Hydrogen Sulfide Increases Drought Tolerance by Modulating Carbon and Nitrogen Metabolism in Foxtail Millet Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Drought and NaHS Treatment
2.2. Determination of Biomass and Leaf Area
2.3. Extraction and Determination of H2S
2.4. Determination of Gas Exchange Parameters, Chlorophyll Content, Fv/Fm, and Relative Water Content
2.5. Enzyme Extraction and Activity Assay
2.6. Quantitative Analysis of Carbohydrates
2.7. Determination of the Content of Metabolites Related to Nitrogen Metabolism
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
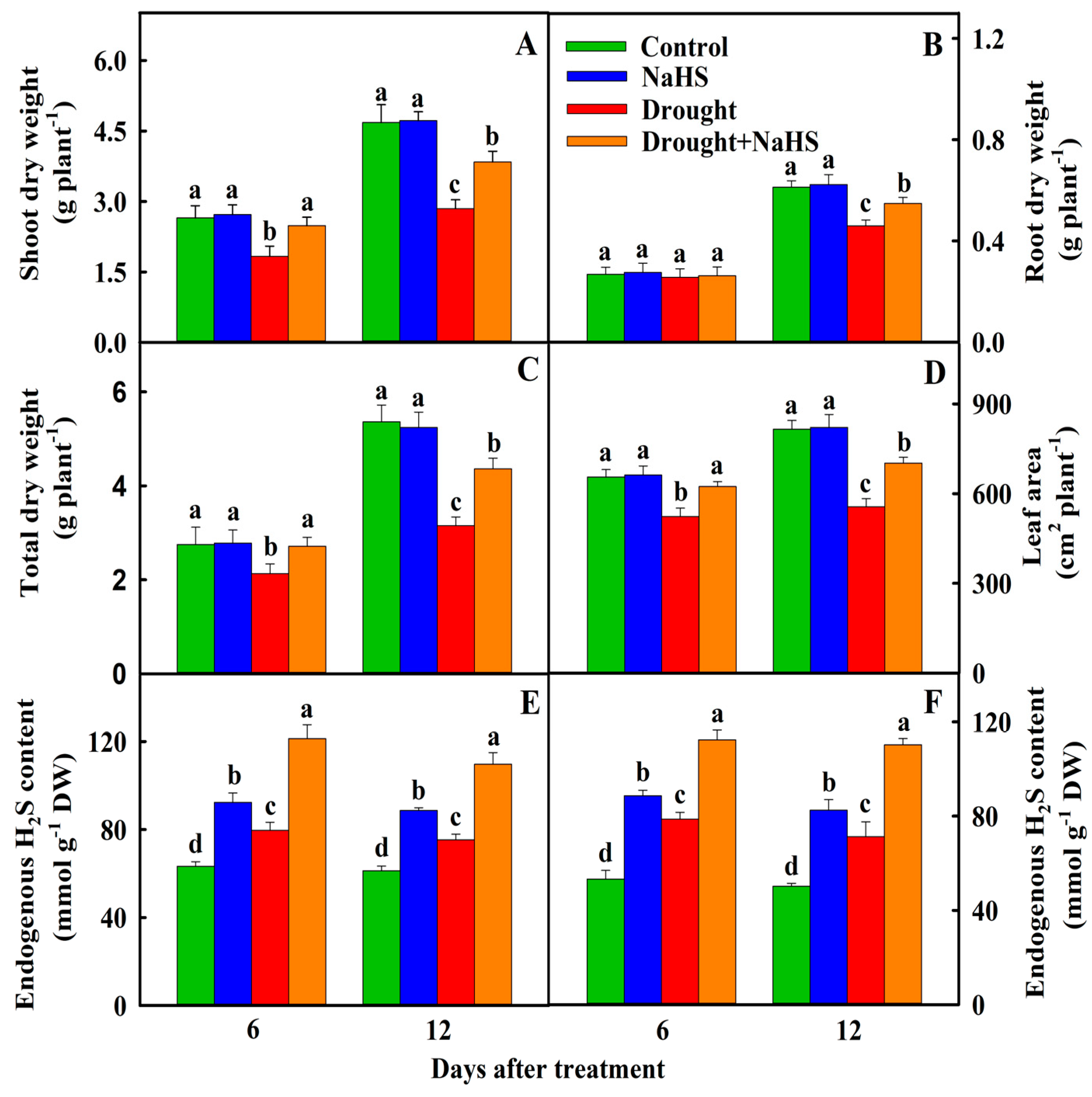
3.1. Effects of Exogenous NaHS on the Growth of Foxtail Millet under Drought Stress
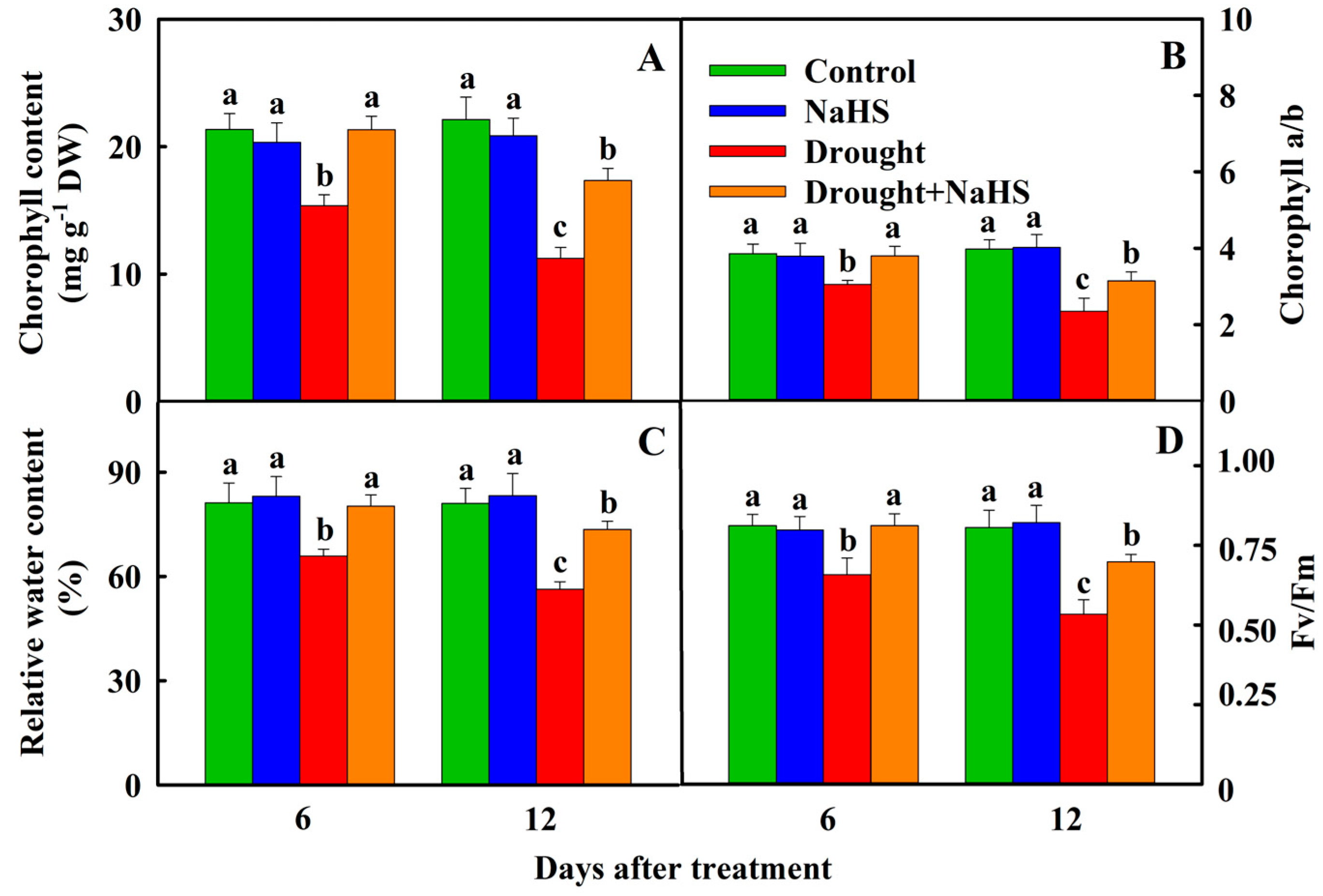
3.2. Effects of Exogenous NaHS on Carbon Assimilation of Foxtail Millet under Drought Stress
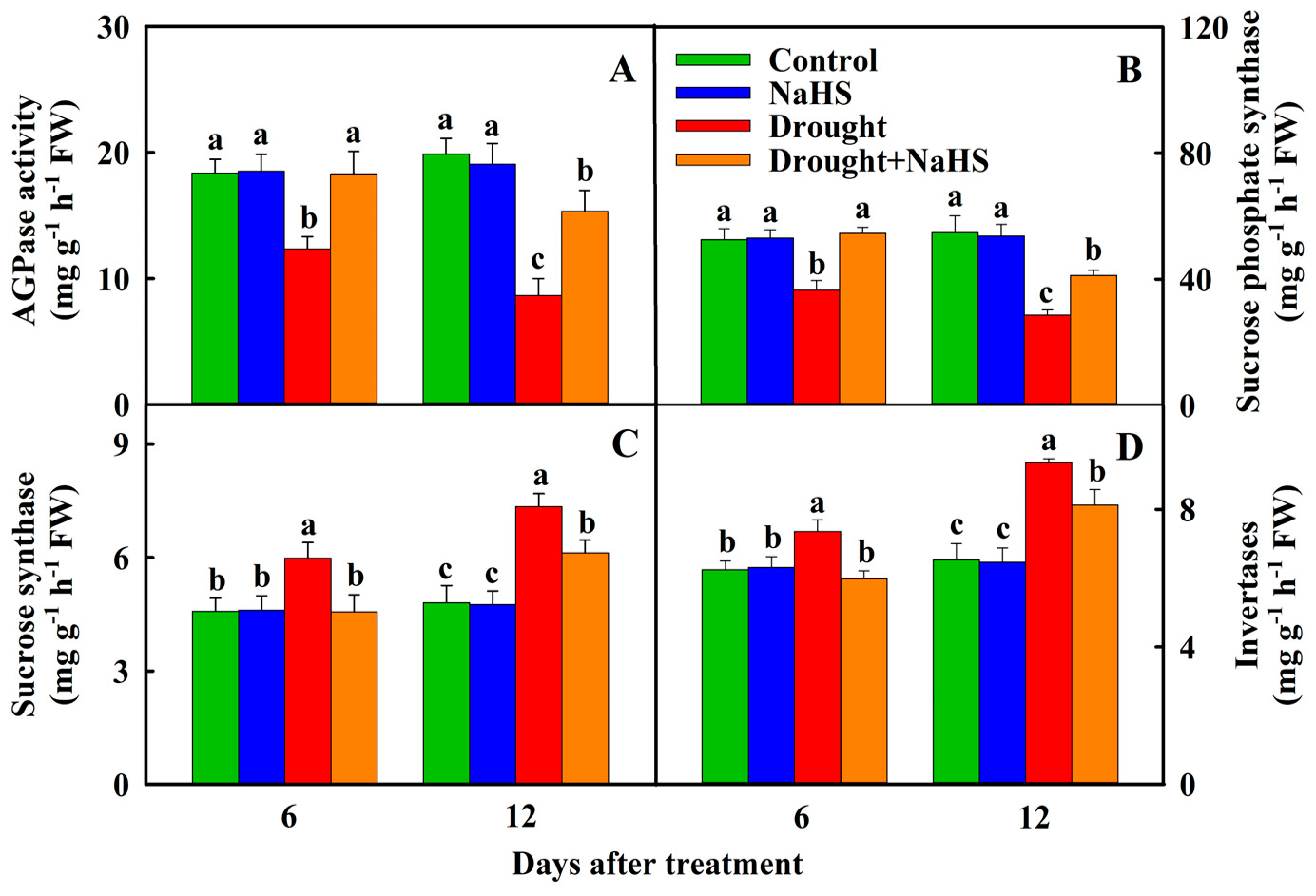
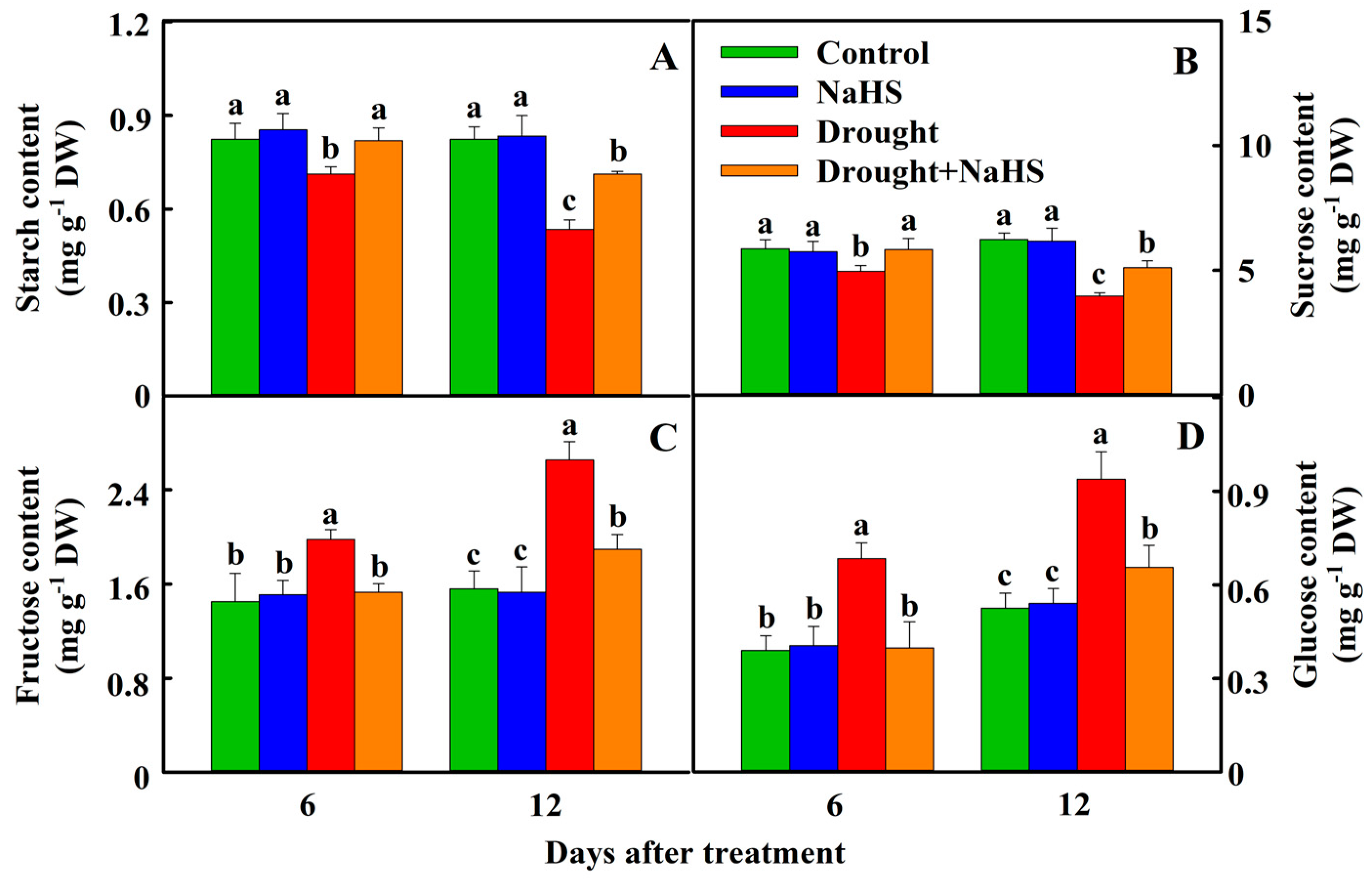
3.3. Effects of Exogenous NaHS on Carbon Metabolism of Foxtail Millet under Drought Stress
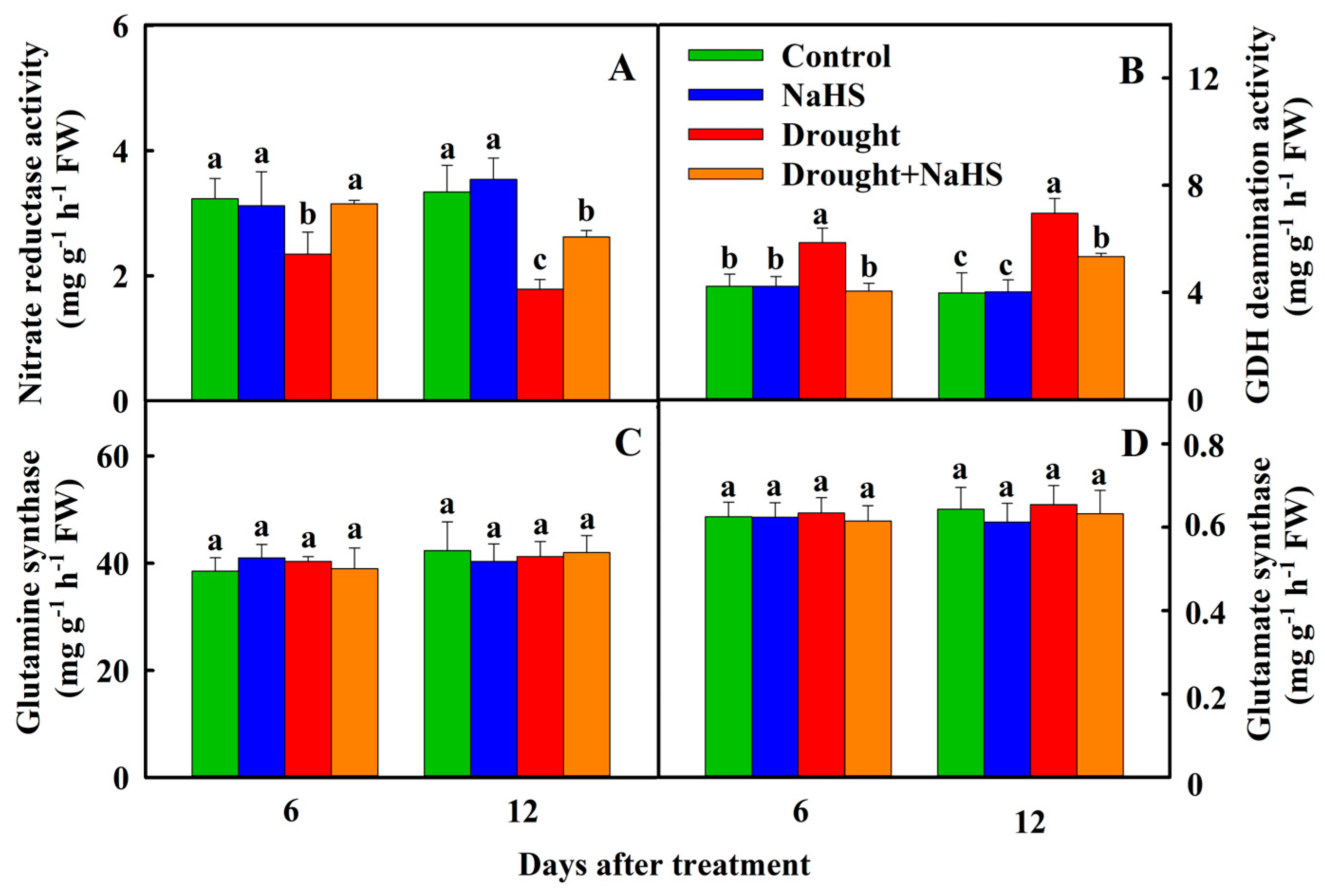
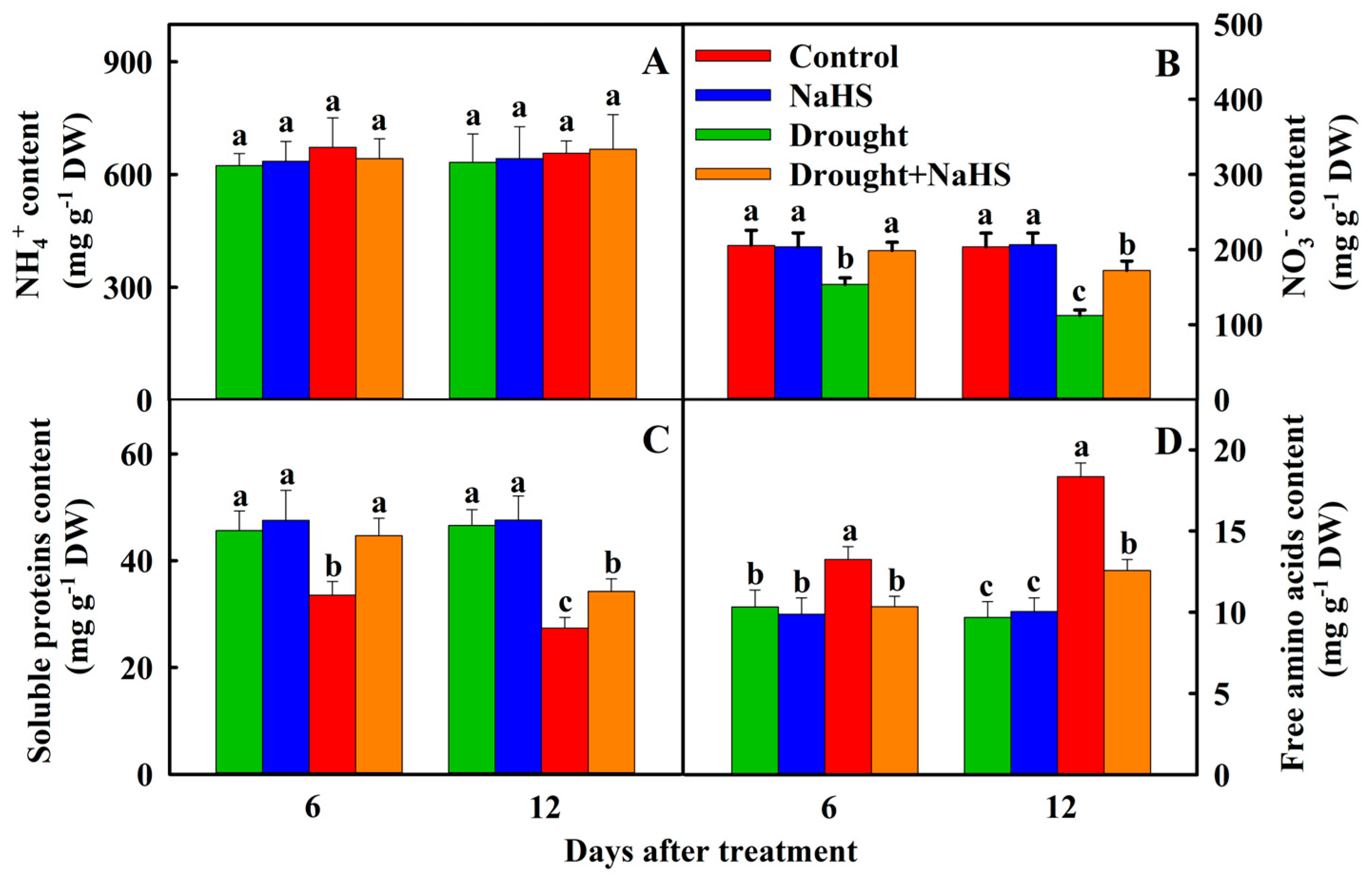
3.4. Effects of Exogenous NaHS on Nitrogen Assimilation and Nitrogen Metabolism of Foxtail Millet under Drought Stress
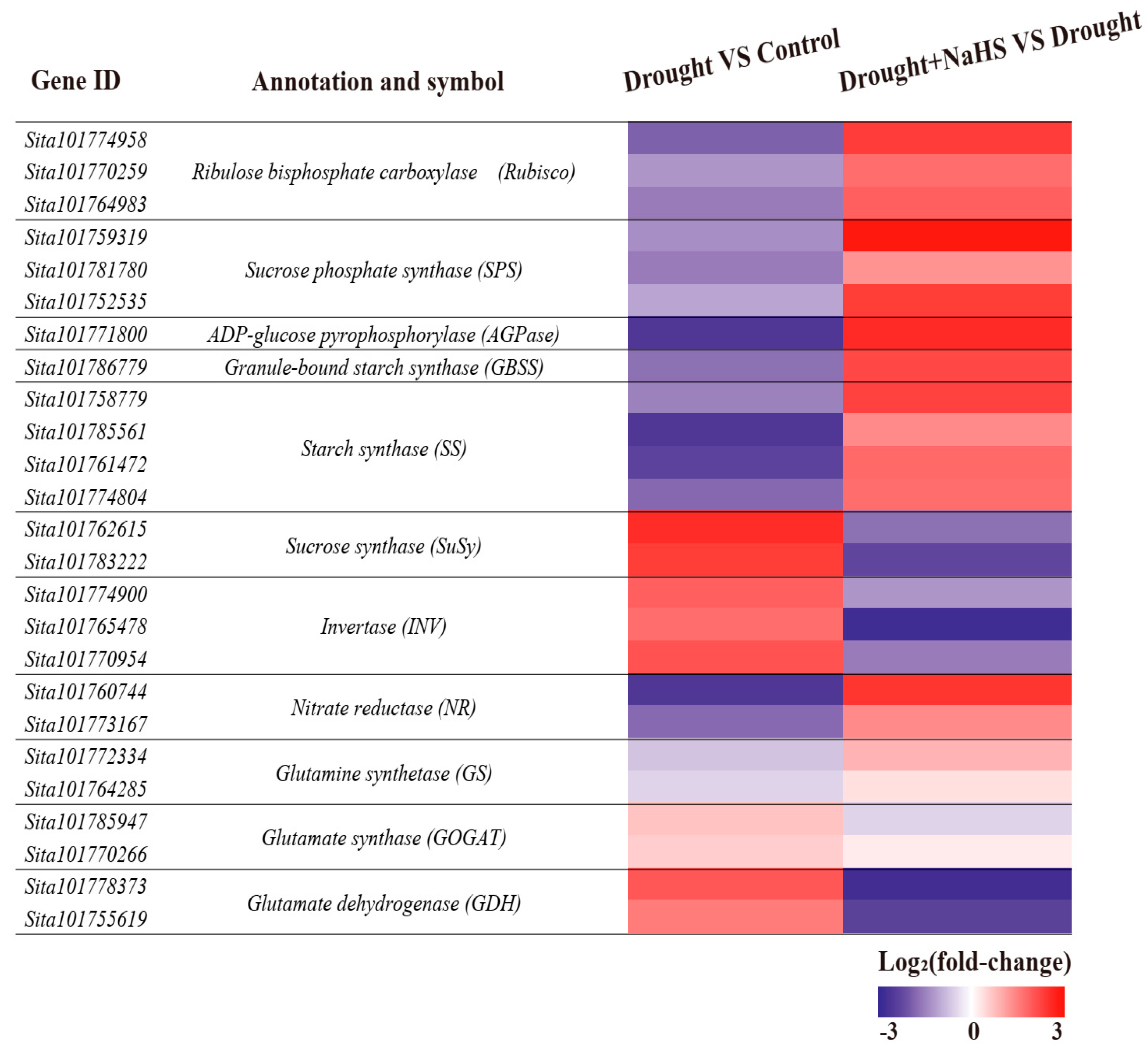
3.5. Effects of Exogenous NaHS on the Expression of Genes Related to Carbon and Nitrogen Metabolism in Foxtail Millet under Drought Stress
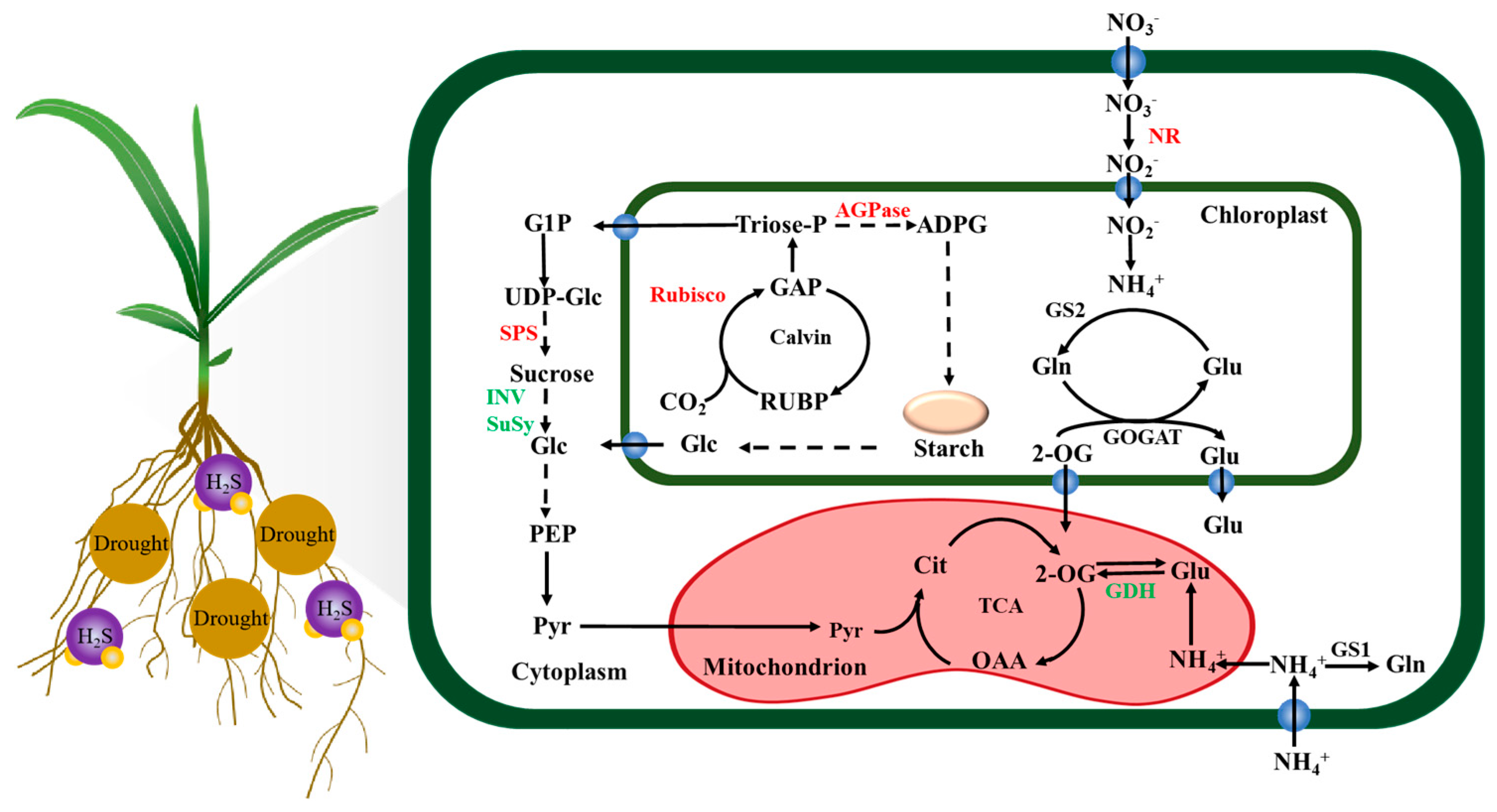
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Yang, P.; Cui, F.L.; Zhang, F.T.; Luo, X.D.; Xie, J.K. Transcriptome analysis of salt stress responsiveness in the seedlings of dongxiang wild rice (Oryza rufipogon Griff.). PLoS ONE 2016, 11, e0146242. [Google Scholar] [CrossRef] [PubMed]
- Rudack, K.; Seddig, S.; Sprenger, H.; Köhl, K.; Uptmoor, R.; Ordon, F. Drought stress-induced changes in starch yield and physiological traits in potato. J. Agron. Crop Sci. 2017, 203, 494–505. [Google Scholar] [CrossRef]
- Omena-Garcia, R.P.; Martins, A.O.; Medeiros, D.B.; Vallarino, J.G.; Ribeiro, D.M.; Fernie, A.R.; Araújo, W.L.; Nunes-Nesi, A. Growth and metabolic adjustments in response to gibberellin deficiency in drought stressed tomato plants. Environ. Exp. Bot. 2019, 159, 95–107. [Google Scholar] [CrossRef]
