PubMed]
- Bunce, J.A. Leaf transpiration efficiency of some drought-resistant maize lines. Crop Sci. 2010, 50, 1409. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Li, X.; Fan, X.; Dong, S.; Liu, P.; Zhao, B. Effects of Waterlogging on the Yield and Growth of Summer Maize under Field Conditions. Can. J. Plant Sci. 2014, 94, 23–31. [Google Scholar] [CrossRef]
- Mangani, R.; Tesfamariam, E.H.; Bellocchi, G.; Hassen, A. Growth, development, leaf gaseous exchange, and grain yield response of maize cultivars to drought and flooding stress. Sustainability 2018, 10, 3492. [Google Scholar] [CrossRef]
- Borrell, A.; van Oosterom, E.; George-Jaeggli, B.; Vadez, V.; Singh, V.; Hammer, G. Physiology of Growth, Development and Yield. In Sorghum in the 21st Century: Food–Fodder–Feed–Fuel for a Rapidly Changing World, 1st ed.; Tonapi, V.A., Talwar, H.S., Are, A.K., Bhat, B.V., Reddy, C.R., Dalton, T.J., Eds.; Springer: Singapore, Singapore, 2020; pp. 127–155. [Google Scholar]
- Yahaya, M.A.; Shimelis, H. Drought stress in sorghum: Mitigation strategies, breeding methods and technologies—A review. J. Agron. Crop Sci. 2022, 208, 127–142. [Google Scholar] [CrossRef]
- Zhang, R.; Yue, Z.; Chen, X.; Huang, R.; Zhou, Y.; Cao, X. Effects of waterlogging at different growth stages on the photosynthetic characteristics and grain yield of sorghum (Sorghum bicolor L.). Sci. Rep. 2023, 13, 7212. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.L. Effects of Flooding or Ponding on Corn prior to Tasseling. Corny News Network 2024. Available online: http://www.Kingcorn.Org/news/timeless/pondingyoungcorn.html (accessed on 19 April 2024).
- Kaur, G.; Zurweller, B.; Motavalli, P.P.; Nelson, K.A. Screening Corn Hybrids for Soil Waterlogging Tolerance at an Early Growth Stage. Agriculture 2019, 9, 33. [Google Scholar] [CrossRef]
- Gerhardt, V.; Bodemer, U. Delayed fluorescence excitation spectroscopy: A method for determining phytoplankton composition. Archiv. Für. Hydrobiol. 2000, 55, 101–120. [Google Scholar]
