The Effects of Ammonium Phosphate with Different Sulfur Additions on Crop Yield and Nutrient Uptake in Calcareous Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Fertilizer Manufacturing
2.2. Pot Experiment
2.3. Statistical Analysis
3. Results
3.1. Effect of Different S Fertilizer Additions on Fertilizer Properties
| Treatment | N a (%) | P2O5 b (%) | SO4−S c (%) | Elemental S d (%) | Total S (%) | pH e |
|---|---|---|---|---|---|---|
| MAP | 8.93 | 45.5 | 2.39 | 0.00 | 2.39 | 4.81 |
| MAP+3%S | 9.34 | 41.2 | 3.69 | 1.43 | 5.12 | 4.86 |
| MAP+6%S | 9.95 | 38.1 | 4.98 | 2.99 | 7.97 | 4.98 |
| MAP+9%S | 10.6 | 34.8 | 6.09 | 4.41 | 10.5 | 4.96 |
| MAP+12%S | 11.2 | 31.7 | 7.47 | 6.03 | 13.5 | 5.04 |
| DAP | 14.2 | 42.9 | 2.32 | 0.00 | 2.32 | 7.29 |
| DAP+3%S | 14.2 | 39.0 | 3.48 | 1.58 | 5.06 | 7.26 |
| DAP+6%S | 14.6 | 36.4 | 5.05 | 2.91 | 7.96 | 7.24 |
| DAP+9%S | 14.7 | 33.0 | 6.51 | 4.41 | 10.9 | 7.23 |