- De Roo, L.; Salomón, R.L.; Oleksyn, J.; Steppe, K. Woody tissue photosynthesis delays drought stress in Populus tremula trees and maintains starch reserves in branch xylem tissues. New Phytol. 2020, 228, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhou, H.; Shen, J.; Zhang, Z.; Gotor, C.; Romero, L.C.; Yuan, X.X.; Xie, Y. H2S action in plant life cycle. Plant Growth Regul. 2021, 94, 1–9. [Google Scholar] [CrossRef]
- Papanatsiou, M.; Scuffi, D.; Blatt, M.; Garcia-Mata, C. Hydrogen sulfide regulates inward-rectifying K+ channels in conjunction with stomatal closure. Plant Physiol. 2015, 168, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Asaeda, T.; Rashid, M. Hydrogen sulfide induced growth, photosynthesis and biochemical responses in three submerged macrophytes. Flora 2017, 230, 1–11. [Google Scholar] [CrossRef]
- Liu, F.J.; Fu, X.; Wu, G.X.; Feng, Y.Q.; Li, F.D.; Bi, H.G.; Ai, X.Z. Hydrogen peroxide is involved in hydrogen sulfide-induced carbon assimilation and photoprotection in cucumber seedlings. Environ. Exp. Bot. 2020, 175, 104052. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Ejaz, S.; Haider, S.T.A.; Alam, M.W.; Javed, H.U. Effects of hydrogen sulfide on postharvest physiology of fruits and vegetables: An overview. Sci. Hortic-Amst. 2019, 243, 290–299. [Google Scholar] [CrossRef]
- Guan, M.Y.; Zhang, H.H.; Pan, W.; Jin, C.W.; Lin, X.Y. Sulfide alleviates cadmium toxicity in Arabidopsis plants by altering the chemical form and the subcellular distribution of cadmium. Sci. Total Environ. 2018, 627, 663–670. [Google Scholar] [CrossRef]
- Jin, Z.P.; Wang, Z.Q.; Ma, Q.X.; Sun, L.M.; Zhang, L.P.; Liu, Z.Q.; Liu, D.M.; Hao, X.F.; Pei, Y.X. Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in Arabidopsis thaliana. Plant Soil 2017, 419, 141–152. [Google Scholar] [CrossRef]
- Chen, S.S.; Jia, H.L.; Wang, X.F.; Shi, C.; Wang, X.; Ma, P.Y.; Wang, J.; Ren, M.J.; Li, J.S. Hydrogen Sulfide Positively Regulates Abscisic Acid Signaling through Persulfidation of SnRK2.6 in Guard Cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wan, R.; Shi, Y.; Xue, S. Hydrogen Sulfide Activates S-Type Anion Channel via OST1 and Ca2+ Modules. Mol. Plant 2016, 9, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.M.; Lu, Y.T.; Qiu, Q.L.; Fan, D.M.; Wang, X.C.; Zheng, X.Q. Influence of different nitrogen sources on carbon and nitrogen metabolism and gene expression in tea plants (Camellia sinensis L.). Plant Physiol. Bioch. 2021, 167, 561–566. [Google Scholar] [CrossRef]
