Effect of Phosphorus Application on Subcellular Distribution and Chemical Morphology of Cadmium in Eggplant Seedlings under Cadmium Stress
Abstract
1. Introduction
2. Material and Methods
2.1. Cd-Polluted Soil Preparation
2.2. Experimental Design
2.3. Measurement Methods
2.4. Calculations and Statistical Analysis
3. Results
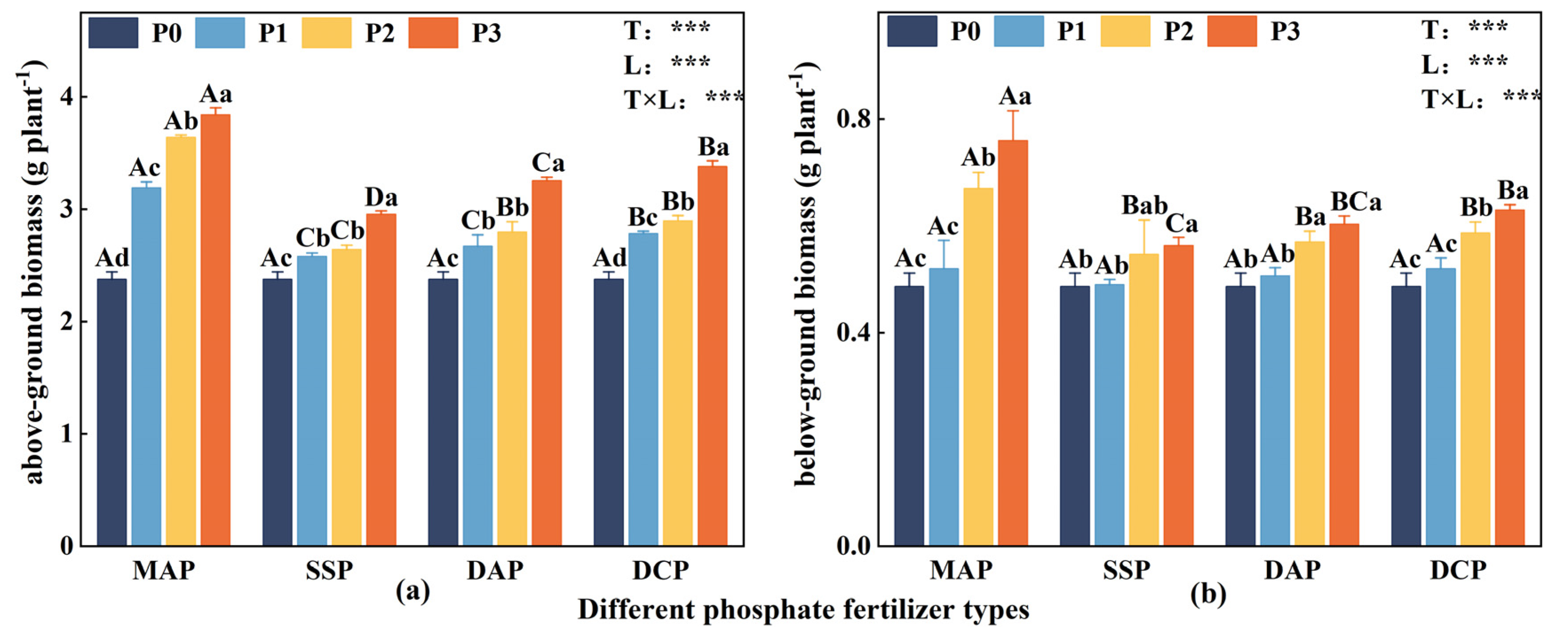
3.1. Eggplant Seedling Biomass
3.2. Cd Content in Eggplant Seedlings
3.3. Bioconcentration and Translocation Factors for Eggplant Seedlings