| DAP+12%S | 14.9 | 29.7 | 7.73 | 5.88 | 13.6 | 7.21 |
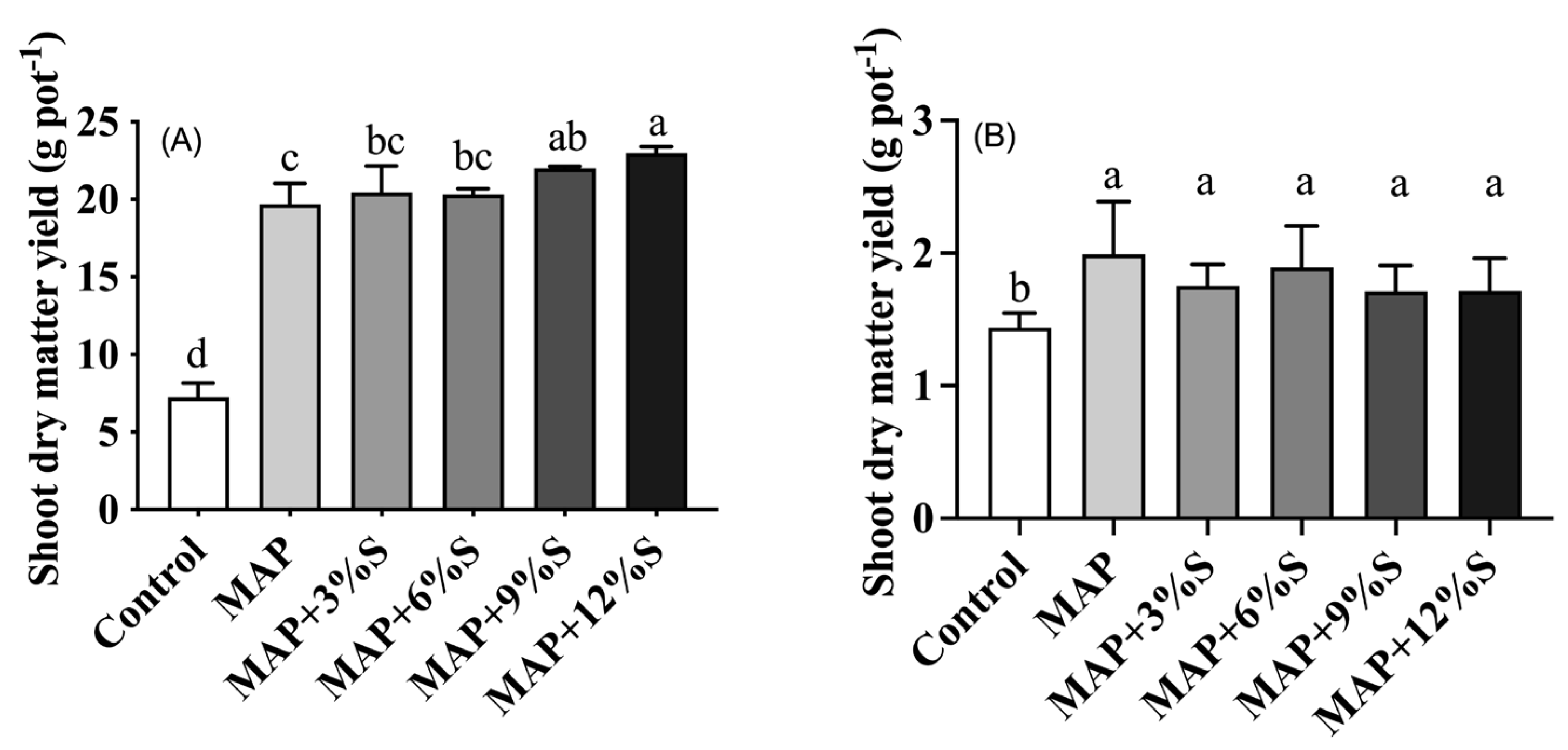
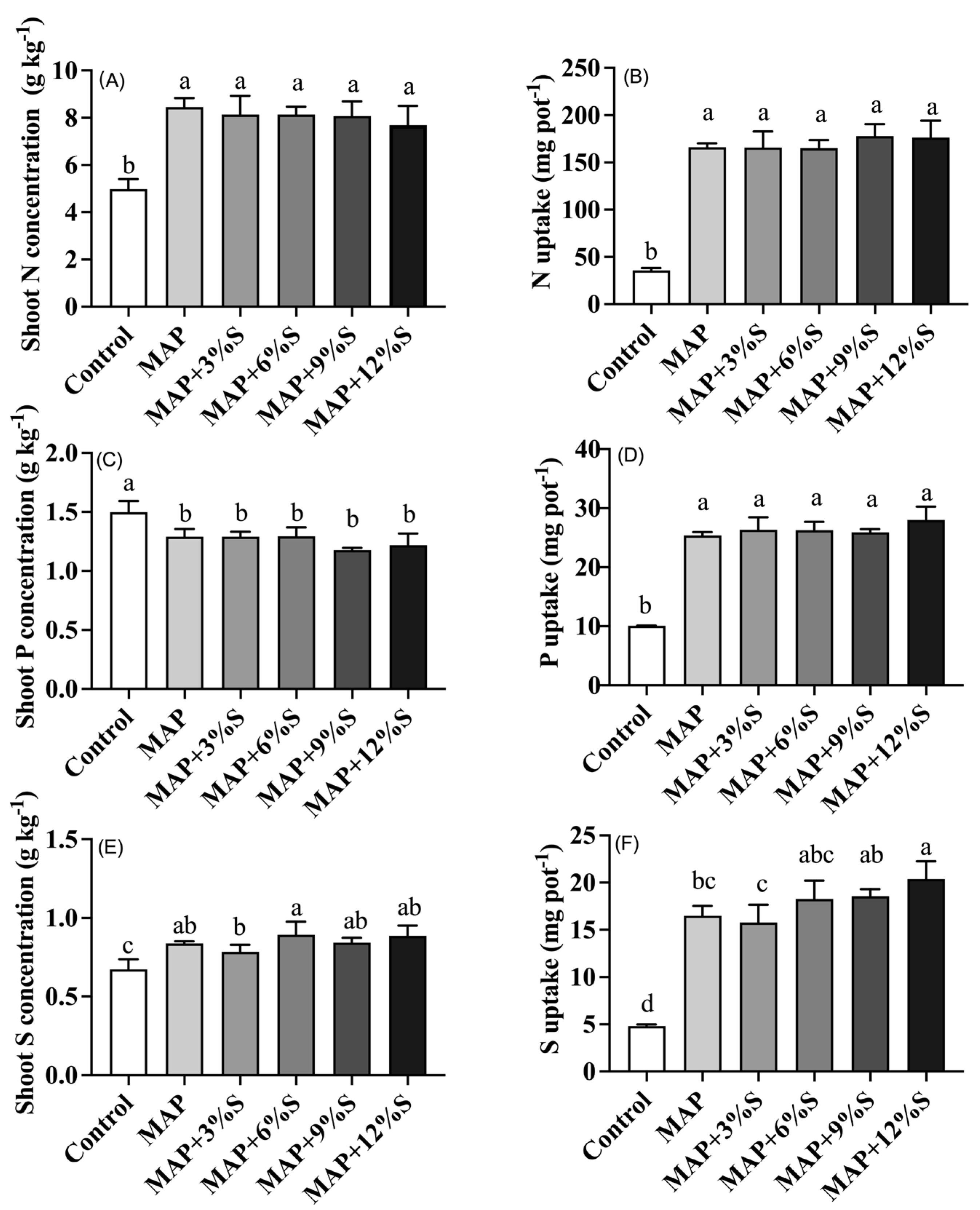
3.2. Effect of Different S Additions to MAP on Crop Yield and Nutrient Uptake
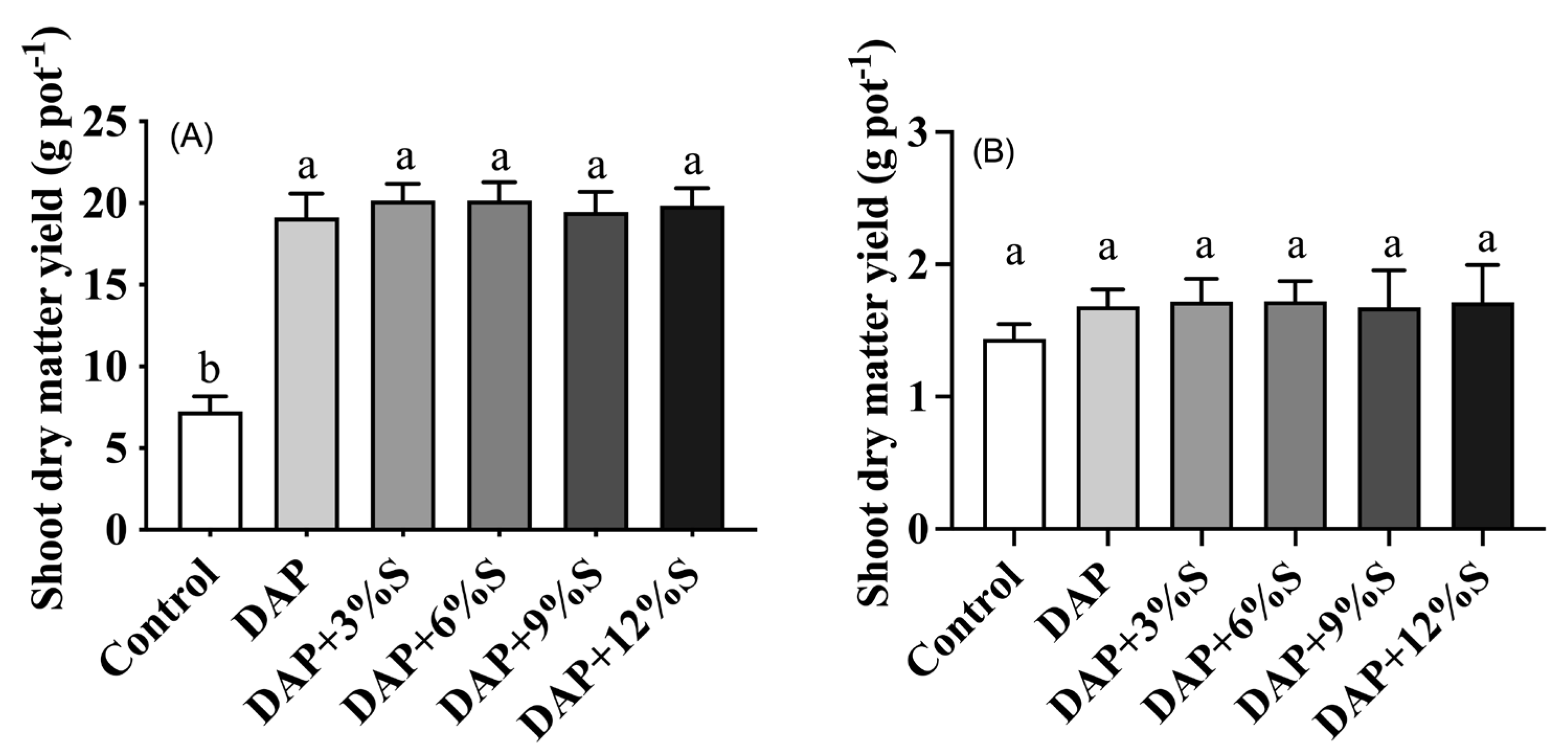
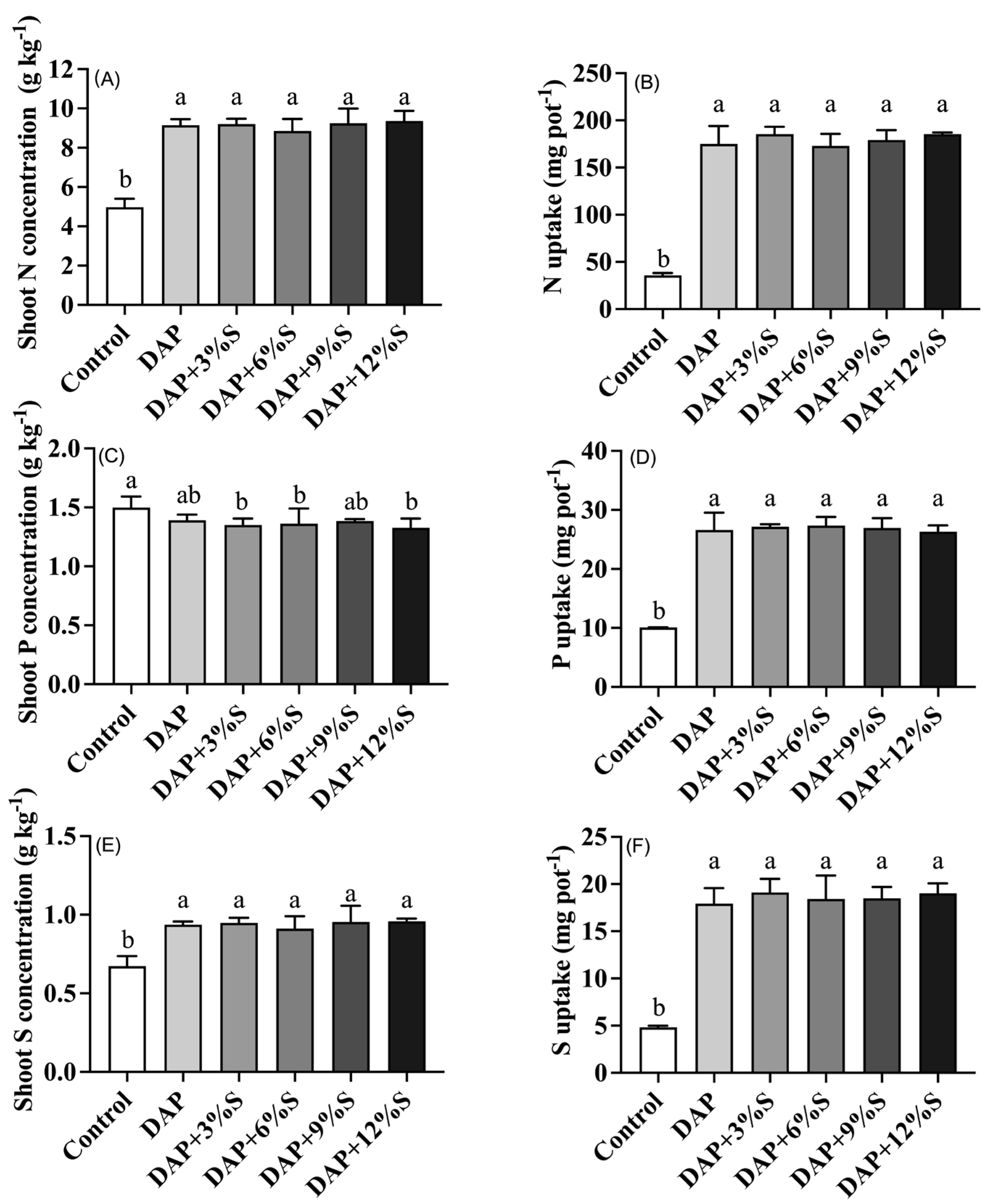
3.3. Effect of Different S Additions to DAP on Crop Yield and Nutrient Uptake
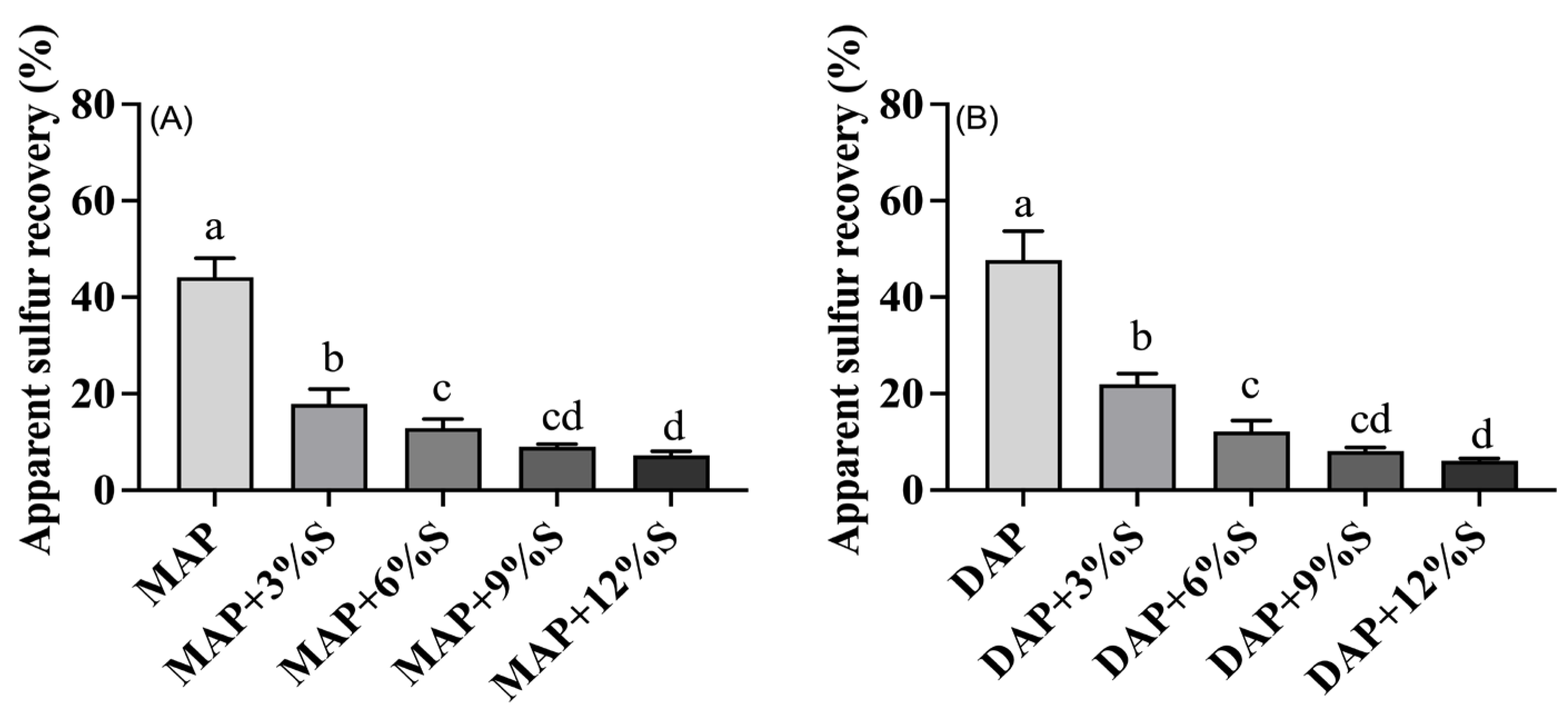
3.4. The Effect of Different S Additions to MAP and DAP on Apparent Sulfur Recovery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feinberg, A.; Stenke, A.; Peter, T.; Hinckley, E.L.S.; Driscoll, C.T.; Winkel, L.H. Reductions in the deposition of sulfur and selenium to agricultural soils pose risk of future nutrient deficiencies. Commun. Earth Environ. 2021, 2, 101. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Malagoli, M.; Avice, J.C.; Bloem, E. Advances in plant sulfur research. Plants 2020, 9, 256. [Google Scholar] [CrossRef]
