Comparison of the Effect of Pruning on Plant Growth and Transcriptome Profiles in Different Tea Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sample Preparation
2.2. Measurement of Shoot Growth Parameters
2.3. Statistical Analysis
2.4. Transcriptomic and Bioinformatic Analyses
3. Results
3.1. The Growth of the Shoots after the Pruning Was Measured in 20 Different Varieties of Tea Plant Cultivars
3.2. Transcriptome Data Analysis of Two Tea Plant Cultivars
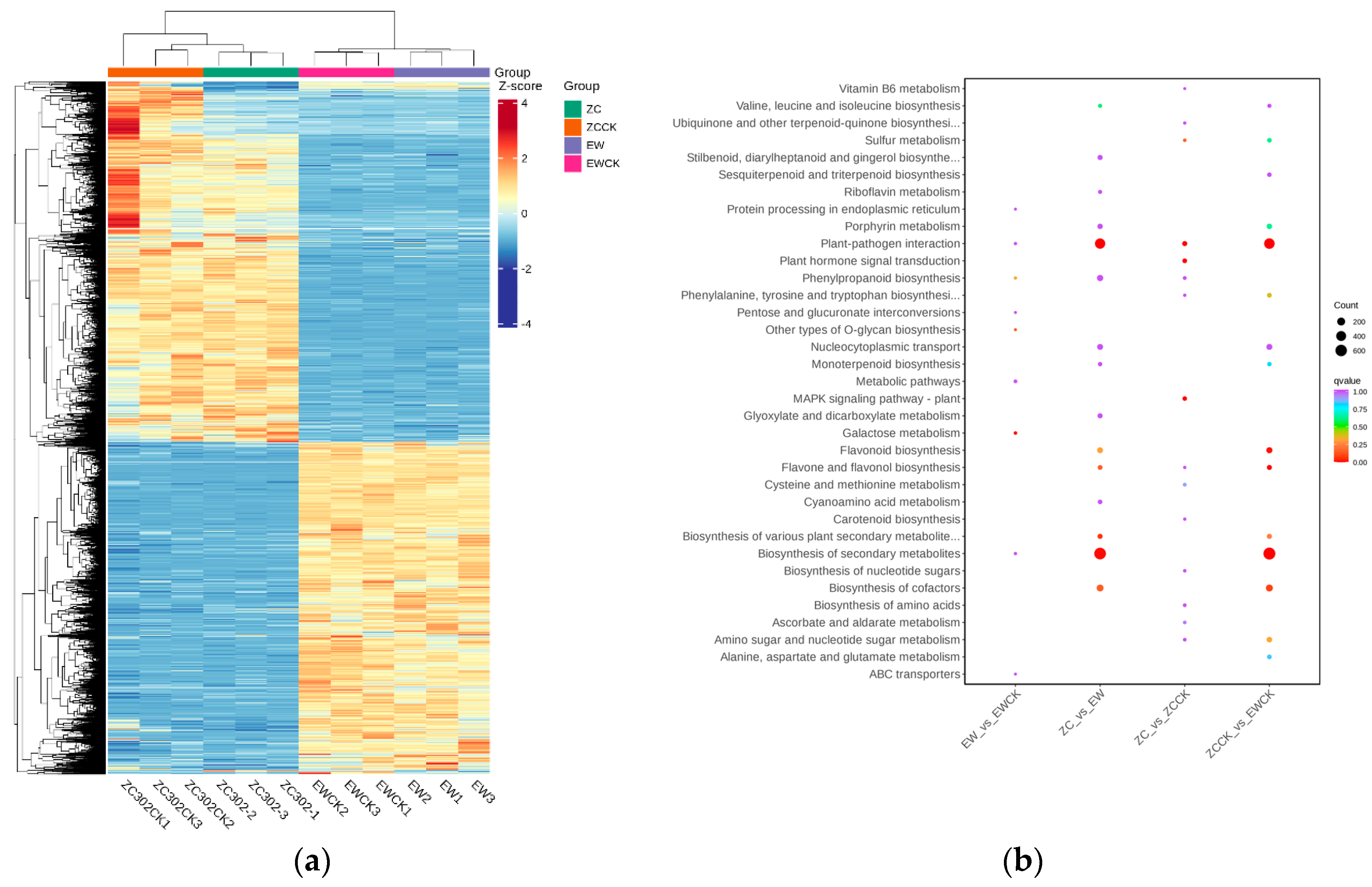
3.2.1. Overall Distribution of Gene Expression in Young Tea Shoots of ‘Zhongcha 302’ and ‘Emei Wenchun’
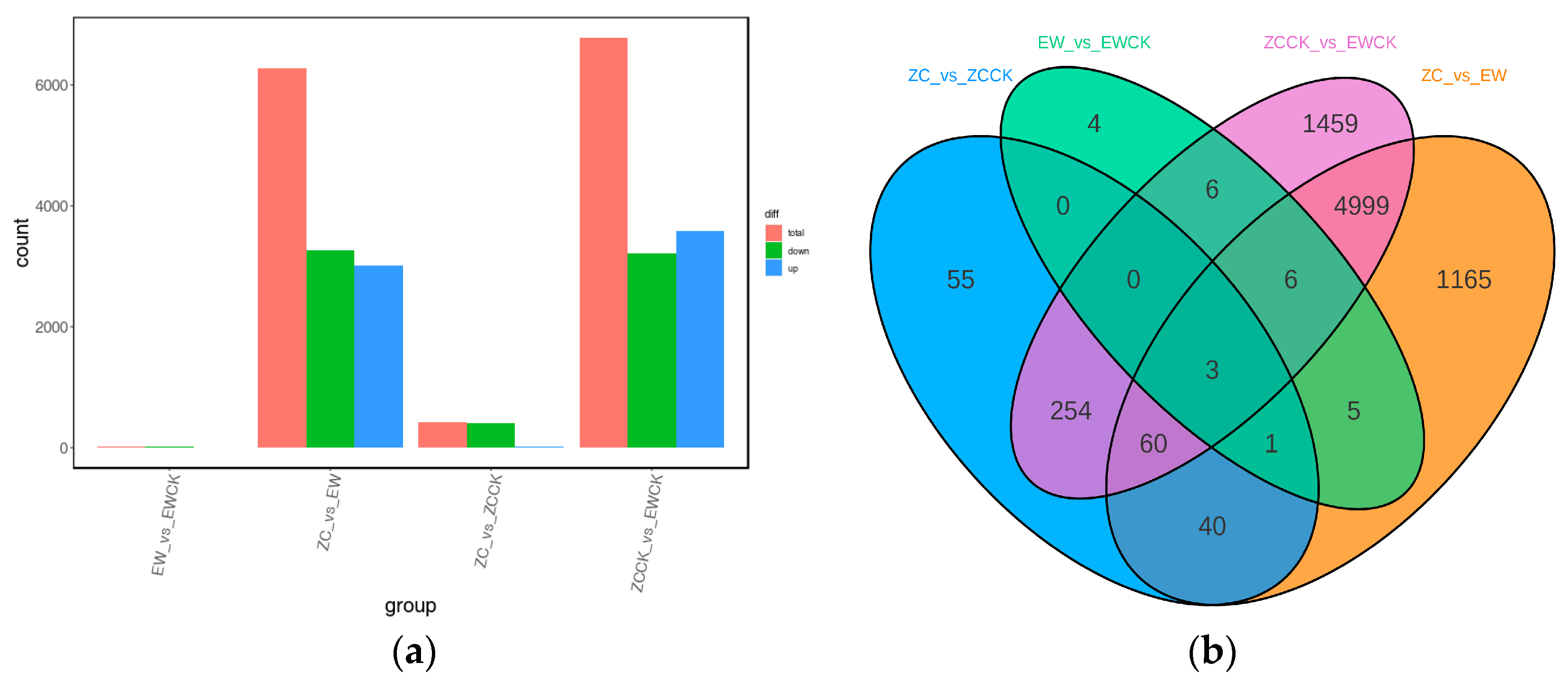
3.2.2. Differential Gene Expression Analysis
3.2.3. KEGG Pathway Enrichment Analysis
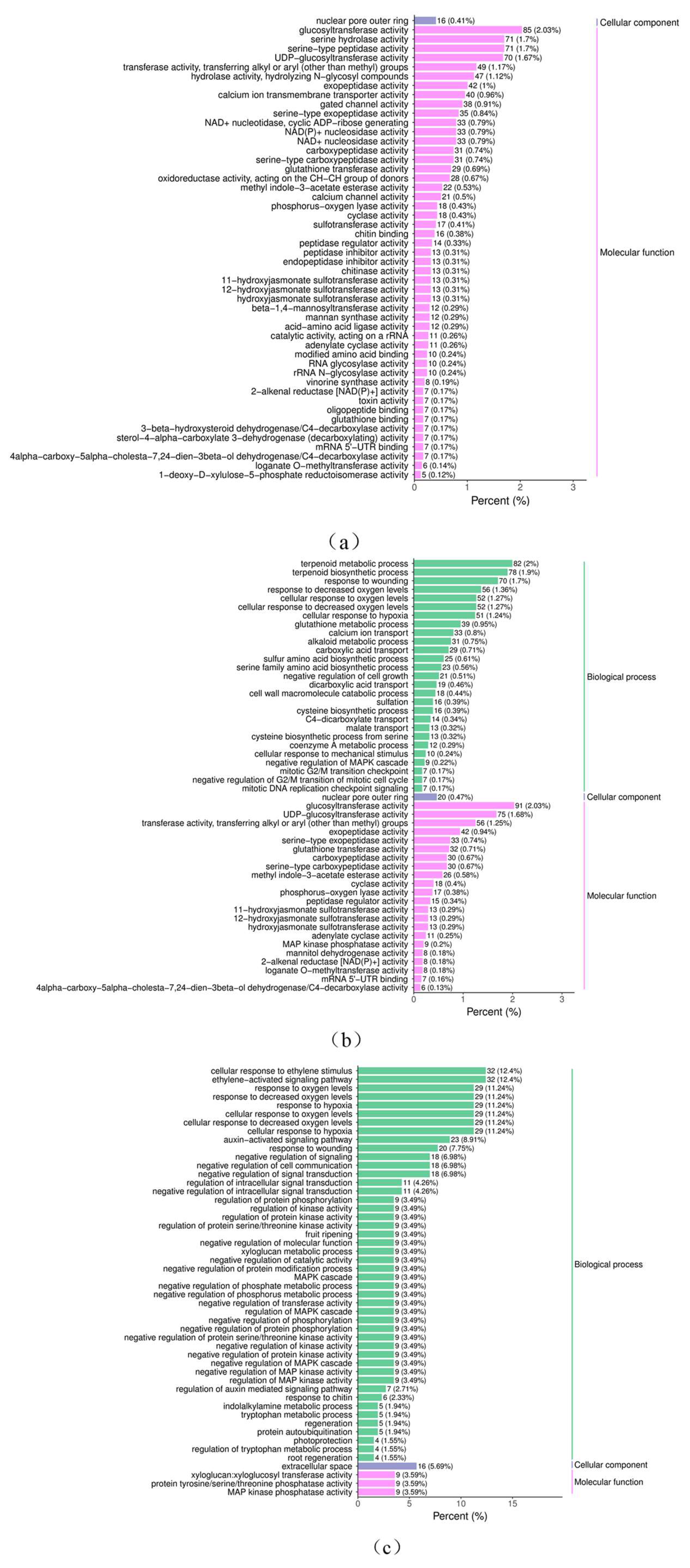
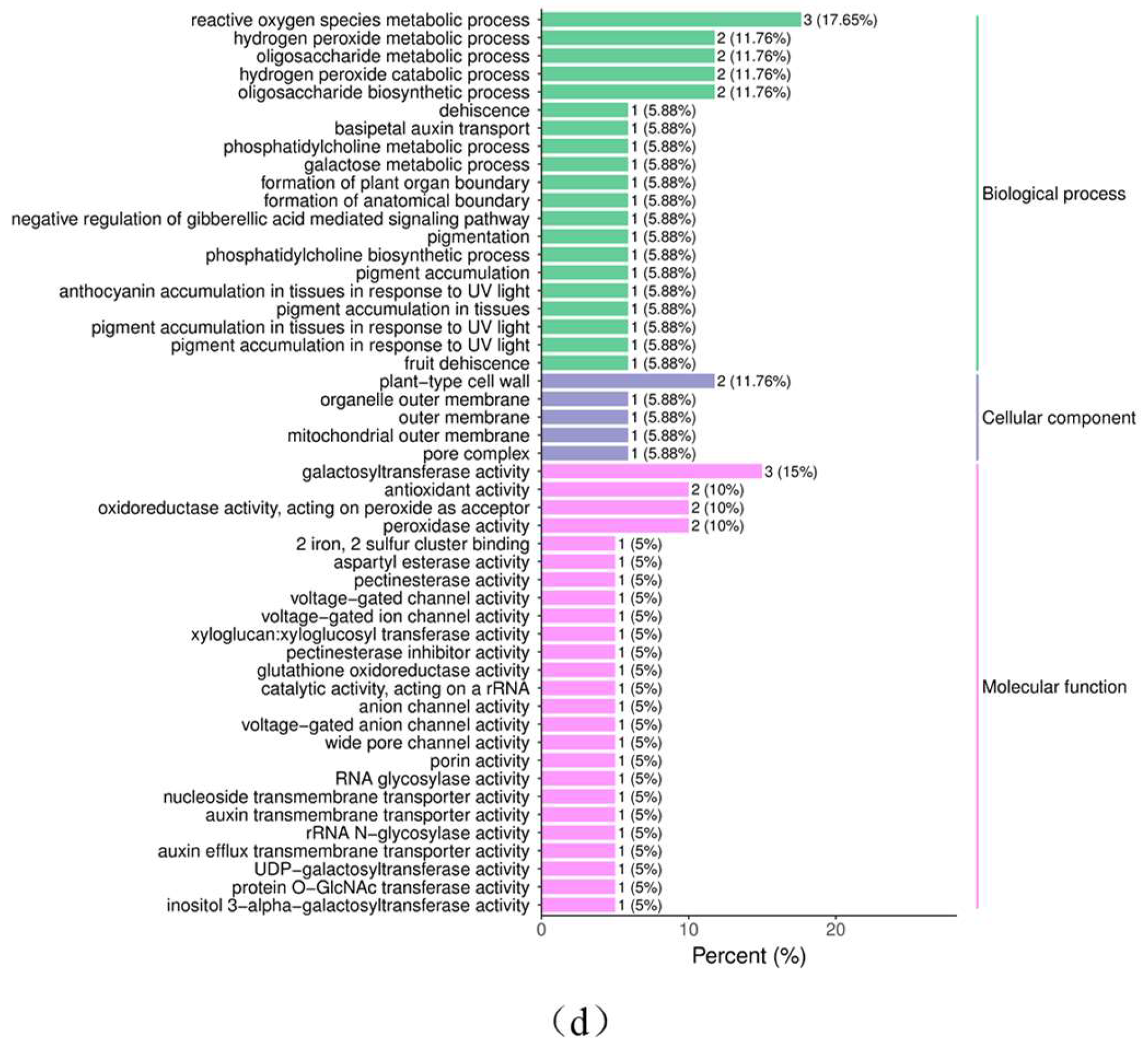
3.2.4. GO Enrichment Analysis
3.2.5. Column Chart of the Differential Gene GO Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Drew, L. The growth of tea. Nature 2019, 566, S2–S4. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, H.; Mischke, S.; Meinhardt, L.W.; Zhang, D.; Zhu, X.; Li, X.; Fang, W. Exogenous abscisic acid significantly affects proteome in tea plant (Camellia sinensis) exposed to drought stress. Hortic. Res. 2014, 1, 14029. [Google Scholar] [CrossRef] [PubMed]