- Jócsák, I.; Lukács, H.; Varga-Visi, É.; Somfalvi-Tóth, K.; Keszthelyi, S. Identification and investigation of barley powdery mildew (Blumeria graminis f. sp. tritici) infection in winter wheat with conventional stress reactions and non-invasive biophoton emission parameters. J. Biosci. 2023, 49, 6. [Google Scholar] [CrossRef]
- Bodemer, U.; Gerhardt, V.; Yacobi, Y.Z.; Zohary, Z.; Friedrich, G.; Pohlmann, M. Phytoplankton abundance and composition of freshwaters systems determined by DF excitation spectroscopy and conventional methods. Arch. Hydrobiol. Spec. Issues Adv. Limnol. 2000, 55, 101–120. [Google Scholar]
- Salvatori, E.; Fusaro, L.; Gottardini, E.; Pollastrini, M.; Goltsev, V.; Strasser, R.J.; Bussotti, F. Plant stress analysis: Application of prompt, delayed chlorophyll fluorescence and 820 nm modulated reflectance. Insights from independent experiments. Plant Physiol. Biochem. 2014, 85, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lukács, H.; Jócsák, I.; Somfalvi-Tóth, K.; Keszthelyi, S. Physiological responses manifested by some conventional stress parameters and biophoton emission in winter wheat as a consequence of cereal leaf beetle infestation. Front. Plant Sci. 2022, 13, 839855. [Google Scholar] [CrossRef] [PubMed]
- Pónya, Z.; Somfalvi-Tóth, K. Modelling biophoton emission kinetics based on the initial intensity value in Helianthus annuus plants exposed to different types of stress. Sci. Rep. 2022, 12, 2317. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.Y.; Bai, Y.W.; Camberato, J.J.; Xue, J.Q.; Zhang, R.H. Effects of drought stress on the photosynthesis in maize. Russ. J. Plant Physiol. 2018, 65, 849–856. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Bi, W.; Zuo, S.; Li, L.; Li, W.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) under field conditions. Agric. Water Manag. 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Kamal, A.H.M.; Komatsu, S. Proteins involved in biophoton emission and flooding-stress responses in soybean under light and dark conditions. Mol. Biol. Rep. 2016, 43, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Daku, E.K.; Salack, S.; Worou, O.N.; Ogunjobi, K. Maize response to temporary floods under ambient on-farm conditions of the West African Sahel. Environ. Res. Commun. 2022, 4, 045004. [Google Scholar] [CrossRef]