- Droux, M. Sulfur assimilation and the role of sulfur in plant metabolism: A survey. Photosynth. Res. 2004, 79, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, Y.; Yang, A. Sulfur homeostasis in plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef] [PubMed]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav. 2023, 18, 2030082. [Google Scholar] [CrossRef]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef]
- Hill, C.R.; Shafaei, A.; Balmer, L.; Lewis, J.R.; Hodgson, J.M.; Millar, A.H.; Blekkenhorst, L.C. Sulfur compounds: From plants to humans and their role in chronic disease prevention. Crit. Rev. Food Sci. 2023, 63, 8616–8638. [Google Scholar] [CrossRef]
- Jez, J.M.; Fukagawa, N.K. Plant sulfur compounds and human health. In Sulfur: A Missing Link between Soils, Crops, and Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 50, pp. 281–291. [Google Scholar]
- Pasricha, N.S.; Abrol, Y.P. Food Production and Plant Nutrient Sulphur. Sulphur in Plants; Springer: Dordrecht, The Netherlands, 2003; pp. 29–44. [Google Scholar]
- Fan, M.X.; Messick, D.L. Advances in sulfur fertilizer requirement and research for Chinese agriculture: Summary of field trial data from TSI’s China project from 1997 to 2003. Landbauforsch. Völkenrode FAL Agric. Res. 2005, 283, 23–27. [Google Scholar]
- Hawkesford, M.J. Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J. Exp. Bot. 2000, 51, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; Kimura, H. Nutritional essentiality of sulfur in health and disease. Nutr. Rev. 2013, 71, 413–432. [Google Scholar] [CrossRef]
- Raffan, S.; Oddy, J.; Halford, N.G. The sulphur response in wheat grain and its implications for acrylamide formation and food safety. Int. J. Mol. Sci. 2020, 21, 3876. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.T.; Dong, A.Y.; Duan, H.J. Revisiting sulphur—The once neglected nutrient: It’s roles in plant growth, metabolism, stress tolerance and crop production. Agriculture 2021, 11, 626. [Google Scholar] [CrossRef]
- Degryse, F.; Baird, R.; da Silva, R.C.; Holzapfel, C.B.; Kappes, C.; Tysko, M.; McLaughlin, M.J. Sulfur uptake from fertilizer fortified with sulfate and elemental S in three contrasting climatic zones. Agronomy 2020, 10, 1035. [Google Scholar] [CrossRef]
- Hinckley, E.L.S.; Crawford, J.T.; Fakhraei, H.; Driscoll, C.T. A shift in sulfur-cycle manipulation from atmospheric emissions to agricultural additions. Nat. Geosci. 2020, 13, 597–604. [Google Scholar] [CrossRef]
- Aulakh, M.S. Crop Responses to Sulphur Nutrition. Sulphur in Plants; Springer: Dordrecht, The Netherlands, 2003; pp. 341–358. [Google Scholar]
- The Sulphur Institute. Sulphur—A Dynamic Nutrient in Chinese Agriculture. 2015. Available online: https://www.sulphurinstitute.org (accessed on 12 April 2024).