- Ren, J.H.; Xie, T.; Wang, Y.L.; Li, H.B.; Liu, T.T.; Zhang, S.Q.; Yin, L.N.; Wang, S.W.; Deng, X.P.; Ke, Q.B. Coordinated regulation of carbon and nitrogen assimilation confers drought tolerance in maize (Zea mays L.). Environ. Exp. Bot. 2020, 176, 104086. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y.; Zhai, F.C.; Zhang, J.; Zhang, F.; Yuan, X.X.; Xie, Y.J. Hydrogen sulfide promotes rice drought tolerance via reestablishing redox homeostasis and activation of ABA biosynthesis and signaling. Plant Physiol. Bioch. 2020, 155, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Zhang, J.P.; Liu, K.B.; Wu, N.Q.; Li, Y.M.; Zhou, K.S.; Ye, M.L.; Zhang, T.Y.; Zhang, H.J.; Yang, X.Y.; et al. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc. Natl. Acad. Sci. USA 2009, 106, 7367–7372. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Huang, X.; Zhi, H.; Zhao, Y.; Zhao, Q.; Li, W. A haplotypemap of genomic variations and genome-wide association studies of agronomic traits in foxtail millet (Setaria italica). Nat. Genet. 2013, 45, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Serba, D.D.; Ligaba-Osena, A. Alternative Strategies for Multi-Stress Tolerance and Yield Improvement in Millets. Genes 2021, 12, 739. [Google Scholar] [CrossRef]
- Thakur, M.; Anand, A. Hydrogen sulfide: An emerging signaling molecule regulating drought stress response in plants. Physiol. Plant. 2021, 172, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Qiao, Z.; Zhang, L.; Li, H.; Pei, Y. Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedings. Plant Physiol. Bioch. 2016, 109, 293–299. [Google Scholar] [CrossRef]