- Nhmrm, A.; Khh, B.; Msl, C.; Ehk, D.; Ysh, A. Metabolomic understanding of the difference between unpruning and pruning cultivation of tea (Camellia sinensis) plants—ScienceDirect. Food Res. Int. 2020, 104, 109978. [Google Scholar]
- Zhang, Q.; Zhang, Y.; Wang, Y.; Zou, J.; Lin, S.; Chen, M.; Miao, P.; Jia, X.; Cheng, P.; Pang, X.; et al. Transcriptomic Analysis of the Effect of Pruning on Growth, Quality, and Yield of Wuyi Rock Tea. Plants 2023, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, M.; Li, X.; Yan, P.; Han, W. Summer pruning improves the branch growth and tea quality of tea plants (Camellia sinensis). Acta Physiol. Plant. 2021, 43, 65. [Google Scholar] [CrossRef]
- Maurin, V.; Desrochers, A. Physiological and growth responses to pruning season and intensity of hybrid poplar. For. Ecol. Manag. 2013, 304, 399–406. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Miao, P.; Chen, M.; Du, M.; Pang, X.; Ye, J.; Wang, H.; Jia, X. Effects of pruning on tea plant growth, soil enzyme activity and microbial diversity. Agronomy 2023, 13, 1214. [Google Scholar] [CrossRef]
- Liechti, R. The Jasmonate Pathway. Science 2002, 296, 1649–1650. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. Jasmonate signaling: Toward an integrated view. Plant Physiol. 2008, 146, 1459–1468. [Google Scholar] [CrossRef]