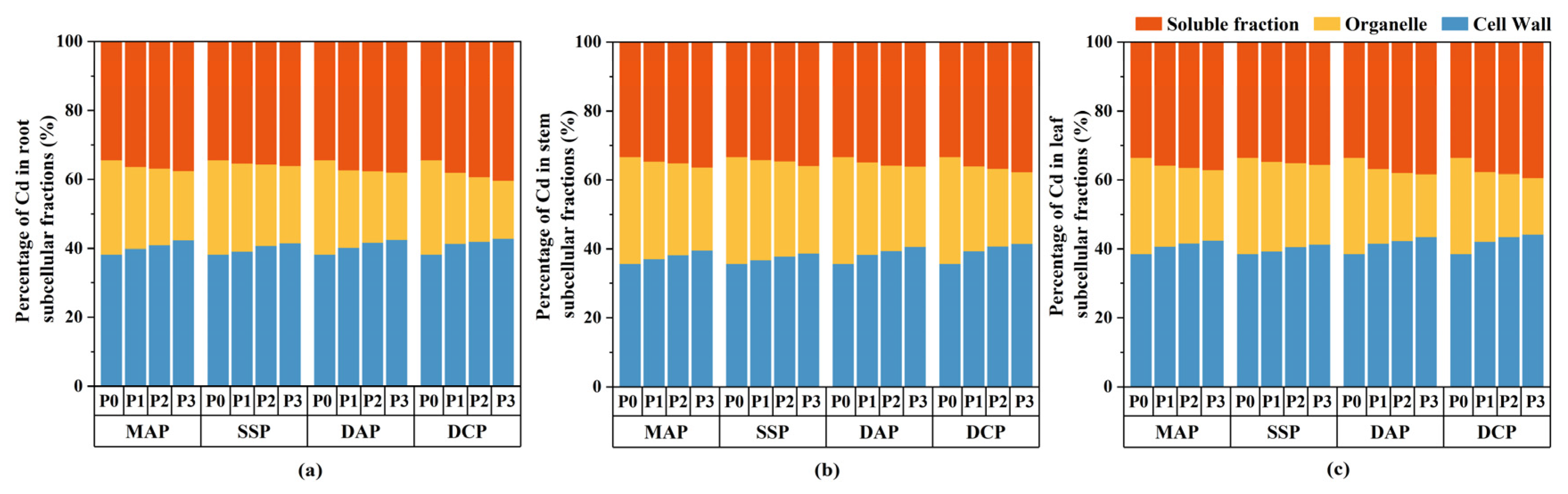
3.4. Subcellular Distribution of Cd in Various Parts of Eggplant
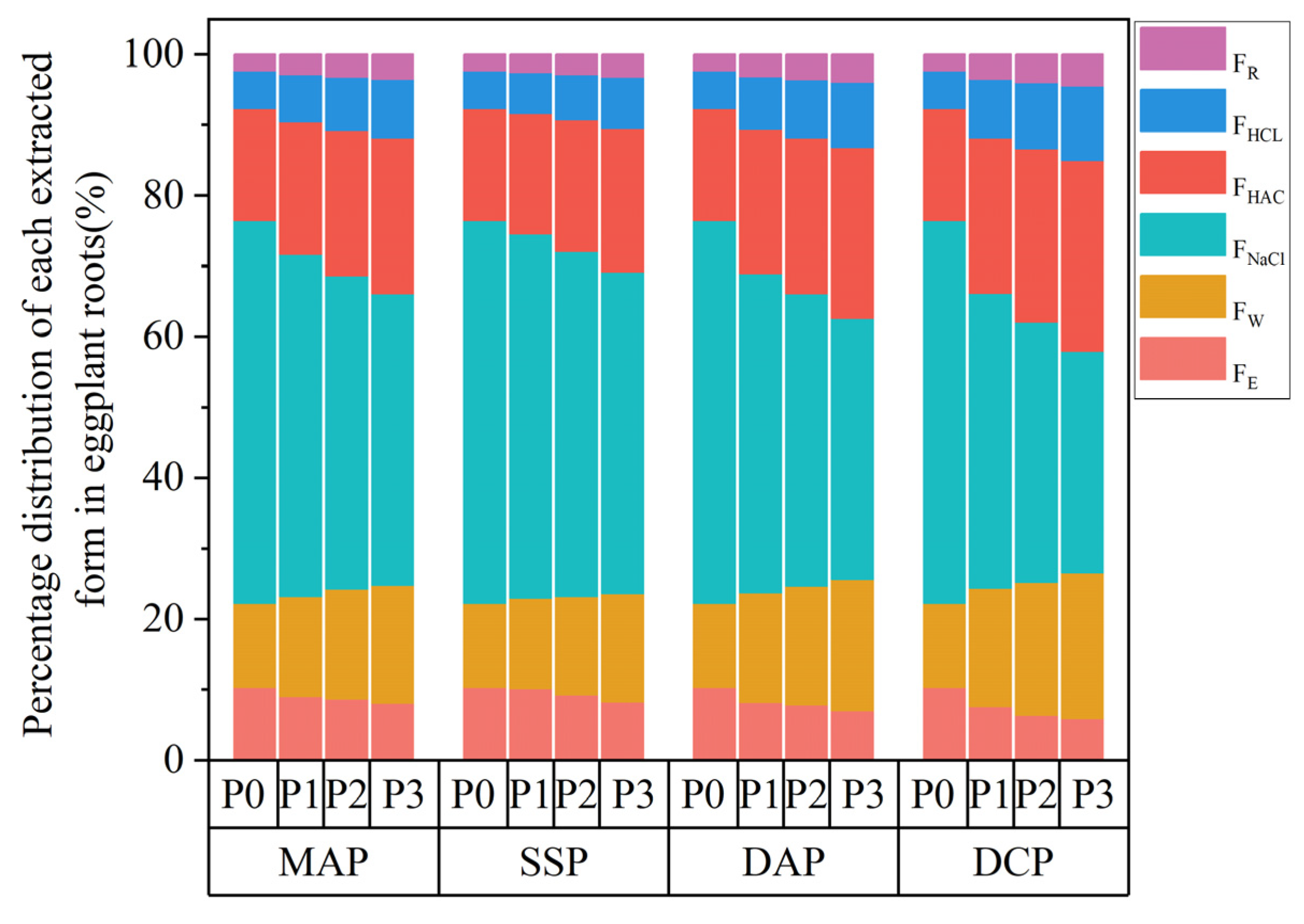
3.5. Chemical Morphology of Cd in the Roots of Eggplant
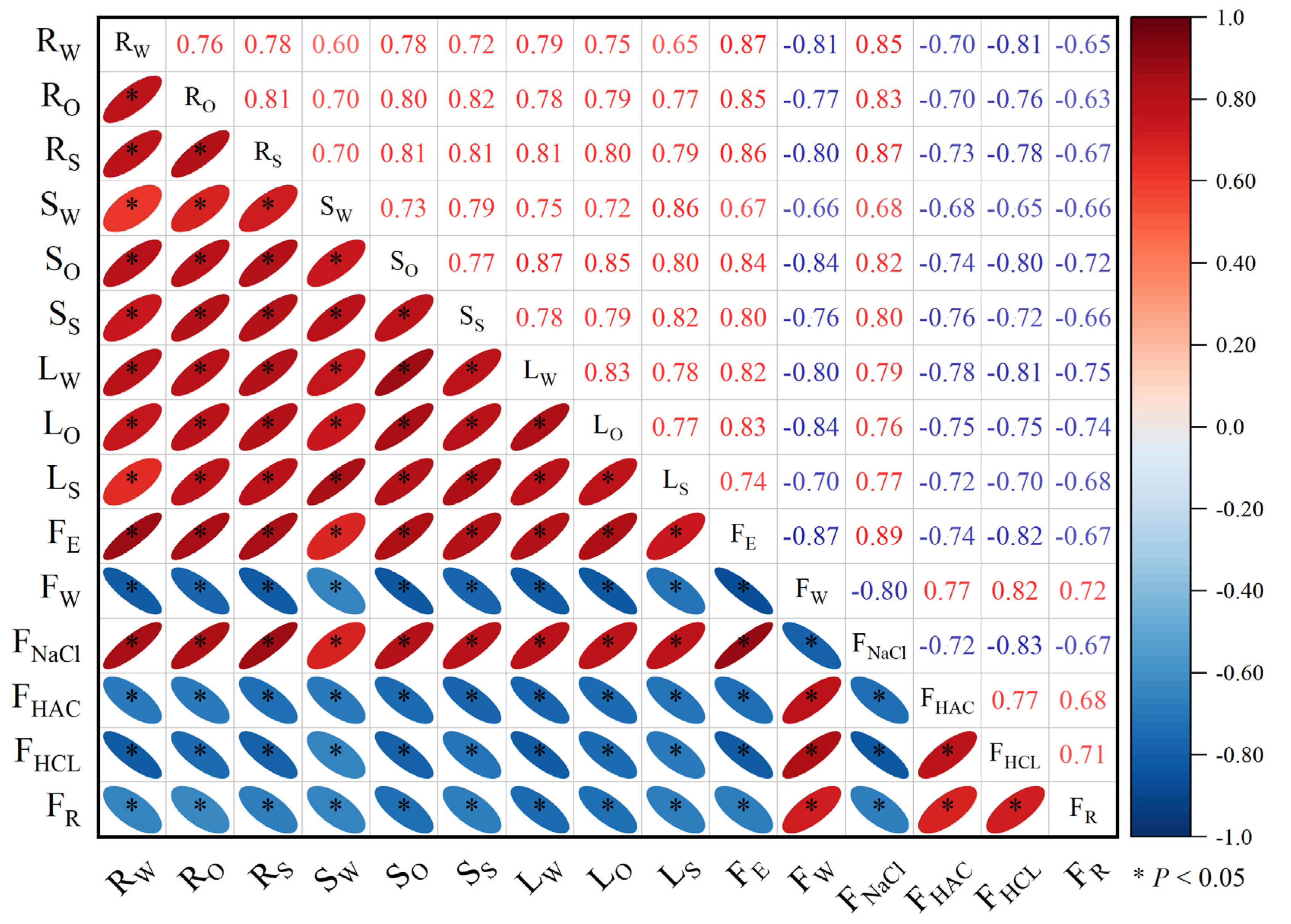
3.6. Correlation Analysis of Subcellular and Chemical Morphologies in Eggplant Seedlings
4. Discussion
4.1. Effect of Phosphorus Application on Eggplant Biomass and Cadmium Content under Cadmium Stress
4.2. Effect of P Application under Cd Stress on the Subcellular Distribution and Chemical Morphology of Cd in Eggplants
4.3. Mechanisms of Applying Different P Fertilizers to Mitigate Cd Pollution
5. Conclusions
6. Prospects
- (1)
- Only the chemical morphology of cadmium in roots was investigated; the chemical morphology in stems and leaves during the seedling stage remained unstudied.
- (2)
- The study focused solely on the seedling stage of eggplant and lacked a systematic examination of the mature stage, thus presenting limitations in the exploration.