- Prathap, V.; Ali, K.; Singh, A.; Vishwakarma, C.; Krishnan, V.; Chinnusamy, V.; Tyagi, A. Starch accumulation in rice grains subjected to drought during grain filling stage. Plant Physiol. Bioch. 2019, 142, 440–451. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Sun, M.; Peng, F. Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front. Plant Sci. 2020, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Liu, J.R.; Ma, Y.N.; Lv, F.J.; Chen, J.; Zhou, Z.G.; Wang, Y.H. Changes of sucrose metabolism in leaf subtending to cotton boll under cool temperature due to late planting. Field Crop Res. 2013, 144, 200–211. [Google Scholar] [CrossRef]
- Schaffer, A.A.; Petreikov, M. Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol. 1997, 113, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Cao, Y.; Loka, D.A.; Harris-Shultz, K.R.; Reiter, R.J.; Ali, S.; Liu, Y.; Zhou, Z. Exogenous melatonin improves cotton (Gossypium hirsutum L.) pollen fertility under drought by regulating carbohydrate metabolism in male tissues. Plant Physiol. Bioch. 2020, 151, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Barro, F.; Fontes, A.G.; Maldonado, J.M. Organic nitrogen content and nitrate and nitrite reductase activities in tritordeum and wheat grown under nitrate or ammonium. Plant Soil 1991, 135, 251–256. [Google Scholar] [CrossRef]
- O’neal, D.; Joy, K.W. Glutamine synthetase of pea leaves. I. purification, stabilization, and pH optima. Arch. Biochem. Biophys. 1973, 159, 113–122. [Google Scholar] [CrossRef]
- Matoh, T.; Takahashi, E. Changes in the activities of ferredoxin-and NADH-glutamate synthase during seedling development of peas. Planta 1982, 154, 289–294. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; de Jimenez, E.S. Differential role of glutamate dehydrogenase in nitrogen metabolism of maize tissues. Plant Physiol. 1984, 76, 536–540. [Google Scholar] [CrossRef]
- Xu, H.F.; Zou, Q.Y.; Yang, G.X.; Jiang, S.H.; Fang, H.C.; Wang, Y.C.; Zhang, J.; Zhang, Z.Y.; Wang, N.; Chen, X. MdMYB6 regulates anthocyanin formation in apple both through direct inhibition of the biosynthesis pathway and through substrate removal. Hortic. Res-Engl. 2020, 7, 72. [Google Scholar] [CrossRef]
- Hansen, J.; Moller, I.B. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Analy. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hajlaoui, H.; El Ayeb, N.; Garrec, J.P.; Denden, M. Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind. Crop Prod. 2010, 31, 122–130. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Shen, J.; Zhou, M.J.; Yuan, X.X.; Xie, Y.J. Redox-based protein persulfidation in guard cell ABA signaling. Plant Signal. Behav. 2020, 15, 1741987. [Google Scholar] [CrossRef]