- Koo, A.J.; Gao, X.; Jones, A.D.; Howe, G.A. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J. 2010, 59, 974–986. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; McCaig, B.C.; Wingerd, B.A.; Wang, J.; Whalon, M.E.; Pichersky, E.; Howe, G.A. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell Online 2004, 16, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.H.; Khan, A.R.; Salam, A.; Neelam, A.; Azhar, W.; Ulhassan, Z.; Gan, Y. Exploring the adaptive responses of plants to abiotic stresses using transcriptome data. Agriculture 2022, 12, 211. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action mechanisms of effectors in plant-pathogen interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Luo, W.; Zheng, Y.; Jin, J.; Liu, R.; Lv, Y.; Ye, Y.; Ye, J. Effect of different pruning operations on the plant growth, phytohormones and transcriptome profiles of the following spring tea shoots. Beverage Plant Res. 2022, 2, 12. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, C.; Lu, M.; Gan, N.; Chen, Z.; Deng, W.-W.; Zhang, Z.-Z. Metabolic Flux Enhancement and Transcriptomic Analysis Displayed the Changes of Catechins Following Long-Term Pruning in Tea plants (Camellia sinensis). J. Agric. Food Chem. 2018, 66, 8566–8573. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Q.; Gong, X.; Chen, J.; Li, Q.Y.; Wang, L.J.; Zhou, Y.F. Research and analysis on optimal adjustment of planting tea varieties in guizhou. Seed 2014, 33, 97–100. [Google Scholar]