- (3)
- Further investigation of the mechanism by which phosphorus fertilizer mitigates cadmium toxicity is warranted.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, C.; Zhang, N.; Tao, R.; Zheng, J.; Hu, H.; Li, J.; Ma, Y.; Liao, X. Screening for Low-Cadmium Accumulation in Maize Varieties Based on Species Sensitivity Distribution and Research on Soil Environmental Thresholds. Agronomy 2023, 13, 1960. [Google Scholar] [CrossRef]
- Wang, C.-C.; Zhang, Q.-C.; Yan, C.-A.; Tang, G.-Y.; Zhang, M.-Y.; Ma, L.Q.; Gu, R.-H.; Xiang, P. Heavy metal(loid)s in agriculture soils, rice, and wheat across China: Status assessment and spatiotemporal analysis. Sci. Total Environ. 2023, 882, 163361. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Huang, X.; Sun, L.; Li, S.; Chen, Y.; Cao, X.; Wang, W.; Dai, J.; Rinnan, R. Screening stably low cadmium and moderately high micronutrients wheat cultivars under three different agricultural environments of China. Chemosphere 2020, 241, 125065. [Google Scholar] [CrossRef]
- Chen, R.; de Sherbinin, A.; Ye, C.; Shi, G. China’s Soil Pollution: Farms on the Frontline. Science 2014, 344, 691. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.-J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Y.; Hou, J.; Wu, L.; Christie, P.; Liu, W. Microbial community assembly of the hyperaccumulator plant Sedum plumbizincicola in two contrasting soil types with three levels of cadmium contamination. Sci. Total Environ. 2023, 863, 160917. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Yang, W.; Chen, Y.; Yang, L.; Zhou, H.; Yang, Y.; Zhao, Z.; Wu, P.; Zia-ur-Rehman, M. Exploring the mechanism of Cd uptake and translocation in rice: Future perspectives of rice safety. Sci. Total Environ. 2023, 897, 165369. [Google Scholar] [CrossRef]
- Wang, X.; Ai, S.; Liao, H. Deciphering Interactions between Phosphorus Status and Toxic Metal Exposure in Plants and Rhizospheres to Improve Crops Reared on Acid Soil. Cells 2023, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Barrow, N.J. How understanding soil chemistry can lead to better phosphate fertilizer practice: A 68 year journey (so far). Plant Soil 2022, 476, 117–131. [Google Scholar] [CrossRef]
- Wielgusz, K.; Praczyk, M.; Irzykowska, L.; Świerk, D. Fertilization and soil pH affect seed and biomass yield, plant morphology, and cadmium uptake in hemp (Cannabis sativa L.). Ind. Crops Prod. 2022, 175, 114245. [Google Scholar] [CrossRef]
- Dai, M.; Liu, W.; Hong, H.; Lu, H.; Liu, J.; Jia, H.; Yan, C. Exogenous phosphorus enhances cadmium tolerance by affecting cell wall polysaccharides in two mangrove seedlings Avicennia marina (Forsk.) Vierh and Kandelia obovata (S., L.) Yong differing in cadmium accumulation. Mar. Pollut. Bull. 2018, 126, 86–92. [Google Scholar] [CrossRef]
- Wu, C.; Yan, S.; Zhang, H.; Luo, Y. Chemical forms of cadmium in a calcareous soil treated with different levels of phosphorus-containing acidifying agents. Soil Res. 2015, 53, 105. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Rehman, M.Z.U.; Ali, S.; Rizwan, M.; Naeem, A.; Maqsood, M.A.; Khalid, H.; Rinklebe, J.; Ok, Y.S. Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 2017, 174, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Zhao, B.; Peng, Y.; El-Badri, A.M.; Batool, M.; Wang, C.; Wang, Z.; Huang, W.; Wang, T.; Li, Z.; et al. Auxin plays a key role in nitrogen and plant density-modulated root growth and yield in different plant types of rapeseed. Field Crops Res. 2023, 302, 109066. [Google Scholar] [CrossRef]
- Han, X.-q.; Xiao, X.-y.; Guo, Z.-h.; Xie, Y.-h.; Zhu, H.-w.; Peng, C.; Liang, Y.-q. Release of cadmium in contaminated paddy soil amended with NPK fertilizer and lime under water management. Ecotoxicol. Environ. Saf. 2018, 159, 38–45. [Google Scholar] [CrossRef]
