Disentangling the Physiological Responses of Sweet Orange Citrus Trees to Optimize the Design of Deficit Irrigation Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Irrigation Treatments
- -
- A full irrigation (FI) treatment, where trees received 100% of the irrigation requirements (IRs).
- -
- A low-frequency deficit irrigation (LFDI) treatment, which subjected trees to irrigation restriction cycles. This treatment kept the crops within a previously defined range of physiological threshold values; hence, its application required crop water monitoring. During restriction periods, irrigation was suppressed until reaching stem water potential (ΨStem) values between −1.8 and −2.0 MPa (the threshold values for mature citrus trees presented by García-Tejero et al. [11]). Then, irrigation withholding was finished, and the trees were irrigated with the same frequency as in the FI treatment until reaching similar ΨStem values. The length of the irrigation restriction cycles varied depending on the weather conditions, ranging between 8 and 10 days on average, with 4–5 days of water restriction and a similar period for recovery.
- -
- A sustained deficit irrigation (SDI) treatment, where trees were irrigated at 75% of the FI treatment.
2.2. Physiological Measurements
2.2.1. Stem Water Potential and Stomatal Conductance
2.2.2. Canopy Temperature
2.2.3. Chlorophyll Fluorescence Measurements
2.2.4. Proline and Malondialdehyde Content Determination
2.3. Measurement Timetable
2.4. Statistical Analysis