- Zhang, X.; Chen, S.; Shi, L.; Gong, D.; Zhang, S.; Zhao, Q.; Zhan, D.; Vasseur, L.; Wang, Y.; Yu, J.; et al. Haplotype-resolved genome assembly provides insights into evolutionary history of the tea plant Camellia sinensis. Nat. Genet. 2021, 53, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.A.-O. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Yang, L.; Smyth, G.K.; Wei, S. Feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar]
- Zhang, W.; Li, H.; Li, Q.; Wang, Z.; Zeng, W.; Yin, H.; Qi, K.; Zou, Y.; Hu, J.; Huang, B. Genome-wide identification, comparative anal-ysis and functional roles in flavonoid biosynthesis of cytochromeP450superfamily in pear (Pyruss spp.). BMC Genom. Data 2023, 24, 58. [Google Scholar] [CrossRef] [PubMed]
- Shohat, H.; Cheriker, H.; Cohen, A.; Weiss, D. Tomato ABA-IMPORTING TRANSPORTER 1.1 inhibits seed germi-nation under high salinity conditions. Plant Physiol. 2023, 191, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, C.; Du, T.; Li, A.; Qin, Z.; Zhang, L.; Dong, S.; Wang, Q.; Hou, F. Overexpression of 9-cis-Epoxycarotenoid Dioxygen-ase Gene, IbNCED1, Negatively Regulates Plant Height in Transgenic Sweet Potato. Int. J. Mol. Sci. 2023, 24, 10421. [Google Scholar] [CrossRef]