- Staggenborg, S.A.; Dhuyvetter, K.C.; Gordon, W.B. Grain sorghum and corn comparisons: Yield, economic, and environmental responses. Agron. J. 2008, 100, 1600–1604. [Google Scholar] [CrossRef]
- Choudhary, S.; Guha, A.; Kholova, J.; Pandravada, A.; Messina, C.D.; Cooper, M.; Vadez, V. Maize, sorghum, and pearl millet have highly contrasting species strategies to adapt to water stress and climate change-like conditions. Plant Sci. 2020, 295, 110297. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, A.; Hura, T. Physiological Comparison of Wheat and Maize Seedlings Responses to Water Stresses. Sustainability 2022, 14, 7932. [Google Scholar] [CrossRef]
- Promkhambut, A.; Younger, A.; Polthanee, A.; Akkasaeng, C. Morphological and physiological responses of sorghum (Sorghum bicolor L. Moench) to waterlogging. Asian J. Plant Sci. 2010, 9, 183. [Google Scholar] [CrossRef]
- Promkhambut, A.; Polthanee, A.; Akkasaeng, C.; Younger, A. Growth, yield and aerenchyma formation of sweet and multipurpose sorghum (’Sorghum bicolor’ L. Moench) as affected by flooding at different growth stages. Aust. J. Crop Sci. 2011, 5, 954–965. [Google Scholar]
- Djanaguiraman, M.; Prasad, P.V.V.; Ciampitti, I.A.; Talwar, H.S. Impacts of abiotic stresses on sorghum physiology. In Sorghum in the 21st Century: Food–Fodder–Feed–Fuel for a Rapidly Changing World; Springer: Singapore, Singapore, 2020; pp. 157–188. [Google Scholar]
- Keten, M.; Değirmenci, H. Comparison of stress susceptibility index of silage corn and sorghum crops. Infrastrukt. i Ekol. Teren. Wiej. 2022, 1, 21–28. [Google Scholar]
- Pásztor, L.; Laborczi, A.; Bakacsi, Z.; Szabó, J.; Illés, G. Compilation of a national soil-type map for Hungary by sequential classification methods. Geoderma 2018, 311, 93–108. [Google Scholar] [CrossRef]
- Schad, P. World Reference Base for Soil Resources—Its fourth edition and its history. JPNSS 2023, 186, 151–163. [Google Scholar] [CrossRef]