- Bräutigam, A.; Gagneul, D.; Weber, A.P. High-throughput colorimetric method for the parallel assay of glyoxylic acid and ammonium in a single extract. Anal. Biochem. 2007, 362, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct signalling pathways and transcriptome response signatures differentiate ammonium-and nitrate-supplied plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.J.; Huo, J.Q.; Liao, W.B. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, M.J.; Zhou, H.; Zhao, D.D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.L.; Zhang, Z.R.; Shen, W.B.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef]
- Amir, S.B.; Rasheed, R.; Ashraf, M.A.; Hussain, I.; Iqbal, M. Hydrogen sulfide mediates defense response in safflower by regulating secondary metabolism, oxidative defense and elemental uptake under drought. Physiol. Plant. 2020, 172, 795–808. [Google Scholar] [CrossRef]
- Ma, D.; Ding, H.; Wang, C.; Qin, H.; Han, Q.; Hou, J.; Lu, H.; Xie, Y.; Guo, T. Alleviation of drought stress by hydrogen sulfide is partially related to the Abscisic Acid signaling pathway in Wheat. PLoS ONE 2016, 11, e0163082. [Google Scholar] [CrossRef]
- Kaya, C.; Shabala, S. Sodium hydrosulfide-mediated upregulation of nitrogen metabolism improves drought stress tolerance in pepper plants. Environ. Exp. Bot. 2023, 209, 105305. [Google Scholar] [CrossRef]
- Jin, Z.; Sun, L.; Yang, G.; Pei, Y. Hydrogen Sulfide Regulates Energy Production to Delay Leaf Senescence Induced by Drought Stress in Arabidopsis. Front. Plant Sci. 2018, 9, 405332. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Garcia-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef]
- Pilon, C.; Snider, J.L.; Sobolev, V.; Chastain, D.R.; Sorensen, R.B.; Meeks, C.D.; Massa, A.N.; Walk, T.; Singh, B.; Earl, H.J. Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). J. Plant Physiol. 2018, 231, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Yarkhunova, Y.; Guadagno, C.R.; Rubin, M.J.; Davis, S.J.; Ewers, B.E.; Weinig, C. Circadian rhythms are associated with variation in photosystem II function and photoprotective mechanisms. Plant Cell Environ. 