- Samreen, T.; Degryse, F.; Baird, R.; Da Silva, R.C.; Zahir, Z.A.; Nazir, M.Z.; Wakeel, A.; Muntaha, S.T.; McLaughlin, M.J. Development and testing of improved efficiency boron-enriched diammonium phosphate fertilizers. J. Soil Sci. Plant Nutr. 2021, 21, 1134–1143. [Google Scholar] [CrossRef]
- Carciochi, W.D.; Salvagiotti, F.; Pagani, A.; Calvo, N.I.R.; Eyherabide, M.; Rozas, H.R.S.; Ciampitti, I.A. Nitrogen and sulfur interaction on nutrient use efficiencies and diagnostic tools in maize. Eur. J. Agron. 2020, 116, 126045. [Google Scholar] [CrossRef]
- Guelfi, D.; Nunes, A.P.P.; Sarkis, L.F.; Oliveira, D.P. Innovative phosphate fertilizer technologies to improve phosphorus use efficiency in agriculture. Sustainability 2022, 14, 14266. [Google Scholar] [CrossRef]
- Mustafa, A.; Athar, F.; Khan, I.; Chattha, M.U.; Nawaz, M.; Shah, A.N.; Mahmood, A.; Batool, M.; Aslam, M.T.; Jaremko, M.; et al. Improving crop productivity and nitrogen use efficiency using sulfur and zinc-coated urea: A review. Front. Plant Sci. 2022, 13, 942384. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Teixeira, L.A.; Cantarella, H.; Rehm, G.W.; Grant, C.A.; Gearhart, M.M. Agronomic effectiveness of granular nitrogen/phosphorus fertilizers containing elemental sulfur with and without ammonium sulfate: A review. Agron. J. 2016, 108, 1203–1213. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Gasparatos, D.; Zechmann, B.; Bouranis, L.D.; Chorianopoulou, S.N. The effect of granular commercial fertilizers containing elemental sulfur on wheat yield under mediterranean conditions. Plants 2018, 8, 2. [Google Scholar] [CrossRef]
- Degryse, F.; Da Silva, R.C.; Baird, R.; Beyrer, T.; Below, F.; McLaughlin, M.J. Uptake of elemental or sulfate-S from fall-or spring-applied co-granulated fertilizer by corn—A stable isotope and modeling study. Field Crop Res. 2018, 221, 322–332. [Google Scholar] [CrossRef]
- Karimizarchi, M.; Aminuddin, H.; Khanif, M.Y.; Radziah, O. Elemental sulphur application effects on nutrient availability and sweet maize (Zea mays L.) response in a high pH soil of Malaysia. Malay. J. Soil Sci. 2014, 18, 75–86. [Google Scholar]
- Kulczycki, G. The effect of elemental sulfur fertilization on plant yields and soil properties. Adv. Agron. 2021, 167, 105–181. [Google Scholar]
- Yasmin, N.; Blair, G.; Till, R. Effect of elemental sulfur, gypsum, and elemental sulfur coated fertilizers, on the availability of sulfur to rice. J. Plant Nutr. 2007, 30, 79–91. [Google Scholar] [CrossRef]
- Degryse, F.; Ajiboye, B.; Baird, R.; Da Silva, R.C.; McLaughlin, M.J. Availability of fertiliser sulphate and elemental sulphur to canola in two consecutive crops. Plant Soil. 2016, 398, 313–325. [Google Scholar] [CrossRef]
- Francisco, E.A.; Chien, S.H.; Ono, F.; Gearhart, M.M.; Cruz, A.P. Comparing various sulfur sources for soybean grown on an acid Oxisol in Brazil. Agron. J. 2022, 114, 3420–3428. [Google Scholar] [CrossRef]
- Solberg, E.D.; Malhi, S.S.; Nyborg, M.; Henriquez, B.; Gill, K.S. Crop response to elemental S and sulfate-S sources on S-deficient soils in the parkland region of Alberta and Saskatchewan. J. Plant Nutr. 2007, 30, 321–333. [Google Scholar] [CrossRef]
- Degryse, F.; Baird, R.; Andelkovic, I.; McLaughlin, M.J. Long-term fate of fertilizer sulfate-and elemental S in co-granulated fertilizers. Nutr. Cycl. Agroecosystems 2021, 120, 31–48. [Google Scholar] [CrossRef]
- Friesen, D.K. Influence of co-granulated nutrients and granule size on plant responses to elemental sulfur in compound fertilizers. Nutr. Cycl. Agroecosystems 1996, 46, 81–90. [Google Scholar] [CrossRef]
- Malik, K.M.; Khan, K.S.; Billah, M.; Akhtar, M.S.; Rukh, S.; Alam, S.; Munir, A.; Aulakh, A.M.; Rahim, M.; Qaisrani, M.M.; et al. Organic amendments and elemental sulfur stimulate microbial biomass and sulfur oxidation in alkaline subtropical soils. Agronomy 2021, 11, 2514. [Google Scholar] [CrossRef]