- Haqiqi, I.; Grogan, D.S.; Hertel, T.W.; Schlenker, W. Quantifying the impacts of compound extremes on agriculture. HESS 2021, 25, 551–564. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G. de How and why to measure the germination process? Rev. Bras. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Gianinetti, A. Basic features of the analysis of germination data with generalized linear mixed models. Data 2020, 5, 6. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2023. Available online: https://www.R-project.org/ (accessed on 18 April 2024).
- Wickham, H.; Hester, J.; Chang, W.; Bryan, J. devtools: Tools to Make Developing R Packages Easier. 2022. Available online: https://cran.r-project.org/web/packages/devtools/devtools.pdf (accessed on 18 April 2024).
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of Salinity on Seed Germination and Seedling Development of Sorghum (Sorghum bicolor (L.) Moench) Genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Glob. Change Biol. 2013, 20, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.; Beltrami, E.; El-Khadem, R.; Khajeh-Hosseini, M.; Nasehzadeh, M.; Urso, G. Evidence that time for repair during early germination leads to vigour differences in maize. SST 2011, 39, 501–509. [Google Scholar] [CrossRef]
- Brar, G.S.; Stewart, B.A. Germination under controlled temperature and field emergence of 13 sorghum cultivars. Crop Sci. 1994, 34, 1336–1340. [Google Scholar] [CrossRef]
- Khaeim, H.; Kende, Z.; Jolánkai, M.; Kovács, G.P.; Gyuricza, C.; Tarnawa, Á. Impact of Temperature and Water on Seed Germination and Seedling Growth of Maize (Zea mays L.). Agronomy 2022, 12, 397. [Google Scholar] [CrossRef]
- Yasin, M.; Andreasen, C. Effect of reduced oxygen concentration on the germination behavior of vegetable seeds. Hortic. Environ. Biotechnol. 2016, 57, 453–461. [Google Scholar] [CrossRef]
- Christianson, J.A.; Wilson, I.W.; Llewellyn, D.J.; Dennis, D.J. The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis seeds following low-oxygen treatment. Plant Physiol. 2009, 149, 1724–1738. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.; Qi, X.; Qiang, X.; Chen, X. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci. Hortic. 2014, 179, 388–395. [Google Scholar]