- Arkorful, E.; Yu, Y.; Chen, C.; Lu, L.; Hu, S.; Yu, H.; Ma, Q.; Thangaraj, K.; Periakaruppan, R.; Jeyaraj, A. Untargeted metabolomic anal-ysis using UPLC-MS/MS identifies metabolites involved in shoot growth and development in pruned tea plants (Camellia sinensis (L.) O. Kuntz). Sci. Hortic. 2020, 264, 109164. [Google Scholar] [CrossRef]
- Ashburner, M.M.; Ball, C.A.C.; Blake, J.A.J.; Botstein, D.D.; Sherlock, G. Gene Ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Matias, P.; Barrote, I.; Azinheira, G.; Continella, A.; Duarte, A. Citrus Pruning in the Mediterranean Climate: A Review. Plants 2023, 12, 3360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, K.; Wei, Q.P.; Yue, Y.L.; Wang, X.W.; Zhang, J.X. Effects of different pruning modes on the light distribution characters and fruit yield and quality in densely planted ‘Red Fuji’ apple orchard. Ying Yong Sheng Tai Xue Bao 2010, 21, 1224–1230. (In Chinese) [Google Scholar]
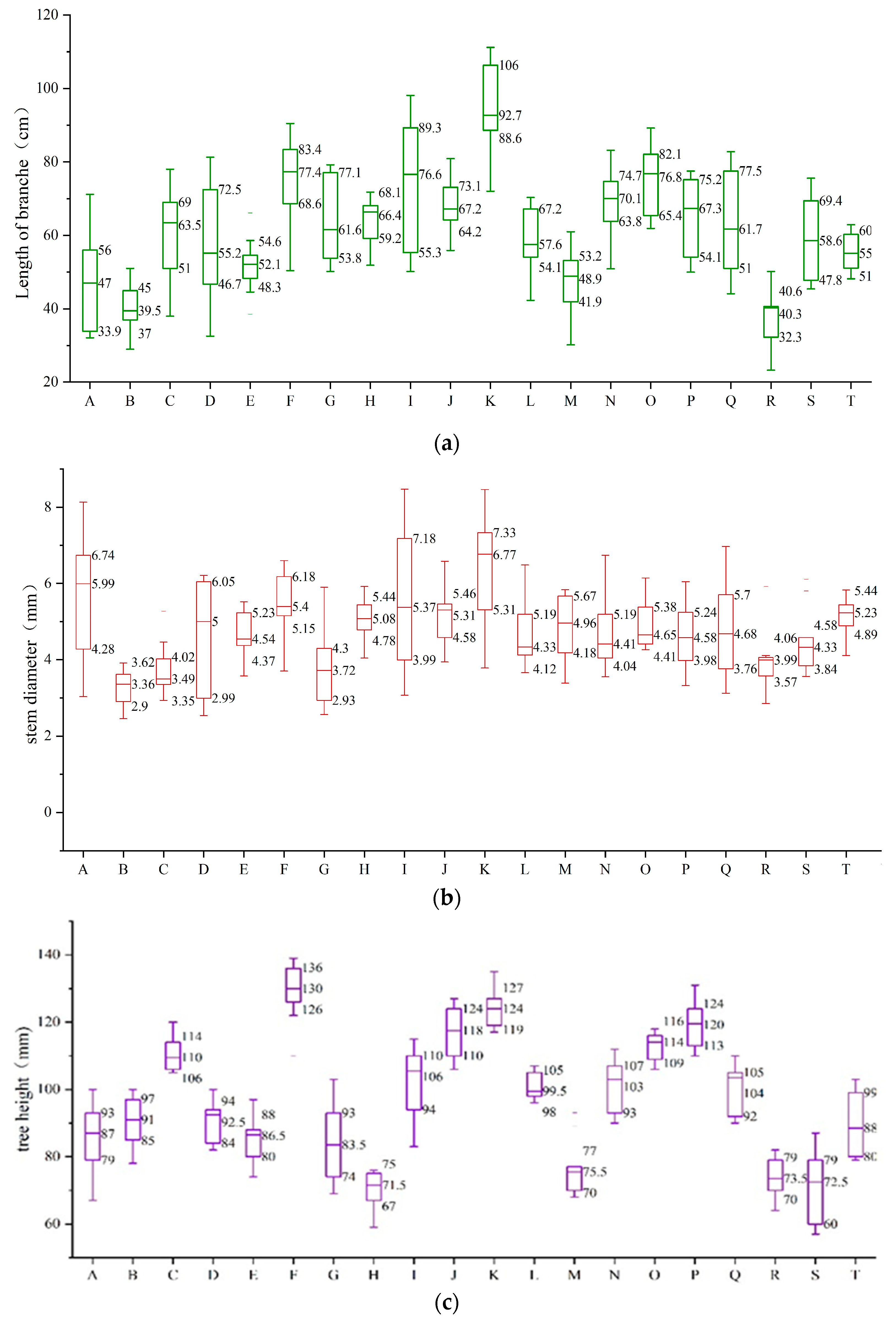
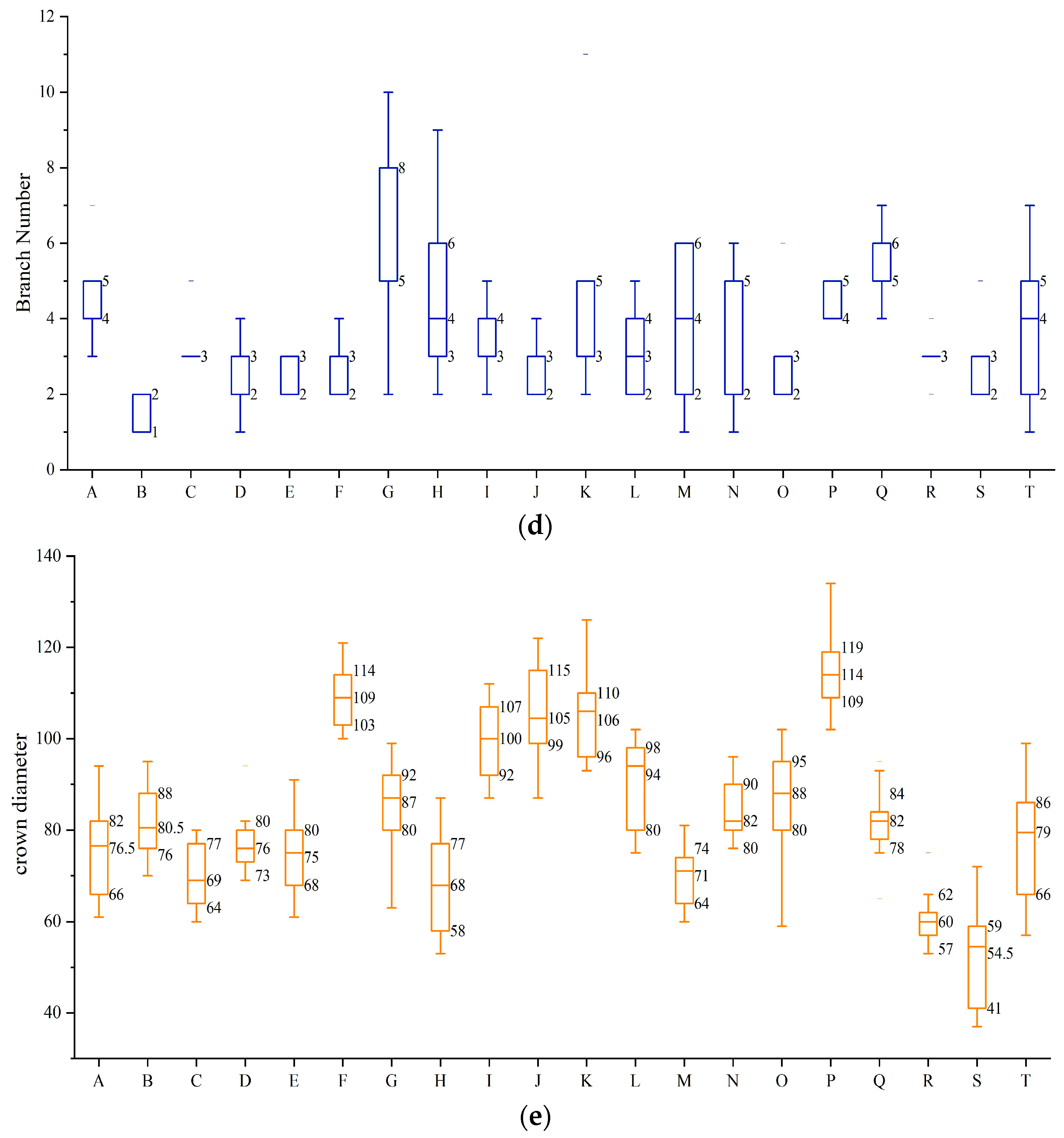




| Variety | Number of Branches (pcs) | Branch Weight (kg) | Branch Length (cm) | Diamethickness (mm) | Tree Height (cm) | Tree Spread (cm) |
|---|---|---|---|---|---|---|