- Maharjan, B.; Das, S.; Shapiro, C.A. Effects of fused and blended fertilizers on maize yield and soil properties. Agron. J. 2022, 114, 3429–3444. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.W.; Li, Z.X. Production and development trends of phosphate and compound fertilizer industry in China in 2022. Phosphate Comp. Fert. 2023, 38, 1–8. (In Chinese) [Google Scholar]
- Yang, L.; Stulen, I.; De Kok, L.J. Sulfur status of Chinese soils and response of Chinese cabbage to sulfur fertilization in the Beijing area. Landbauforsch. Völkenrode FAL Agric. Res. 2005, 283, 163–170. [Google Scholar]
- Khan, M.J.; Khan, M.H.; Khattak, R.A.; Jan, M.T. Response of maize to different levels of sulfur. Commun. Soil Sci. Plan. 2006, 37, 41–51. [Google Scholar] [CrossRef]
- Wainwright, M.; Nevell, W.; Grayston, S.J. Effects of organic matter on sulphur oxidation in soil and influence of sulphur oxidation on soil nitrification. Plant Soil. 1986, 96, 369–376. [Google Scholar] [CrossRef]
- Kovar, J.L. Maize response to sulfur fertilizer in three Iowa soils. Commun. Soil Sci. Plan. 2021, 52, 905–915. [Google Scholar] [CrossRef]
- Lu, Z. The Development and Evaluation of Magnesium-fortified Compound Fertilizer. Ph.D. Thesis, China Agricultural University, Beijing, China, 2021. [Google Scholar]
- Lu, Z.; Wang, Y.; Degryse, F.; Huang, C.; Hou, C.; Wu, L.; Mclaughlin, M.; Zhang, F. Magnesium-fortified phosphate fertilizers improve nutrient uptake and plant growth without reducing phosphorus availability. Pedosphere 2022, 32, 744–751. [Google Scholar] [CrossRef]
- Zhao, T.K.; Zhang, G.Y.; Ma, L.M.; Sun, Z.Y. The contents, forms and distribution of sulfur in soils of Hebei. Plant Nutr. Fert. Sci. 2001, 7, 178–182. (In Chinese) [Google Scholar]
- Jones, J. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Everaert, M.; Duboc, O.; Willems, E.; Soja, G.; Pfeifer, C.; Van Velthoven, N.; de Oliveira-Silva, R.; Sakellariou, D.; Santner, J. Thermochemical processing of boron-impregnated cellulose insulation waste for upcycling to slow-release boron fertilizers. J. Clean. Prod. 2023, 399, 136684. [Google Scholar] [CrossRef]
- Matamwa, W.; Blair, G.; Guppy, C.; Yunusa, I. Plant availability of sulfur added to finished fertilizers. Commun. Soil. Sci. Plan. 2018, 49, 433–443. [Google Scholar] [CrossRef]
- Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 Relating to Fertilisers; Office for Official Publications of the European Communities: Luxembourg, 2003.
- Ministry of Agriculture of the People’s Republic of China. Water-Soluble Fertilizers Determination of Water Insoluble Matter and pH; China Agriculture Press: Beijing, China, 2011. [Google Scholar]
- Sample, E.; Soper, R.; Racz, G. Reactions of phosphate fertilizer in soils. In Role of Phosphorus in Agriculture; Khasawneh, F.E., et al., Eds.; SSSA: Madison, WI, USA, 1980; pp. 263–310. [Google Scholar]
- Degryse, F.; Da Silv, R.C.; Baird, R.; McLaughlin, M.J. Effect of cogranulation on oxidation of elemental sulfur: Theoretical model and experimental validation. Soil Sci. Soc. Am. J. 2016, 80, 1244–1253. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Fu, X. Characteristics of soil salt crust formed by mixing calcium chloride with sodium sulfate and the possibility of inhibiting wind-sand flow. Sci. Rep. 2021, 11, 9746. [Google Scholar] [CrossRef]
- Li, W.; Wang, T.; Yang, Y.; Fang, M.; Gao, X. Calcium recovery from waste carbide slag via ammonium sulfate leaching system. J. Clean. Prod. 2022, 377, 134308. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, X.; Chen, C.; Wang, W.; Dang, W. Effect of sulfuric acid concentration on morphology of calcium sulfate hemihydrate crystals. Mater. Res. Express 2020, 7, 105501. [Google Scholar] [CrossRef]