- You, Y.; Ju, C.; Wang, L.; Wang, X.; Ma, F.; Wang, G.; Wang, Y. The mechanism of arbuscular mycorrhizal enhancing cadmium uptake in Phragmites australis depends on the phosphorus concentration. J. Hazard. Mater. 2022, 440, 129800. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Wang, Y.; Yang, Z.; Yuan, J. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem. Toxicol. 2011, 49, 2260–2267. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Wang, H.; Zhang, B.; He, Y.; Wang, H.; Zhu, Y.; Holm, P.E.; Shi, Y. Comparing cadmium uptake kinetics, xylem translocation, chemical forms, and subcellular distribution of two tobacco (Nicotiana tabacum L.) cultivars. Ecotoxicol. Environ. Saf. 2023, 254, 114738. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Zhang, Y.; Wang, H.; Wei, S.; Zhang, X.; Zhang, D.; Ma, H.; Ding, Q.; Ma, L. Morphophysiological, proteomic and metabolomic analyses reveal cadmium tolerance mechanism in common wheat (Triticum aestivum L.). J. Hazard. Mater. 2023, 445, 130499. [Google Scholar] [CrossRef]
- Grüter, R.; Costerousse, B.; Mayer, J.; Mäder, P.; Thonar, C.; Frossard, E.; Schulin, R.; Tandy, S. Long-term organic matter application reduces cadmium but not zinc concentrations in wheat. Sci. Total Environ. 2019, 669, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhu, Y.; Shao, K.; Zhang, Q.; Ye, G.; Shen, J. Metals source apportionment in farmland soil and the prediction of metal transfer in the soil-rice-human chain. J. Environ. Manag. 2020, 260, 110092. [Google Scholar] [CrossRef]
- Nishigaki, T.; Tsujimoto, Y.; Rinasoa, S.; Rakotoson, T.; Andriamananjara, A.; Razafimbelo, T. Phosphorus uptake of rice plants is affected by phosphorus forms and physicochemical properties of tropical weathered soils. Plant Soil 2019, 435, 27–38. [Google Scholar] [CrossRef]
- Chevallier, T.; Cournac, L.; Hamdi, S.; Gallali, T.; Bernoux, M. Temperature dependence of CO2 emissions rates and isotopic signature from a calcareous soil. J. Arid Environ. 2016, 135, 132–139. [Google Scholar] [CrossRef]
- Ma, S.; Nan, Z.; Hu, Y.; Chen, S.; Yang, X.; Su, J. Phosphorus supply level is more important than wheat variety in safe utilization of cadmium-contaminated calcareous soil. J. Hazard. Mater. 2022, 424, 127224. [Google Scholar] [CrossRef] [PubMed]
- Azzi, V.; Kanso, A.; Kazpard, V.; Kobeissi, A.; Lartiges, B.; El Samrani, A. Lactuca sativa growth in compacted and non-compacted semi-arid alkaline soil under phosphate fertilizer treatment and cadmium contamination. Soil Tillage Res. 2017, 165, 1–10. [Google Scholar] [CrossRef]
- Barchi, L.; Aprea, G.; Rabanus-Wallace, M.T.; Toppino, L.; Alonso, D.; Portis, E.; Lanteri, S.; Gaccione, L.; Omondi, E.; van Zonneveld, M.; et al. Analysis of >3400 worldwide eggplant accessions reveals two independent domestication events and multiple migration-diversification routes. Plant J. 2023, 116, 1667–1680. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Tai, P.; Liu, W.; Li, X.; Hao, L. Effects of grafting on root-to-shoot cadmium translocation in plants of eggplant (Solanum melongena) and tomato (Solanum lycopersicum). Sci. Total Environ. 2019, 652, 989–995. [Google Scholar] [CrossRef]
- Cao, F.; Cai, Y.; Liu, L.; Zhang, M.; He, X.; Zhang, G.; Wu, F. Differences in photosynthesis, yield and grain cadmium accumulation as affected by exogenous cadmium and glutathione in the two rice genotypes. Plant Growth Regul. 2015, 75, 715–723. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Liang, L.; Lin, Z.; Su, X.; Zhang, W. Immobilization of cadmium in contaminated soils using sulfidated nanoscale zero-valent iron: Effectiveness and remediation mechanism. J. Hazard. Mater. 2021, 420, 126605. [Google Scholar] [CrossRef]
- Yu, S.; Sheng, L.; Mao, H.; Huang, X.; Luo, L.; Li, Y. Physiological response of Conyza Canadensis to cadmium stress monitored by Fourier transform infrared spectroscopy and cadmium accumulation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 118007. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; He, D.; He, X.; Yan, Y.; Wu, K.; Wei, H. Effects of Exogenous Organic Acids on Cd Tolerance Mechanism of Salix variegata Franch. Under Cd Stress. Front. Plant Sci. 2020, 11, 594352. [Google Scholar] [CrossRef]