| Chunmu 28 | 27.67 ± 4.84 cde | 27.67 ± 4.84 cde | 53.75 ± 713 def | 5.31 ± 0.11 abc | 84.33 ± 3.18 hr | 83.67 ± 5.55 cdef |
| Zizhu | 30.33 ± 2.33 cde | 0.75 ± 0.15 bcde | 51.52 ± 7.14 ef | 3.80 ± 0.30 d | 90.33 ± 0.67 fgh | 81.00 ± 3.61 def |
| Zijuan | 43.00 ± 1.53 ab | 0.73 ± 0.04 bcde | 58.58 ± 3.63 bcdef | 3.95 ± 0.26 d | 114.33 ± 2.60 bcd | 70.67 ± 5.21 fg |
| Huangyazao | 33.67 ± 4.33 abcde | 0.68 ± 0.07 bcde | 63.07 ± 3.23 bcdef | 4.99 ± 0.54 abcd | 93.00 ± 0.57 efgh | 78.00 ± 2.08 def |
| Dangui | 30.00 ± 2.08 cde | 0.70 ± 0.05 bcde | 56.13 ± 3.50 cdef | 4.44 ± 0.10 bcd | 82.67 ± 4.67 hr | 78.33 ± 3.53 def |
| Chuancha 3 | 38.00 ± 1.73 abc | 0.90 ± 0.05 bc | 69.68 ± 1.18 abc | 5.41 ± 0.07 ab | 134.00 ± 4.04 a | 109.33 ± 5.49 ab |
| Zhongcha 102 | 31.33 ± 4.91 cde | 0.82 ± 0.04 bcde | 54.43 ± 5.50 def | 4.46 ± 0.40 bcd | 88.67 ± 3.38 gh | 91.00 ± 4.04 cd |
| Fudingdabaicha | 30.33 ± 4.18 cde | 0.60 ± 0.03 bcde | 60.65 ± 1.08 bcdef | 5.04 ± 0.13 abcd | 72.00 ± 2.65 r | 80.00 ± 3.51 def |
| Mabianlv 1 | 25.33 ± 3.18 de | 0.91 ± 0.2 bc | 78.55 ± 3.33 a | 4.99 ± 0.50 abcd | 109.00 ± 3.46 cd | 97.67 ± 7.45 bc |
| Xiangshanzao | 30.33 ± 1.67 cde | 0.91 ± 0.06 bc | 67.78 ± 3.59 abcd | 4.55 ± 0.24 bcd | 112.00 ± 4.58 bcd | 109.00 ± 5.29 ab |
| ‘Zhongcha 302’ | 33.67 ± 1.45 | 1.58 ± 0.06 a | 72.64 ± 5.99 ab | 5.59 ± 0.37 ab | 124.67 ± 0.88 ab | 108.00 ± 1.15 ab |