- Simojoki, A.; Jaakkola, A.; Alakukku, L. Effect of compaction on soil air in a pot experiment and in the field. Soil Tillage Res. 1991, 19, 175–186. [Google Scholar] [CrossRef]
- Khodarahmpour, Z. Evaluation of maize (Zea mays L.) hybrids, seed germination and seedling characters in water stress conditions. Afr. J. Agric. Res. 2012, 7, 6049–6053. [Google Scholar]
- Tolk, J.A.; Howell, T.A.; Steiner, J.L.; Evett, S.R. Grain sorghum growth, water use and yield in contrasting soils. Agric. Water Manag. 1997, 35, 29–42. [Google Scholar] [CrossRef]
- Wang, G. Agricultural drought in a future climate: Results from 15 global climate models participating in the IPCC 4th assessment. Clim. Dyn. 2005, 25, 739–753. [Google Scholar] [CrossRef]
- Hänsel, S.; Ustrnul, Z.; Łupikasza, E.; Skalak, P. Assessing seasonal drought variations and trends over Central Europe. Adv. Water Resour. 2019, 127, 53–75. [Google Scholar] [CrossRef]
- Somfalvi-Tóth, K. A kukoricatermesztés feltételeinek változása 1901-től napjainkig. Agrofórum Extra 2020, 87, 16–18. [Google Scholar]
- Conley, M.M.; Kimball, B.A.; Brooks, T.J.; Pinter, P.J., Jr.; Hunsaker, D.J.; Wall, G.W.; Adam, N.R.; LaMorte, R.L.; Matthias, A.D.; Thompson, T.L.; et al. CO2 enrichment increases water use efficiency in sorghum. New Phytol. 2001, 151, 407–412. [Google Scholar] [CrossRef]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Strasser, R. Delayed chlorophyll fluorescence as a monitor for physiological state of photosynthetic apparatus. Biotechn. Biotechn. Equip. 2009, 23, 452–457. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Araniti, F. Imaging of chlorophyll a fluorescence in natural compound-induced stress detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef] [PubMed]
- Jócsák, I.; Gyalog, H.; Hoffmann, R.; Somfalvi-Tóth, K. In-Vivo Biophoton Emission, Physiological and Oxidative Responses of Biostimulant-Treated Winter Wheat (Triticum eastivum L.) as Seed Priming Possibility, for Heat Stress Alleviation. Plants 2022, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.A.; Ali, I.A.; Lux, A.; Luxová, M.; Cohen, Y.; Sugimoto, Y.; Inanaga, S. Rooting, water uptake and xylem structure adaptation to drought of two sorghum cultivars. Crop Sci. 1999, 39, 168–173. [Google Scholar] [CrossRef]