- Weigel, H.J.; Jäger, H.J. Subcellular Distribution and Chemical Form of Cadmium in Bean Plants. Plant Physiol. 1980, 65, 480–482. [Google Scholar] [CrossRef]
- Wu, F.-B.; Dong, J.; Qian, Q.Q.; Zhang, G.-P. Subcellular distribution and chemical form of Cd and Cd–Zn interaction in different barley genotypes. Chemosphere 2005, 60, 1437–1446. [Google Scholar] [CrossRef]
- Marchiol, L.; Assolari, S.; Sacco, P.; Zerbi, G. Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ. Pollut. 2004, 132, 21–27. [Google Scholar] [CrossRef]
- Meng, Q.; Fan, W.; Liu, F.; Wang, G.; Di, X. Effect of Phosphorus Application on Eggplant Cadmium Accumulation and Soil Cadmium Morphology. Sustainability 2023, 15, 16236. [Google Scholar] [CrossRef]
- Santoro, V.; Schiavon, M.; Celi, L. Role of soil abiotic processes on phosphorus availability and plant responses with a focus on strigolactones in tomato plants. Plant Soil 2023, 194, 1–49. [Google Scholar] [CrossRef]
- Li, Z.; Guo, F.; Cornelis, J.-T.; Song, Z.; Wang, X.; Delvaux, B. Combined Silicon-Phosphorus Fertilization Affects the Biomass and Phytolith Stock of Rice Plants. Front. Plant Sci. 2020, 11, 67. [Google Scholar] [CrossRef]
- Dai, M.; Lu, H.; Liu, W.; Jia, H.; Hong, H.; Liu, J.; Yan, C. Phosphorus mediation of cadmium stress in two mangrove seedlings Avicennia marina and Kandelia obovata differing in cadmium accumulation. Ecotoxicol. Environ. Saf. 2017, 139, 272–279. [Google Scholar] [CrossRef]
- Jia, H.; Wu, Y.; Zhang, M.; Ye, J.; Du, D.; Wang, H. Role of phosphorus on the biogeochemical behavior of cadmium in the contaminated soil under leaching and pot experiments. J. Environ. Sci. 2024, 137, 488–499. [Google Scholar] [CrossRef]
- Wang, K.; Qiao, Y.; Zhang, H.; Yue, S.; Li, H.; Ji, X.; Liu, L. Influence of metal-contamination on distribution in subcellular fractions of the earthworm (Metaphire californica) from Hunan Province, China. J. Environ. Sci. 2018, 73, 127–137. [Google Scholar] [CrossRef]
- Wang, J.; Su, L.; Yang, J.; Yuan, J.; Yin, A.; Qiu, Q.; Zhang, K.; Yang, Z. Comparisons of cadmium subcellular distribution and chemical forms between low-Cd and high-Cd accumulation genotypes of watercress (Nasturtium officinale L. R. Br.). Plant Soil 2015, 396, 325–337. [Google Scholar] [CrossRef]
- Veach, A.M.; Yip, D.; Engle, N.L.; Yang, Z.K.; Bible, A.; Morrell-Falvey, J.; Tschaplinski, T.J.; Kalluri, U.C.; Schadt, C.W. Modification of plant cell wall chemistry impacts metabolome and microbiome composition in Populus PdKOR1 RNAi plants. Plant Soil 2018, 429, 349–361. [Google Scholar] [CrossRef]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal Interactions between Cadmium-Induced Cell Wall Responses and Oxidative Stress in Plants. Front. Plant Sci. 2017, 8, 286473. [Google Scholar] [CrossRef]
- Yu, H.; Guo, J.; Li, Q.; Zhang, X.; Huang, H.; Huang, F.; Yang, A.; Li, T. Characteristics of cadmium immobilization in the cell wall of root in a cadmium-safe rice line (Oryza sativa L.). Chemosphere 2020, 241, 125095. [Google Scholar] [CrossRef]
- Sabella, E.; Aprile, A.; Tenuzzo, B.A.; Carata, E.; Panzarini, E.; Luvisi, A.; De Bellis, L.; Vergine, M. Effects of Cadmium on Root Morpho-Physiology of Durum Wheat. Front. Plant Sci. 2022, 13, 936020. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef]
- Guo, J.; Ye, D.; Zhang, X.; Huang, H.; Wang, Y.; Zheng, Z.; Li, T.; Yu, H. Characterization of cadmium accumulation in the cell walls of leaves in a low-cadmium rice line and strengthening by foliar silicon application. Chemosphere 2022, 287, 132374. [Google Scholar] [CrossRef]
- Ma, P.; Zang, J.; Shao, T.; Jiang, Q.; Li, Y.; Zhang, W.; Liu, M. Cadmium distribution and transformation in leaf cells involved in detoxification and tolerance in barley. Ecotoxicol. Environ. Saf. 2023, 249, 114391. [Google Scholar] [CrossRef]