| Chunmu 217 | 27.67 ± 4.10 cde | 0.65 ± 0.03 bcde | 64.50 ± 3.51 abcdef | 4.94 ± 0.24 abcd | 102.00 ± 1.73 def | 90.00 ± 5.68 cde |
| Jianhexiangcha | 28.33 ± 3.71 cde | 0.58 ± 0.02 cde | 50.40 ± 2.10 f | 4.82 ± 0.12 bcd | 75.00 ± 1.53 r | 69.67 ± 3.48 |
| Chuancha 2 | 29.33 ± 2.96 cde | 0.53 ± 0.14 de | 65.71 ± 0.32 abcde | 5.41 ± 0.69 ab | 104.00 ± 5.69 de | 83.33 ± 5.46 cdef |
| Huangjingui | 32.33 ± 1.45 bcde | 0.78 ± 0.04 bcde | 71.10 ± 4.03 abc | 5.28 ± 0.26 abc | 112.67 ± 2.03 bcd | 88.33 ± 4.41 cde |
| Zhongcha 108 | 45.00 ± 6.66 a | 0.95 ± 0.19 b | 65.04 ± 3.09 abcdef | 5.61 ± 0.63 ab | 118.67 ± 1.86 bc | 114.67 ± 2.96 a |
| Taicha 2 | 22.33 ± 0.88 e | 0.70 ± 0.06 bcde | 71.02 ± 5.80 abc | 5.38 ± 0.52 ab | 101.67 ± 6.01 defg | 80.00 ± 2.00 def |
| Ziyan | 36.00 ± 3.06 abcd | 0.67 ± 0.06 bcde | 51.25 ± 6.70 ef | 4.76 ± 0.40 bcd | 72.33 ± 4.41 r | 61.33 ± 2.90 gh |
| Emei wenchun | 27.33 ± 2.40 cde | 0.47 ± 0.03 e | 51.99 ± 3.96 ef | 4.02 ± 0.34 cd | 74.33 ± 8.97 r | 52.00 ± 5.03 h |
| Mingke 1 | 30.00 ± 5.77 cde | 0.88 ± 0.27 bcd | 64.54 ± 4.83 abcdef | 6.14 ± 0.51 a | 89.13 ± 7.05 fgh | 75.67 ± 5.81 ef |