3. Results and Discussion
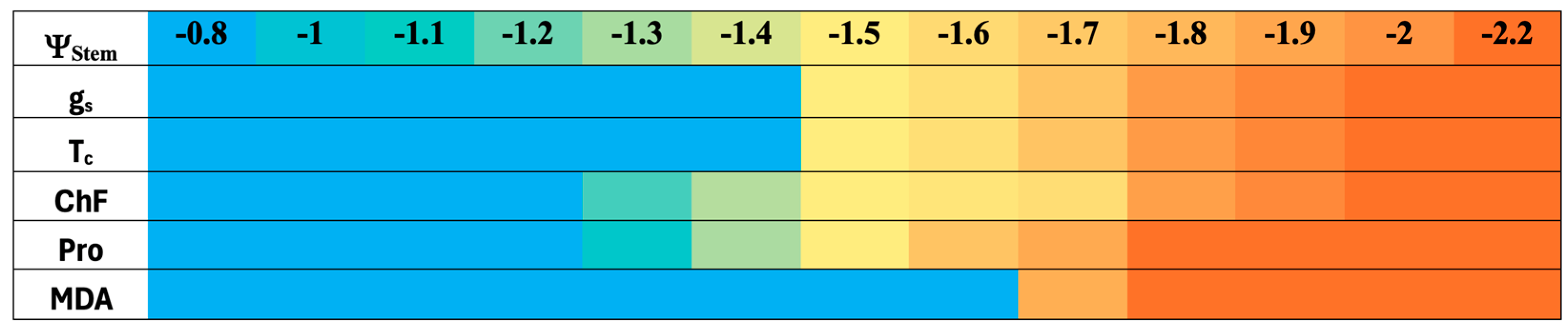
3.1. Water Potential, Stomatal Conductance, and Canopy Temperature Readings
3.2. Chlorophyll Fluorescence
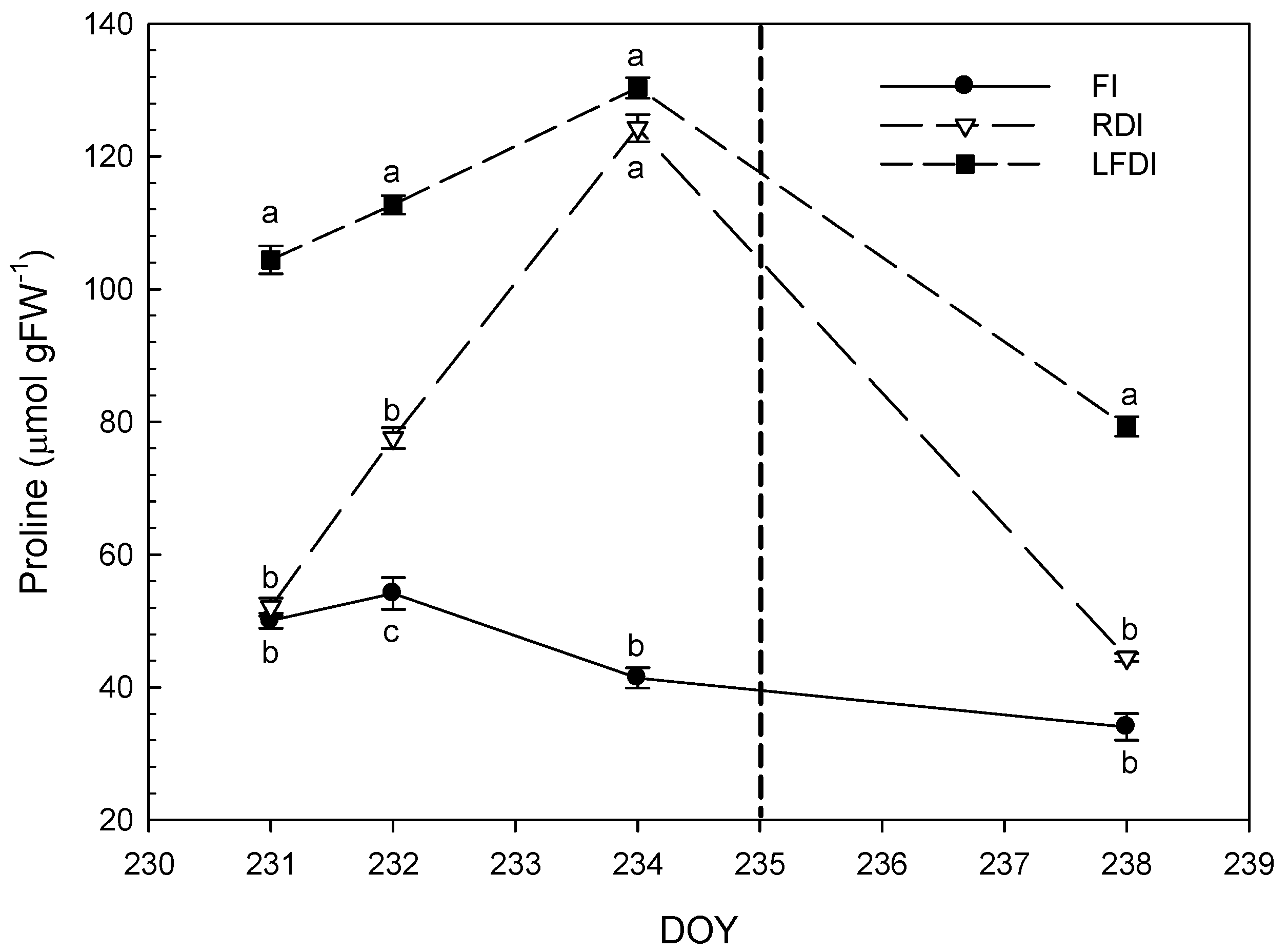
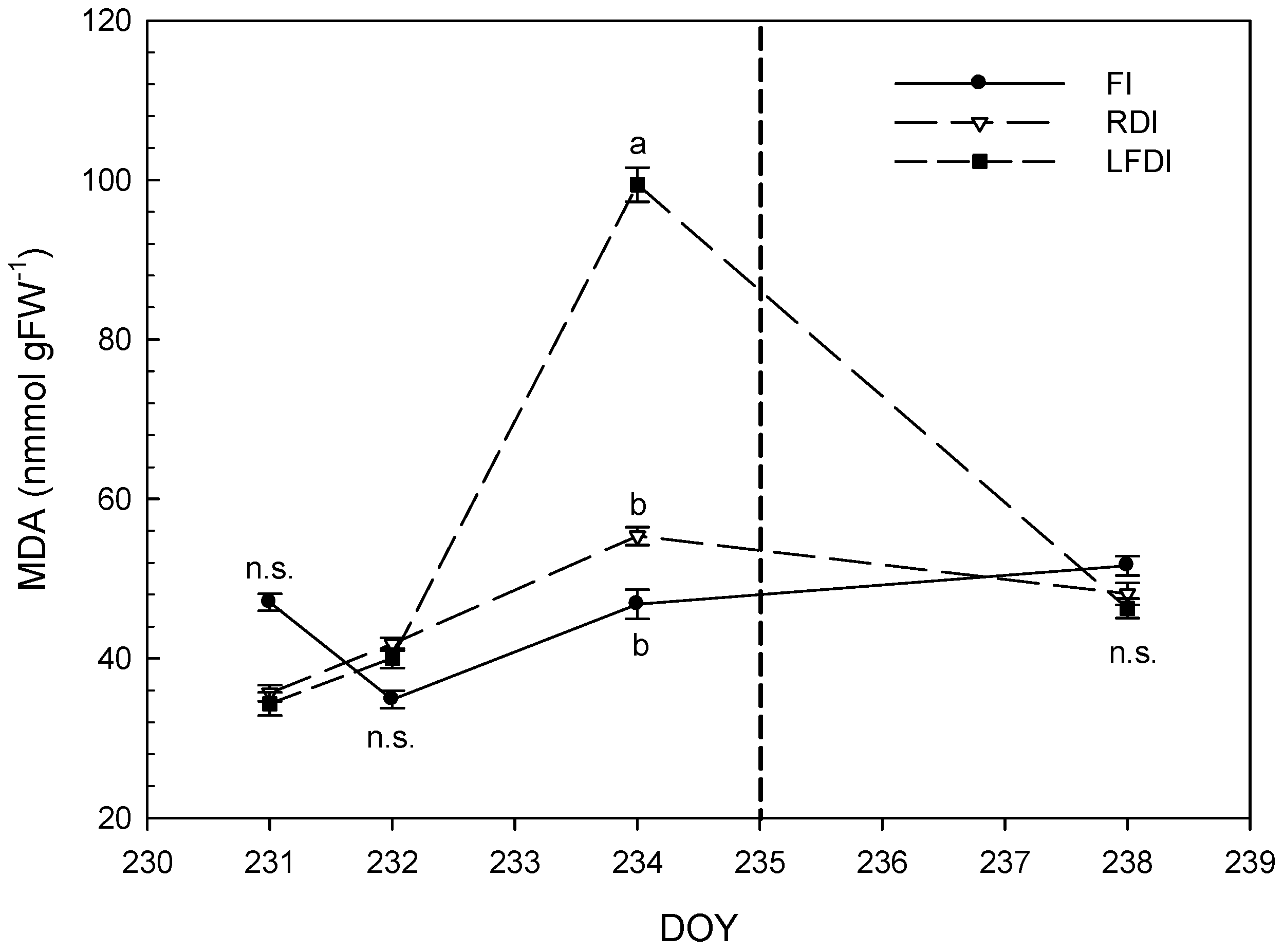
3.3. Proline and Malondialdehyde Contents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ESYRCE 2023. Encuesta Sobre Superficies y Rendimientos Cultivos (ESYRCE). Encuesta de Marco de Áreas de España. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/esyrce/ (accessed on 1 March 2024).
- EEA 2019. Climate Change Adaptation in the Agriculture Sector in Europe. EEA Report 4/2019. p. 112. Available online: https://www.eea.europa.eu/publications/cc-adaptation-agriculture/ (accessed on 1 March 2024).