- Przybylska, A.; Wieczorek, P.; Obrępalska-Stęplowska, A. Meloidogyne arenaria candidate effector MaMsp4 interacts with maize (Zea mays L.) proteins involved in host defense response and cell wall modifications. Plant Soil 2023, 491, 501–523. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Wang, C.; Huang, Y.; Liu, Z. Increasing phosphate inhibits cadmium uptake in plants and promotes synthesis of amino acids in grains of rice. Environ. Pollut. 2020, 257, 113496. [Google Scholar] [CrossRef] [PubMed]
- Siebers, N.; Siangliw, M.; Tongcumpou, C. Cadmium uptake and subcellular distribution in rice plants as affected by phosphorus: Soil and hydroponic experiments. J. Soil Sci. Plant Nutr. 2013, 13, 833–844. [Google Scholar] [CrossRef]
- Sterckeman, T.; Thomine, S. Mechanisms of Cadmium Accumulation in Plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, G.; Guo, H.; Yang, M.; Yang, Q. Accumulation and sub cellular distribution of lead (Pb) in industrial hemp grown in Pb contaminated soil. Ind. Crops Prod. 2021, 161, 113220. [Google Scholar] [CrossRef]
- Gu, T.; Lu, Y.; Li, F.; Zeng, W.; Shen, L.; Yu, R.; Li, J. Microbial extracellular polymeric substances alleviate cadmium toxicity in rice (Oryza sativa L.) by regulating cadmium uptake, subcellular distribution and triggering the expression of stress-related genes. Ecotoxicol. Environ. Saf. 2023, 257, 114958. [Google Scholar] [CrossRef]
- Zheng, M.-M.; Feng, D.; Liu, H.-J.; Yang, G.-L. Subcellular distribution, chemical forms of cadmium and rhizosphere microbial community in the process of cadmium hyperaccumulation in duckweed. Sci. Total Environ. 2023, 859, 160389. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhou, Y.; Yang, Z.; Lin, B.; Yuan, J.; Wu, S. Comparisons in subcellular and biochemical behaviors of cadmium between low-Cd and high-Cd accumulation cultivars of pakchoi (Brassica chinensis L.). Front. Environ. Sci. Eng. 2014, 8, 226–238. [Google Scholar] [CrossRef]
- Thawornchaisit, U.; Polprasert, C. Evaluation of phosphate fertilizers for the stabilization of cadmium in highly contaminated soils. J. Hazard. Mater. 2009, 165, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, L.; Wang, Y.; Cao, Y.; Zhang, S. Synergistic enhanced passivation of phosphorus and cadmium in sediment by Ca/Al co-modified biochar. Chem. Eng. J. 2023, 474, 145539. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Liu, T.; Liu, B.; Huang, D.; Zhu, Q.; Xu, C. The influence of liming on cadmium accumulation in rice grains via iron-reducing bacteria. Sci. Total Environ. 2018, 645, 109–118. [Google Scholar] [CrossRef]
- He, L.-L.; Huang, D.-Y.; Zhang, Q.; Zhu, H.-H.; Xu, C.; Li, B.; Zhu, Q.-H. Meta-analysis of the effects of liming on soil pH and cadmium accumulation in crops. Ecotoxicol. Environ. Saf. 2021, 223, 112621. [Google Scholar] [CrossRef] [PubMed]


| Type | Level | BCFroot | BCFstem | BCFleaf | TFstem/root | TFleaf/root |
|---|---|---|---|---|---|---|
| MAP | P0 | 0.928 ± 0.004 Aa | 0.517 ± 0.005 Aa | 0.354 ± 0.011 Aa | 0.558 ± 0.007 Aa | 0.382 ± 0.013 Aa |
| P1 | 0.870 ± 0.011 Bb | 0.470 ± 0.006 Bb | 0.294 ± 0.005 Bb | 0.540 ± 0.000 Bb | 0.338 ± 0.005 Ab | |
| P2 | 0.849 ± 0.008 Bc | 0.432 ± 0.004 Ac | 0.246 ± 0.004 Bc | 0.509 ± 0.005 Ac | 0.289 ± 0.002 Bc | |
| P3 | 0.794 ± 0.007 Ad | 0.388 ± 0.008 Ad | 0.224 ± 0.002 Bd | 0.489 ± 0.009 Ad | 0.283 ± 0.001 Bc | |
| SSP | P0 | 0.928 ± 0.004 Aa | 0.517 ± 0.005 Aa | 0.354 ± 0.011 Aa | 0.558 ± 0.007 Aa | 0.382 ± 0.013 Aa |
| P1 | 0.903 ± 0.005 Ab | 0.496 ± 0.006 Ab | 0.309 ± 0.002 Ab | 0.550 ± 0.004 Aa | 0.342 ± 0.001 Ab | |
| P2 | 0.874 ± 0.011 Ac | 0.444 ± 0.009 Ac | 0.265 ± 0.003 Ac | 0.508 ± 0.010 Ab | 0.304 ± 0.006 Ac | |
| P3 | 0.784 ± 0.013 Ad | 0.387 ± 0.002 Ad | 0.234 ± 0.002 Ad | 0.494 ± 0.006 Ac | 0.299 ± 0.002 Ac | |
| DAP | P0 | 0.928 ± 0.004 Aa | 0.517 ± 0.005 Aa | 0.354 ± 0.011 Aa | 0.558 ± 0.007 Aa | 0.382 ± 0.013 Aa |
| P1 | 0.862 ± 0.001 Bb | 0.448 ± 0.003 Cb | 0.276 ± 0.002 Cb | 0.519 ± 0.003 Cb | 0.320 ± 0.003 Bb | |
| P2 | 0.811 ± 0.002 Cc | 0.394 ± 0.006 Bc | 0.220 ± 0.001 Cc | 0.486 ± 0.006 Bc | 0.271 ± 0.001 Cc | |