- Tari, I.; Laskay, G.; Takács, Z.; Poór, P. Response of Sorghum to Abiotic Stresses: A Review. J. Agron. Crop Sci. 2012, 199, 264–274. [Google Scholar] [CrossRef]
- Xie, T.; Su, P.; Shan, L. Photosynthetic characteristics and water use efficiency of sweet sorghum under different watering regimes. Pak. J. Bot. 2010, 42, 3981–3994. [Google Scholar]
- Lemaire, G.; Charrier, X.; Hebert, Y. Nitrogen uptake capacities of maize and sorghum crops in different nitrogen and water supply conditions. Agronomie 1996, 16, 231–246. [Google Scholar] [CrossRef]
- Cakir, R. Effect of water stress at different development stages on vegetative and reproductive growth of corn. Field Crops Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Ge, T.; Sui, F.; Bai, L.; Tong, C.; Sun, N. Effects of water stress on growth, biomass partitioning, and water-use efficiency in summer maize (Zea mays L.) throughout the growth cycle. Acta Physiol. Plant. 2012, 34, 1043–1053. [Google Scholar] [CrossRef]
- Zhou, R.; Kan, X.; Chen, J.; Hua, H.; Li, Y.; Ren, J.; Feng, K.; Liu, H.; Deng, D.; Yin, Z. Droughtinduced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ. Exp. Bot. 2019, 158, 51–62. [Google Scholar] [CrossRef]
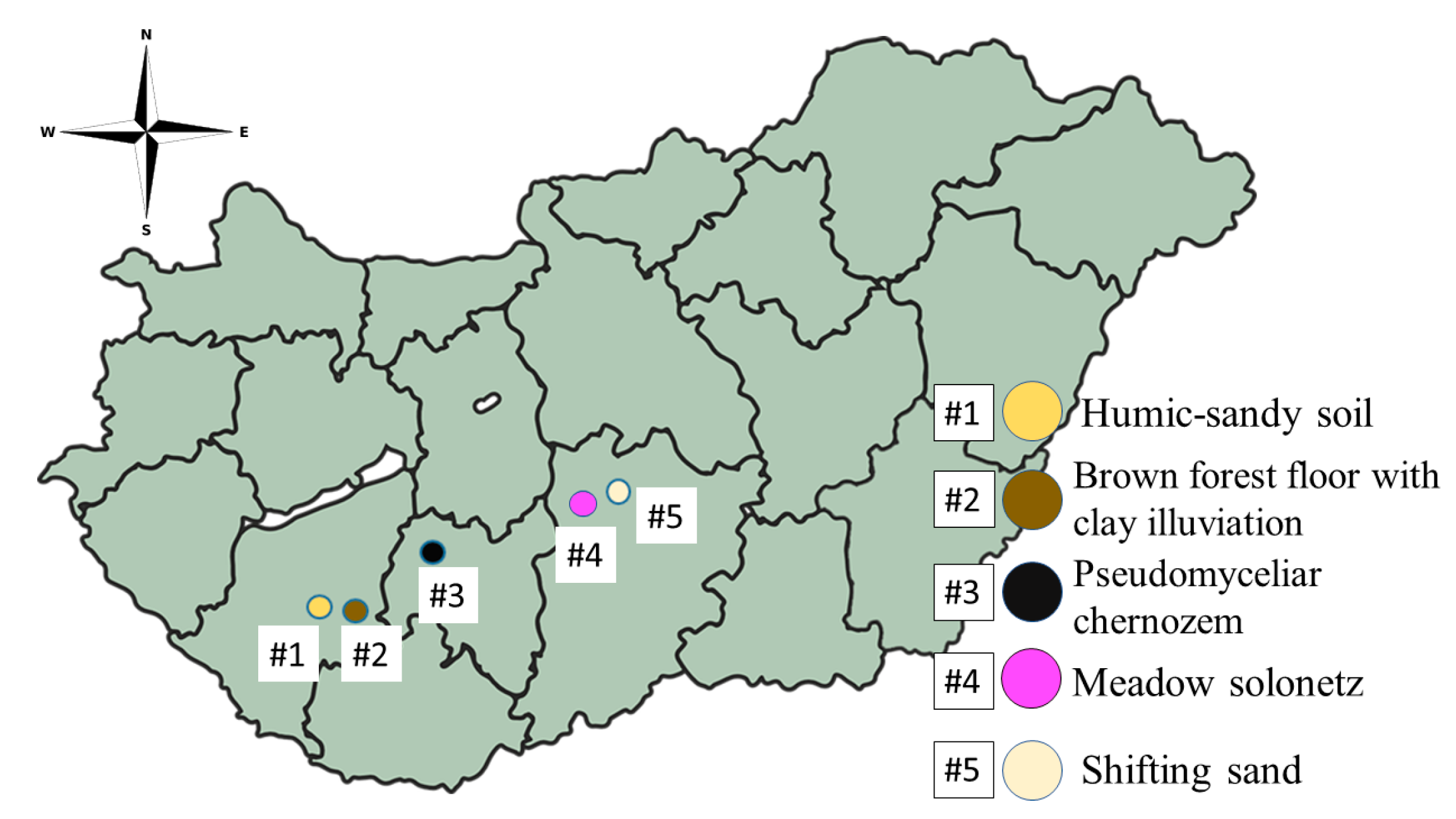
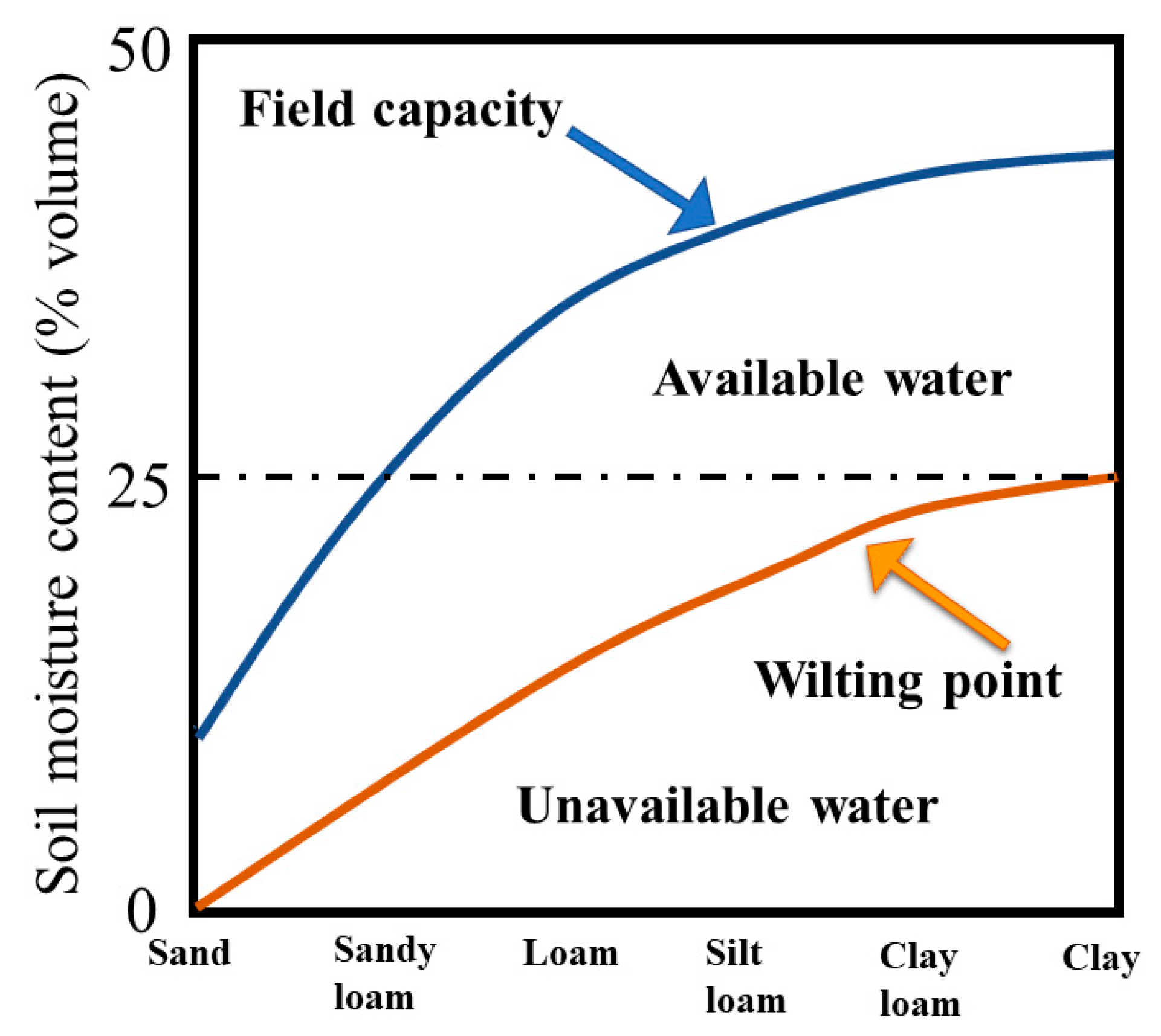
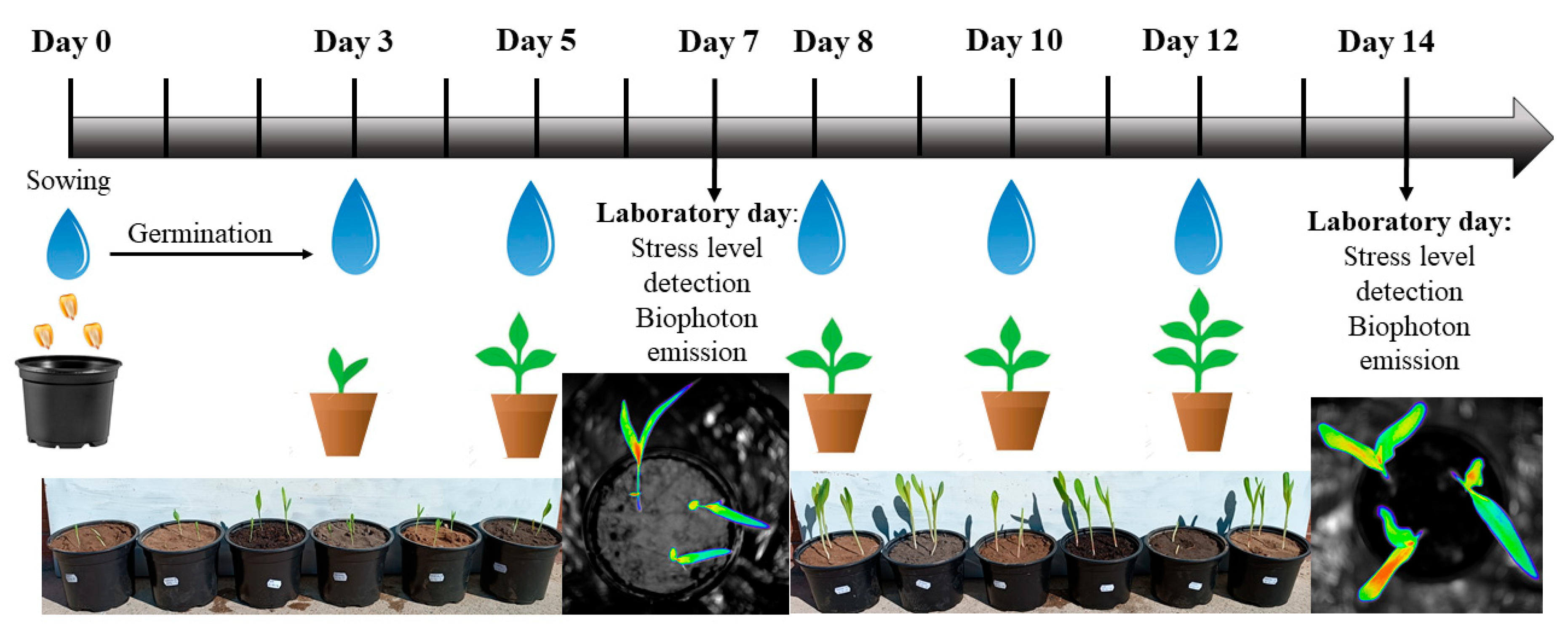
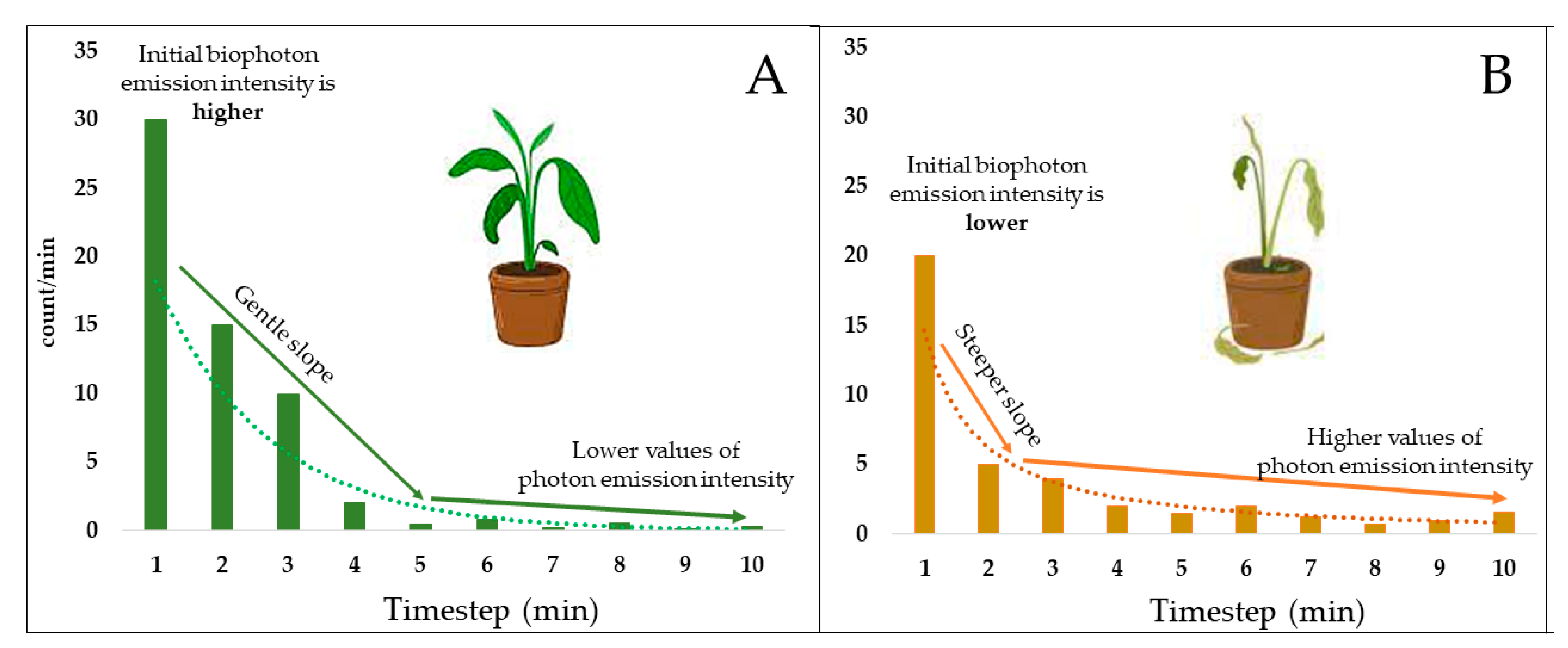
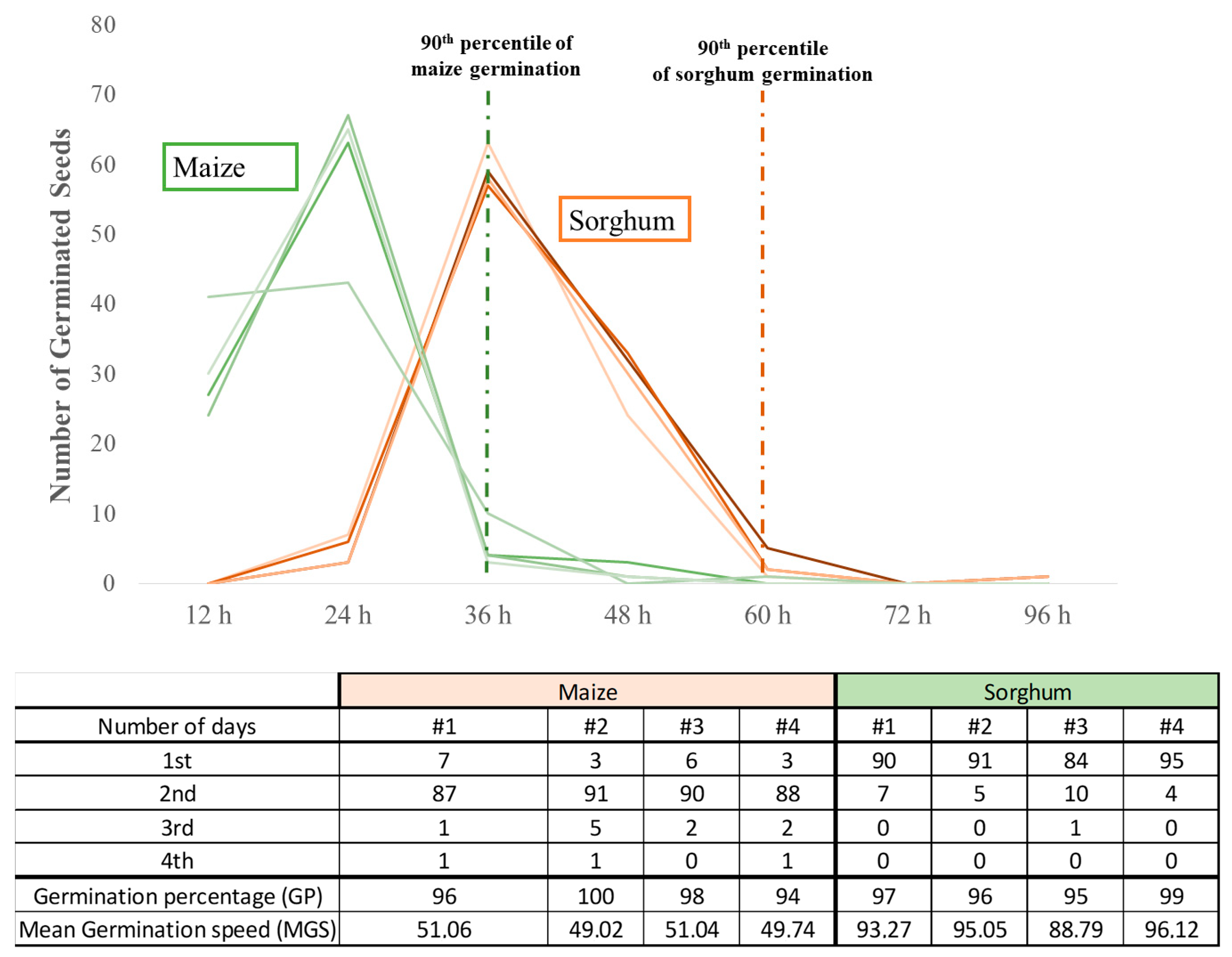


| Sample Number | #1 | #2 | #3 | #4 | #5 | #6 |
|---|---|---|---|---|---|---|
| Location | Kaposfő | Kaposvár | Tamási | Izsák | Fülöpháza | Control |
| Coordinates (latitude, longitude) | 46.371819, 17.619180 | 46.371887, 17.811380 | 46.648951, 18.272295 | 46.821052, 19.206805 | 46.865305, 19.398247 | - |
| Sampling depth (m) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | - |
| Soil type by Pásztor et al. [35] | Humic-sandy soil | Brown forest floor with clay illuviation | Pseudo-myceliar chernozem | Meadow solonetz | Shifting sand | Control |