- Iglesias, A.; Garrote, L. Local and collective actions for adaptation to use less water for agriculture in the Mediterranean region. In Water Scarcity and Sustainable Agriculture in Semiarid Environment: Tools, Strategies and Challenges for Woody Crops; García-Tejero, I.F., Durán-Zuazo, V.H., Eds.; Academic Press: London, UK, 2018; pp. 73–86. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Morinau, G.; Kourgialas, N. Drought and salinity in citriculture: Optimal practices to alleviate salinity and drought stress. Agronomy 2021, 11, 1283. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Lloyd, J. CO2 assimilation of citrus leaves: From mesophyll conductance to gross primary productivity of trees in different climates. International symposium on computer modelling in fruit research and orchard management. Acta Hortic. 1995, 416, 147–154. [Google Scholar]
- Syvertsen, J.P.; Lloyd, J.; Mcconchie, C.; Kriedemann, P.E.; Farquhar, G.D. On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant Cell. Environ. 1995, 18, 149–157. [Google Scholar] [CrossRef]
- Berger, J.; Palta, J.; Vadez, V. Review: An integrated framework for crop adaptation to dry environments: Responses to transient and terminal drought. Plant Sci. 2016, 253, 58–67. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.G.; Robles, J.M.; García-Sánchez, F.; Botía, P. Comparison of deficit and saline irrigation strategies to confront water restriction in lemon trees grown in semi-arid regions. Agric. Water Manag. 2016, 164, 46–57. [Google Scholar] [CrossRef]
- Ningombam, L.; Hazarika, B.N.; Singh, S.; Yumkhaibam, T. Drought stress in Citrus and its related genera: A review. Environ. Ecol. 2022, 40, 2459–2466. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Ayesha, F. Crop production under drought and heat stress: Plant responses and management options. Front. Plants Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- García-Tejero, I.; Durán-Zuazo, V.H.; Muriel-Fernández, J.L.; Jiménez-Bocanegra, J.A. Linking canopy temperature and trunk diameter fluctuations with other physiological water status tools for irrigation scheduling in citrus orchards. Funct. Plant Biol. 2011, 38, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef]
- De Ollas, C.; Hernando, B.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alymeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The Roles of Reactive Oxygen Metabolism in Drought: Not So Cut and Dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.; Deleu, C.; Larher, F. Proline accumulation by tomato leaf tissue in response to salinity. C. R. Acad. Sci. III 2000, 323, 551–557. [Google Scholar] [CrossRef]
- Wong, S.H.; Knight, J.A.; Hopfer, S.M.; Zaharia, O.; Leach, C.N., Jr.; Sunderman, W., Jr. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehide-thiobarbituric acid adduct. Clin. Chem. 1987, 32, 214–220. [Google Scholar] [CrossRef]