2018, 41, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.B.; Ma, Y.H.; Jiang, M.R.; Yang, F.; Ni, L.; Lu, W. Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul. 2015, 75, 33–44. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Wang, L.; Zhang, L.P.; Kong, X.Q.; Zhang, J.; Wang, X.; Pei, Y.X.; Jin, Z.P. H2S-mediated balance regulation of stomatal and non-stomatal factors responding to drought stress in Chinese cabbage. Hortic. Res. 2023, 10, uhac284. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, F.H.; Wang, W.H.; Zheng, C.J.; Lin, G.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J. Exp. Bot. 2011, 62, 4481–4493. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Xu, W.Z.; Deng, X.P.; Xu, B.C.; Gao, Z.J.; Ding, W.L. Photosynthetic activity and efficiency of Bothriochloa ischaemum and Lespedeza davurica in mixtures across growth periods under water stress. Acta Physiol. Plant 2014, 36, 1033–1044. [Google Scholar] [CrossRef]
- Chen, J.; Shang, Y.T.; Wang, W.H.; Chen, X.Y.; He, E.M.; Zheng, H.L.; Shangguan, Z.P. Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in Spinacia oleracea seedlings. Front. Plant Sci. 2016, 7, 1173. [Google Scholar] [CrossRef]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative role of sugar signaling and transport during drought stress responses in plants. Physiol. Plantarum. 2021, 171, 833–848. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.K.; Smith, S.R.; McCrow, J.P.; Tan, M.; Zheng, H.; Beeri, K.; Roth, R.; Lichtle, C.; Goodenoug, U.; Bowler, C.P.; et al. Nitrate reductase knockout uncouples nitrate transport from nitrate assimilation and drives repartitioning of carbon flux in a model pennate diatom. Plant Cell 2017, 29, 2047–2070. [Google Scholar] [CrossRef]
- Tsuji, C.; Dannoura, M.; Desalme, D.; Angeli, N.; Takanashi, S.; Kominami, Y.; Epron, D. Drought affects the fate of non-structural carbohydrates in hinoki cypress. Tree Physiol. 2022, 42, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Alamri, S.; Mukherjee, S.