| Soil type by WRB [36] | Arenosol | Luvisol | Chernozem | Solonetz | Arenosol | |
| Referred to as | HAR | LUV | CH | SN | SAR | Control |
| Soil texture | sand | sandy clay | clay loam | sandy clay | sand | - |
| Humus content (%) | 0.98 | 1.78 | 3.62 | 1.65 | 0.54 | 6.09 |
| P2O5 (mg/kg) | 435 | 582 | 2816 | 259 | 76.1 | 1041 |
| K2O (mg/kg) | 371 | 312 | 750 | 269 | 102 | 1675 |
| CaCO3 (m/m%) | 3.18 | 2.66 | 2.89 | 7.05 | 3.66 | 5.73 |
| pH (KCL) | 6.57 | 7.16 | 7.11 | 7.49 | 6.26 | 6.81 |
| Mg (mg/kg) | 90.3 | 164 | 236 | 229 | 45.4 | 1089 |
| Zn (mg/kg | 1.22 | 1.34 | 6.96 | 0.81 | 0.87 | 15.1 |
| Cu (mg/kg) | 1.28 | 3.42 | 3.7 | 1.94 | 1.4 | 8.28 |
| Mn (mg/kg) | 236 | 265 | 64.1 | 29.6 | 34.7 | 110 |
| (NO2 + NO3)-N content (mg/kg) | 11.2 | 67.2 | 107 | 51.6 | 15.6 | 92.9 |
| Na (mg/kg) | 23.5 | 36.9 | 77.7 | 228 | 39.6 | 170 |
| SO4 (mg/kg) | 22.5 | 43 | 76.5 | 45.9 | 18.3 | 4329 |
| Water-soluble salinity (m/m%) | <0.02 | 0.06 | 0.09 | 0.13 | <0.02 | 0.14 |
| pH (H2O) | 7.23 | 7.75 | 7.46 | 8.57 | 6.9 | 6.98 |
| Control | Flooding | Drought | Number of Pots | ||||
|---|---|---|---|---|---|---|---|
| Soil Type | Maize | Sorghum | Maize | Sorghum | Maize | Sorghum | Σ |
| LUV | 10 | 10 | 10 | 10 | 10 | 10 | 60 |
| CH | 10 | 10 | 10 | 10 | 10 | 10 | 60 |
| SAR | 10 | 10 | 10 | 10 | 10 | 10 | 60 |
| HAR | 10 | 10 | 10 | 10 | 10 | 10 | 60 |
| SN | 10 | 10 | 10 | 10 | 10 | 10 | 60 |
| Control | 10 | 10 | 10 | 10 | 10 | 10 | 60 |
| Soil Type | Water Regime | Field Water Capacity (mL/100 g) | Water Amount (mL/pot) | Dry Soil Mass (g) in a Pot |
|---|---|---|---|---|
| LUV | Control | 36 | 240 | 950 |
| LUV | Drought | 36 | 65 | 950 |
| LUV | Flooding | 36 | 410 | 950 |
| CH | Control | 43 | 290 | 950 |
| CH | Drought | 43 | 80 | 950 |
| CH | Flooding | 43 | 490 | 950 |
| HAR | Control | 27 | 205 | 1080 |
| HAR | Drought | 27 | 60 | 1080 |
| HAR | Flooding | 27 | 350 | 1080 |
| SAR | Control | 26 | 210 | 1150 |
| SAR | Drought | 26 | 65 | 1150 |
| SAR | Flooding | 26 | 360 | 1150 |
| SN | Control | 30 | 230 | 1050 |