- Arbona, V.; Hossain, Z.; López-Climent, M.F.; Pérez-Clemente, M.R.; Gómez-Cadenas, A. Antioxidant enzymatic activity is l inked to waterlogging stress tolerance in citrus. Physiol. Plant. 2008, 132, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; López-Climent, M.F.; Arbona, V.; Pérez-Clemente, M.R.; Gómez-Cadenas, A. Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage. J. Plant Physiol. 2009, 166, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cadenas, A.; Tadeo, F.R.; Talón, M.; Primo-Millo, E. Leaf abscission induced by ethylene in water stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid acumulation in roots. Plant Physiol. 1996, 112, 401–408. [Google Scholar] [CrossRef]
- Brakke, M.; Allen, L.H. Gas-exchange of citrus seedlings at different temperatures, vapor-pressure deficits and soil water contents. J. Am. Soc. Hort. Sci. 1995, 120, 497–504. [Google Scholar] [CrossRef]
- Balfagon, D.; Zandalinas, S.I.; Muriach, M.; Gomez, C.A. Involvent of ascorbate peroxidase and heat shock proteins on citrus tolerance to combine conditions of drought and high temperatures. Plant Physiol. Biochem. 2018, 127, 194–199. [Google Scholar]
- Carvalho, L.M.; Araújo, S.B.; Carvalho, H.W.L.; Girardi, E.A.; Soares Filho, W.S. Leaf proline accumulation and fruit yield of ‘Pera’ sweet orange trees under natural water stress. Bragantia 2021, 80, e1121. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1964, 148, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G.; Stoll, M.; Santos, T.; Sousa, C.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- García-Tejero, I.; Durán-Zuazo, V.H.; Arriaga, J.; Hernández, A.; Vélez, L.M.; Muriel Fernádez, J.L. Approach to assess infrared thermal imaging of almond trees under water-stress conditions. Fruits 2012, 67, 463–474. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Syvertsen, J.P.; Gimeno, V.; Botía, P.; Perez-Perez, J.G. Responses to flooding and drought stress by two citrus rootstock seedlings with different water use efficiency. Physiol. Plant. 2007, 130, 532–542. [Google Scholar] [CrossRef]
- Gonçalves, L.P.; Alves, T.F.O.; Martins, C.P.S.; Souza, A.O.; Santos, I.C.; Pirovani, C.P.; Almeida, A.A.F.; Coelho Filho, M.A.; Gesteira, A.S.; Soares Filho, W.S.; et al. Rootstock-induced physiological and biochemical mechanisms of drought tolerance in sweet orange. Acta Physiol. Plant. 2016, 38, 174. [Google Scholar] [CrossRef]
- Zaher-Ara, T.; Boroomand, N.; Sadat-Hosseini, M. Physiological and morphological response to drought stress in seedlings of ten citrus. Trees 2016, 30, 985–993. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Smith, M.L. Environmental-stress and seasonal-changes in proline concentration of citrus tree tissues and juice. J. Am. Soc. Hortic. Sci. 1983, 108, 861–866. [Google Scholar] [CrossRef]
| DOY | Date | DWW | ΨStem | gs | TC | ChF | Pro | MDA |
|---|---|---|---|---|---|---|---|---|
| Cycle I | ||||||||
| 203 | 22/7 | 0 | • | • | • | • | ||
| 205 | 24/7 | 2 | • | • | • | |||
| 206 | 25/7 | 3 | • | • | • | • | ||
| 210 | 29/7 | 0 | • | • | • | • | ||
| Cycle II | ||||||||
| 231 | 19/8 | 0 | • | • | • | • | • | • |
| 232 | 20/8 | 1 | • | • | • | • | • | • |
| 233 | 21/8 | 2 | • | • | • | |||
| 234 | 22/8 | 3 | • | • | • | • | • | • |
| 238 | 26/8 | 0 | • | • | • | • | • | • |
| DOY | Treatment | ΨStem (MPa) | gs (mmol m−2 s−1) | TC (°C) |
|---|---|---|---|---|
| FI | −1.28 a | 71.8 a | 39.8 a | |
| 203 | RDI | −1.27 a | 71.6 a | 40.5 a |