| P3 | 0.758 ± 0.017 Bd | 0.358 ± 0.007 Bd | 0.200 ± 0.005 Cd | 0.472 ± 0.003 Bd | 0.264 ± 0.004 Cc | |
| DCP | P0 | 0.928 ± 0.004 Aa | 0.517 ± 0.005 Aa | 0.354 ± 0.011 Aa | 0.558 ± 0.007 Aa | 0.382 ± 0.013 Aa |
| P1 | 0.861 ± 0.010 Bb | 0.436 ± 0.006 Db | 0.244 ± 0.005 Db | 0.506 ± 0.005 Db | 0.283 ± 0.002 Cb | |
| P2 | 0.820 ± 0.010 Cc | 0.395 ± 0.008 Bc | 0.207 ± 0.003 Dc | 0.482 ± 0.004 Bc | 0.253 ± 0.002 Dc | |
| P3 | 0.752 ± 0.006 Bd | 0.338 ± 0.003 Cd | 0.186 ± 0.003 Dd | 0.449 ± 0.004 Cd | 0.247 ± 0.003 Dc | |
| T | *** | *** | *** | *** | *** | |
| L | *** | *** | *** | *** | *** | |
| T × L | *** | *** | *** | *** | *** | |
| Type | Level | FE | FW | FNaCl | FHAC | FHCL | FR |
|---|---|---|---|---|---|---|---|
| MAP | P0 | 0.712 ± 0.009 Aa | 0.821 ± 0.004 Aa | 3.738 ± 0.080 Aa | 1.092 ± 0.023 Ab | 0.369 ± 0.038 Ab | 0.159 ± 0.009 Ab |
| P1 | 0.536 ± 0.002 Bb | 0.84 ± 0.007 Bab | 2.883 ± 0.024 Bb | 1.115 ± 0.002 ABa | 0.398 ± 0.006 Cab | 0.171 ± 0.004 BCa | |
| P2 | 0.472 ± 0.007 Bc | 0.847 ± 0.002 Db | 2.42 ± 0.036 Bc | 1.12 ± 0.001 Ca | 0.416 ± 0.004 Ca | 0.174 ± 0.008 ABa | |
| P3 | 0.410 ± 0.007 Bd | 0.852 ± 0.003 Bc | 2.105 ± 0.030 Bd | 1.126 ± 0.005 Ba | 0.423 ± 0.003 Ca | 0.179 ± 0.003 Ba | |
| SSP | P0 | 0.712 ± 0.009 Aa | 0.82 ± 0.004 Aa | 3.738 ± 0.080 Aa | 1.092 ± 0.023 Aa | 0.369 ± 0.038 Ac | 0.159 ± 0.009 Ab |
| P1 | 0.659 ± 0.013 Ab | 0.827 ± 0.003 Cb | 3.354 ± 0.044 Ab | 1.106 ± 0.012 Bab | 0.377 ± 0.008 Dc | 0.167 ± 0.001 Cab | |
| P2 | 0.553 ± 0.009 Ac | 0.834 ± 0.003 Cc | 2.937 ± 0.033 Ac | 1.116 ± 0.003 Cab | 0.387 ± 0.003 Db | 0.17 ± 0.004 Ba | |
| P3 | 0.454 ± 0.005 Ad | 0.847 ± 0.002 Bd | 2.515 ± 0.035 Ad | 1.121 ± 0.003 Bb | 0.404 ± 0.005 Da | 0.176 ± 0.002 Ba | |
| DAP | P0 | 0.712 ± 0.009 Aa | 0.82 ± 0.004 Aa | 3.738 ± 0.080 Aa | 1.092 ± 0.023 Ab | 0.369 ± 0.038 Ad | 0.159 ± 0.009 Ab |
| P1 | 0.450 ± 0.005 Cb | 0.85 ± 0.002 Ab | 2.486 ± 0.038 Cb | 1.122 ± 0.003 Aa | 0.411 ± 0.003 Bc | 0.174 ± 0.002 ABa | |
| P2 | 0.401 ± 0.011 Cc | 0.856 ± 0.003 Bb | 2.118 ± 0.053 Cc | 1.126 ± 0.002 Ba | 0.423 ± 0.001 Bb | 0.181 ± 0.002 Aa | |
| P3 | 0.328 ± 0.003 Cd | 0.867 ± 0.004 Ac | 1.73 ± 0.016 Cd | 1.133 ± 0.002 Aa | 0.434 ± 0.004 Ba | 0.184 ± 0.002 Aa | |
| DCP | P0 | 0.712 ± 0.009 Aa | 0.82 ± 0.004 Aa | 3.738 ± 0.080 Aa | 1.092 ± 0.023 Ab | 0.369 ± 0.038 Ac | 0.159 ± 0.009 Ab |
| P1 | 0.386 ± 0.01 Db | 0.855 ± 0.004 Aa | 2.132 ± 0.053 Db | 1.124 ± 0.003 Aa | 0.426 ± 0.002 Ab | 0.178 ± 0.005 Aa | |
| P2 | 0.291 ± 0.005 Dc | 0.866 ± 0.002 Ab | 1.699 ± 0.02 Dc | 1.13 ± 0.001 Aa | 0.433 ± 0.002 Ab | 0.182 ± 0.001 Aa | |
| P3 | 0.246 ± 0.003 Dd | 0.872 ± 0.001 Ac | 1.321 ± 0.023 Dd | 1.136 ± 0.003 Aa | 0.446 ± 0.003 Aa | 0.187 ± 0.003 Aa | |
| T | *** | *** | *** | ns | ** | ** | |
| L | *** | *** | *** | *** | *** | *** | |
| T × L | *** | *** | *** | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Fan, W.; Liu, F.; Wang, G.; Di, X. Effect of Phosphorus Application on Subcellular Distribution and Chemical Morphology of Cadmium in Eggplant Seedlings under Cadmium Stress. Agronomy 2024, 14, 932. https://doi.org/10.3390/agronomy14050932
Meng Q, Fan W, Liu F, Wang G, Di X. Effect of Phosphorus Application on Subcellular Distribution and Chemical Morphology of Cadmium in Eggplant Seedlings under Cadmium Stress. Agronomy. 2024; 14(5):932. https://doi.org/10.3390/agronomy14050932
Chicago/Turabian StyleMeng, Qinghui, Wenhua Fan, Fenwu Liu, Gailing Wang, and Xiaoying Di. 2024. "Effect of Phosphorus Application on Subcellular Distribution and Chemical Morphology of Cadmium in Eggplant Seedlings under Cadmium Stress" Agronomy 14, no. 5: 932. https://doi.org/10.3390/agronomy14050932
APA StyleMeng, Q., Fan, W., Liu, F., Wang, G., & Di, X. (2024). Effect of Phosphorus Application on Subcellular Distribution and Chemical Morphology of Cadmium in Eggplant Seedlings under Cadmium Stress. Agronomy, 14(5), 932. https://doi.org/10.3390/agronomy14050932