| SN | Drought | 30 | 100 | 1050 |
| SN | Flooding | 30 | 380 | 1050 |
| Soil Type | Maize | Sorghum | Overall Mean | p-Value |
|---|---|---|---|---|
| CH | 72.22 | 88.89 | 80.56 | 0.03 |
| Control | 83.33 | 90.00 | 86.67 | 0.42 |
| HAR | 82.22 | 81.11 | 81.67 | 0.78 |
| SAR | 88.89 | 91.11 | 90.00 | 0.72 |
| SN | 63.33 | 74.44 | 68.89 | 0.05 |
| LUV | 76.67 | 85.56 | 81.11 | 0.06 |
| F-Value | p-Value | ||
|---|---|---|---|
| One-way ANOVA | Soil | 14.75 | <0.0001 |
| Day | 334.36 | <0.0001 | |
| Treatment | 0.21 | 0.648 | |
| Plant | 3.73 | 0.055 | |
| Two-way ANOVA | Soil:Day | 45,520 | <0.0001 |
| Soil:Treatment | 0.99 | 0.42 | |
| Day:Treatment | 0.40 | 0.526 | |
| Soil:Plant | 2.86 | 0.016 | |
| Day:Plant | 16.1 | <0.0001 | |
| Treatment:Plant | 7.05 | 0.008 | |
| Three-way ANOVA | Soil:Day:Treatment | 1.02 | 0.407 |
| Soil:Day:Plant | 3.82 | 0.002 | |
| Soil:Treatment:Plant | 6.82 | <0.0001 | |
| Day:Treatment:Plant | 11.73 | <0.0001 | |
| Four-way ANOVA | Day:Plant:Treatment:Soil | 9.75 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somfalvi-Tóth, K.; Hoffmann, R.; Jócsák, I.; Pitz, A.; Keszthelyi, S. Comparison of Juvenile Development of Maize and Sorghum in Six Temperate Soil Types under Extreme Water Regimes. Agronomy 2024, 14, 862. https://doi.org/10.3390/agronomy14040862
Somfalvi-Tóth K, Hoffmann R, Jócsák I, Pitz A, Keszthelyi S. Comparison of Juvenile Development of Maize and Sorghum in Six Temperate Soil Types under Extreme Water Regimes. Agronomy. 2024; 14(4):862. https://doi.org/10.3390/agronomy14040862
Chicago/Turabian StyleSomfalvi-Tóth, Katalin, Richárd Hoffmann, Ildikó Jócsák, András Pitz, and Sándor Keszthelyi. 2024. "Comparison of Juvenile Development of Maize and Sorghum in Six Temperate Soil Types under Extreme Water Regimes" Agronomy 14, no. 4: 862. https://doi.org/10.3390/agronomy14040862
APA StyleSomfalvi-Tóth, K., Hoffmann, R., Jócsák, I., Pitz, A., & Keszthelyi, S. (2024). Comparison of Juvenile Development of Maize and Sorghum in Six Temperate Soil Types under Extreme Water Regimes. Agronomy, 14(4), 862. https://doi.org/10.3390/agronomy14040862