| ZC302-1 | ZC302-2 | ZC302-3 | ZC302CK-1 | ZC302CK-2 | ZC302CK-3 | EW-1 | EW-2 | EW-3 | EWCK-1 | EWCK-2 | EWCK-3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clean reads | 45,059,022 | 51,229,958 | 46,339,932 | 45,227,996 | 46,918,974 | 41,608,160 | 42,537,080 | 48,000,798 | 43,215,036 | 47,478,840 | 52,396,598 | 47,933,588 |
| GC content | 45.26 | 45.31 | 45.29 | 45.61 | 45.33 | 45.5 | 45.47 | 45.33 | 45.27 | 45.42 | 45.3 | 45.26 |
| Q30 | 93.42 | 93.26 | 92.93 | 92.53 | 92.75 | 92.7 | 92.68 | 92.74 | 92.96 | 92.89 | 92.25 | 92.34 |
| Mapped reads ratio | 91.08% | 90.64% | 90.64% | 89.29% | 91.45% | 90.86% | 91.20% | 90.52% | 90.34% | 90.73% | 91.02% | 90.52% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, S.; Tang, D.; Feng, L.; Tan, X.; Zhang, Y.; Tang, Q. Comparison of the Effect of Pruning on Plant Growth and Transcriptome Profiles in Different Tea Varieties. Agronomy 2024, 14, 1105. https://doi.org/10.3390/agronomy14061105
Kan S, Tang D, Feng L, Tan X, Zhang Y, Tang Q. Comparison of the Effect of Pruning on Plant Growth and Transcriptome Profiles in Different Tea Varieties. Agronomy. 2024; 14(6):1105. https://doi.org/10.3390/agronomy14061105
Chicago/Turabian StyleKan, Shizhuo, Dandan Tang, Lufang Feng, Xiaoqin Tan, Yijing Zhang, and Qian Tang. 2024. "Comparison of the Effect of Pruning on Plant Growth and Transcriptome Profiles in Different Tea Varieties" Agronomy 14, no. 6: 1105. https://doi.org/10.3390/agronomy14061105