| LFDI | −1.19 a | 50.4 a | 40.1 a | |
| FI | −1.05 a | 68.4 a | 38.6 a | |
| 204 | RDI | −1.35 b | 58.8 a | 38.6 a |
| LFDI | −1.28 ab | 76.2 a | 38.4 a | |
| FI | −1.14 a | 74.9 a | 34.5 a | |
| 205 | RDI | −1.23 a | 70.6 a | 34.5 a |
| LFDI | −1.68 b | 43.1 b | 35.5 b | |
| FI | −1.15 a | 82.5 a | 31.6 a | |
| 206 | RDI | −1.33 b | 74.0 a | 33.1 b |
| LFDI | −2.19 c | 35.1 b | 33.9 c | |
| FI | −0.83 a | 70.9 a | 25.2 a | |
| 210 | RDI | −0.84 a | 70.6 a | 24.7 a |
| LFDI | −0.89 a | 66.8 a | 24.8 a |
| DOY | Treatment | ΨStem (MPa) | gs (mmol m−2 s−1) | TC (°C) |
|---|---|---|---|---|
| FI | −1.28 a | 89.4 a | 33.9 a | |
| 231 | RDI | −1.43 a | 77.5 a | 33.4 a |
| LFDI | −1.34 a | 84.9 a | 32.9 a | |
| FI | −1.16 a | 65.6 a | 35.3 a | |
| 232 | RDI | −1.30 ab | 62.8 a | 34.3 a |
| LFDI | −1.51 b | 62.9 a | 34.8 a | |
| FI | −1.25 a | 64.4 a | 38.5 a | |
| 233 | RDI | −1.23 a | 59.1 a | 38.8 a |
| LFDI | −1.58 b | 60.1 a | 39.2 a | |
| FI | −1.15 a | 78.2 a | 37.00 a | |
| 234 | RDI | −1.41 b | 70.4 a | 37.5 a |
| LFDI | −1.76 c | 47.2 b | 39.2 b | |
| FI | −1.33 a | 76.99 a | 38.2 a | |
| 238 | RDI | −1.51 b | 51.80 a | 38.9 a |
| LFDI | −1.34 a | 58.20 a | 38.0 a |
| DOY | Treatment | Fv/Fm | ϕPSII | NPQ |
|---|---|---|---|---|
| FI | 0.715 a | 0.150 a | 1.864 a | |
| 203 | RDI | 0.726 a | 0.186 a | 2.353 a |
| LFDI | 0.729 a | 0.186 a | 2.241 a | |
| FI | 0.742 a | 0.165 a | 1.226 a | |
| 204 | RDI | 0.734 a | 0.204 a | 2.019 b |
| LFDI | 0.717 a | 0.134 a | 2.044 b | |
| FI | 0.710 a | 0.143 a | 1.705 a | |
| 206 | RDI | 0.716 a | 0.188 a | 2.259 b |
| LFDI | 0.726 a | 0.199 a | 2.383 b | |
| FI | 0.731 a | 0.188 a | 1.778 a | |
| 210 | RDI | 0.726 a | 0.216 a | 1.837 a |
| LFDI | 0.762 a | 0.182 a | 1.604 a |
| DOY | Treatment | Fv/Fm | ϕPSII | NPQ |
|---|---|---|---|---|
| FI | 0.755 a | 0.172 a | 1.699 a | |
| 231 | RDI | 0.739 a | 0.163 a | 1.767 a |
| LFDI | 0.767 a | 0.191 a | 1.907 a | |
| FI | 0.737 a | 0.195 a | 2.109 a | |
| 232 | RDI | 0.757 a | 0.188 a | 2.031 a |
| LFDI | 0.750 a | 0.157 a | 2.552 a | |
| FI | 0.666 a | 0.272 a | 2.006 a | |
| 234 | RDI | 0.712 a | 0.238 a | 2.352 a |
| LFDI | 0.666 a | 0.252 a | 2.579 a | |
| FI | 0.699 a | 0.290 a | 0.887 a | |
| 238 | RDI | 0.683 a | 0.302 a | 0.930 a |
| LFDI | 0.638 a | 0.229 a | 0.725 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Casal, A.E.; Durán-Zuazo, V.H.; García-Tejero, I.F. Disentangling the Physiological Responses of Sweet Orange Citrus Trees to Optimize the Design of Deficit Irrigation Strategies. Agronomy 2024, 14, 1149. https://doi.org/10.3390/agronomy14061149
Rubio-Casal AE, Durán-Zuazo VH, García-Tejero IF. Disentangling the Physiological Responses of Sweet Orange Citrus Trees to Optimize the Design of Deficit Irrigation Strategies. Agronomy. 2024; 14(6):1149. https://doi.org/10.3390/agronomy14061149
Chicago/Turabian StyleRubio-Casal, Alfredo Emilio, Víctor Hugo Durán-Zuazo, and Iván Francisco García-Tejero. 2024. "Disentangling the Physiological Responses of Sweet Orange Citrus Trees to Optimize the Design of Deficit Irrigation Strategies" Agronomy 14, no. 6: 1149. https://doi.org/10.3390/agronomy14061149