- Wang, L.Y. Effects of Different Phosphorus Supplies on the Root-Soil-Microbe Interactions and Rhizosphere Regulation Mechanisms for Improving Phosphorus Use Efficiency by Maize. Ph.D. Thesis, China Agricultural University, Beijing, China, 2022. (In Chinese). [Google Scholar]
- Bhattarai, D.; Kumar, S.; Nleya, T. Nitrogen and sulfur fertilizers effects on growth and yield of Brassica carinata in South Dakota. Agron. J. 2021, 113, 1945–1960. [Google Scholar] [CrossRef]
- Sattar, B.; Ahmad, S.; Daur, I.; Hussain, M.B.; Ali, M.; Ul Haq, T.; Arif, M.; Bakhtawer, M. Bioactive-sulfur coated diammonium phosphate improves nitrogen and phosphorus use efficiency and maize (Zea mays L.) yield. J. Environ. Agr. Sci. 2021, 23, 23–29. [Google Scholar]
- Aula, L.; Dhillon, J.S.; Omara, P.; Wehmeyer, G.B.; Freeman, K.W.; Raun, W.R. World sulfur use efficiency for cereal crops. Agron. J. 2019, 111, 2485–2492. [Google Scholar] [CrossRef]
- Bharathi, C.; Poongothai, S. Direct and residual effect of sulphur on growth, nutrient uptake, yield and its use efficiency in maize and subsequent greengram. Res. J. Agr. Biol. Sci. 2008, 4, 368–372. [Google Scholar]
- Gupta, A.K.; Jain, N.K. Sulphur fertilization in a pearl millet (Pennisetum glaucum)-Indian mustard (Brassica juncea) cropping system. Arch. Agron. Soil Sci. 2008, 54, 533–539. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, R.; Singh, M.P.; Singh, V.P. Impact of sulfur fertilization on different forms and balance of soil sulfur and the nutrition of wheat in wheat-soybean cropping sequence in tarai soil. J. Plant Nutr. 2014, 37, 618–632. [Google Scholar] [CrossRef]
- Haque, M.M.; Saleque, M.A.; Shah, A.L.; Biswas, J.C.; Kim, P.J. Long-term effects of sulfur and zinc fertilization on rice productivity and nutrient efficiency in double rice cropping paddy in Bangladesh. Commun. Soil Sci. Plant Anal. 2015, 46, 2877–2887. [Google Scholar] [CrossRef]
- Singh Shivay, Y.; Prasad, R.; Pal, M. Effect of levels and sources of sulfur on yield, sulfur and nitrogen concentration and uptake and S-use efficiency in basmati rice. Commun. Soil Sci. Plant Anal. 2014, 45, 2468–2479. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.B.; Dana, M.; Anderson, G.C. Modelling of sulfur oxidation from elemental sulfur. Plant Soil 1993, 155, 379–382. [Google Scholar] [CrossRef]
- Fontaine, D.; Eriksen, J.; Sørensen, P.; McLaughlin, M.J.; Degryse, F. Application method influences the oxidation rate of biologically and chemically produced elemental sulfur fertilizers. Soil Sci. Soc. Am. J. 2021, 85, 746–759. [Google Scholar] [CrossRef]
- Goyal, D.; Franzen, D.W.; Chatterjee, A. Do crops’ responses to sulfur vary with its forms? Agrosyst. Geosci. Environ. 2021, 4, e20201. [Google Scholar] [CrossRef]
- Mattiello, E.; da Silva, R.; Degryse, F.; Baird, R.; Gupta, V.; Mclaughlin, M. Sulfur and zinc availability from co-granulated Zn-enriched elemental sulfur fertilizers. J. Agric. Food Chem. 2017, 65, 1108–1115. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Liu, J.; Zhu, Y.; Wang, Y.; Huang, C. The Effects of Ammonium Phosphate with Different Sulfur Additions on Crop Yield and Nutrient Uptake in Calcareous Soil. Agronomy 2024, 14, 1145. https://doi.org/10.3390/agronomy14061145
Lu Z, Liu J, Zhu Y, Wang Y, Huang C. The Effects of Ammonium Phosphate with Different Sulfur Additions on Crop Yield and Nutrient Uptake in Calcareous Soil. Agronomy. 2024; 14(6):1145. https://doi.org/10.3390/agronomy14061145
Chicago/Turabian StyleLu, Zhenya, Junjie Liu, Yuanyuan Zhu, Yanyan Wang, and Chengdong Huang. 2024. "The Effects of Ammonium Phosphate with Different Sulfur Additions on Crop Yield and Nutrient Uptake in Calcareous Soil" Agronomy 14, no. 6: 1145. https://doi.org/10.3390/agronomy14061145
APA StyleLu, Z., Liu, J., Zhu, Y., Wang, Y., & Huang, C. (2024). The Effects of Ammonium Phosphate with Different Sulfur Additions on Crop Yield and Nutrient Uptake in Calcareous Soil. Agronomy, 14(6), 1145. https://doi.org/10.3390/agronomy14061145