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedhi, B.M.A.; Ali, H.M.; Kalaji, H.M.; Fahad, S.; Rajput, V.D.; et al. Molybdenum and hydrogen sulfide synergistically mitigate arsenic toxicity by modulating defense system, nitrogen and cysteine assimilation in faba bean (Vicia faba L.) seedlings. Environ. Pollut. 2021, 290, 117953. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.L.; Gu, W.R.; Wang, M.Q.; Zhang, L.G.; Li, C.F.; Li, C.F.; Li, W.H.; Li, L.J.; Wei, S. Exogenous 2-(3, 4-Dichlorophenoxy) triethylamine ameliorates the soil drought effect on nitrogen metabolism in maize during the pre-female inflorescence emergence stage. BMC Plant Biol. 2019, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, D.W.; Flügge, U.I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Zou, H.; Lin, X.Y.; Pan, Q.; Zhang, W.Q.; Zhang, J.H.; Wei, G.H.; Shangguan, Z.P.; Chen, J. Hydrogen sulfide and rhizobia synergistically regulate nitrogen (N) assimilation and remobilization during N deficiency-induced senescence in soybean. Plant Cell Environ. 2020, 43, 1130–1147. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Wang, Y.J.; Pan, K.W.; Zhu, T.T.; Li, W.; Zhang, L. Carbon and nitrogen metabolism in leaves and roots of dwarf bamboo (Fargesia denudata Yi) subjected to drought for two consecutive years during sprouting period. J. Plant Growth Regul. 2014, 33, 243–255. [Google Scholar] [CrossRef]
- Tian, J.; Pang, Y.; Zhao, Z. Drought, salinity, and low nitrogen differentially affect the growth and nitrogen metabolism of Sophora japonica (L.) in a semi-hydroponic phenotyping platform. Front. Plant Sci. 2021, 12, 715456. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolińska, B.; Leszczyńska, J.; Fotopoulos, V. Influence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, proteome regulation and allergen production in basil (Ocimum basilicum L.). Plants Front. Plant Sci. 2018, 9, 374129. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Shah, T.M.; Imran, M.; Atta, B.M.; Ashraf, M.Y.; Hameed, A.; Waqar, I.; Shafiq, M.; Hussain, K.; Naveed, M.; Aslam, M.; et al. Selection and screening of drought tolerant high yielding chickpea genotypes based on physio-biochemical indices and multi-environmental yield trials. BMC Plant Biol. 2020, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.F.; Zhang, X.; Ma, W.J.; Song, J.Y.; Rahman, S.U.; Wang, J.H.; Zhang, Y. Morphological and physiological responses to cyclic drought in two contrasting genotypes of Catalpa bungei. Environ. Exp. Bot. 2017, 138, 77–87. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plantarum. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Coruzzi, G.M.; Zhou, L. Carbon and nitrogen sensing and signaling in plants: Emerging ‘matrix effects’. Curr. Opin. Plant Biol. 2001, 4, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Li, J.; Wang, X.; Wang, C. Crosstalk between hydrogen sulfide and other signal molecules regulates plant growth and development. Int. J. Mol. Sci. 2020, 21, 4593. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Zhou, C.Z.; Burnap, R.L.; Peng, L. Carbon/Nitrogen Metabolic Balance: Lessons from Cyanobacteria. Trends Plant Sci. 2018, 23, 1116–1130. [Google Scholar] [CrossRef]
- Fang, H.; Jing, T.; Liu, Z.; Zhang, L.; Jin, Z.; Pei, Y. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 2014, 56, 472–481. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhang, S.; Yang, X.; Feng, K.; Wang, G.; Shi, Q.; Wang, X.; Yuan, X.; Ren, J. Hydrogen Sulfide Increases Drought Tolerance by Modulating Carbon and Nitrogen Metabolism in Foxtail Millet Seedlings. Agronomy 2024, 14, 1080. https://doi.org/10.3390/agronomy14051080
Zhao J, Zhang S, Yang X, Feng K, Wang G, Shi Q, Wang X, Yuan X, Ren J. Hydrogen Sulfide Increases Drought Tolerance by Modulating Carbon and Nitrogen Metabolism in Foxtail Millet Seedlings. Agronomy. 2024; 14(5):1080. https://doi.org/10.3390/agronomy14051080
Chicago/Turabian StyleZhao, Juan, Shifang Zhang, Xiaoxiao Yang, Ke Feng, Guo Wang, Qifeng Shi, Xinru Wang, Xiangyang Yuan, and Jianhong Ren. 2024. "Hydrogen Sulfide Increases Drought Tolerance by Modulating Carbon and Nitrogen Metabolism in Foxtail Millet Seedlings" Agronomy 14, no. 5: 1080. https://doi.org/10.3390/agronomy14051080
APA StyleZhao, J., Zhang, S., Yang, X., Feng, K., Wang, G., Shi, Q., Wang, X., Yuan, X., & Ren, J. (2024). Hydrogen Sulfide Increases Drought Tolerance by Modulating Carbon and Nitrogen Metabolism in Foxtail Millet Seedlings. Agronomy, 14(5), 1080. https://doi.org/10.3390/agronomy14051080





