Monitoring Land Management Practices Using Vis–NIR Spectroscopy Provides Insights into Predicting Soil Organic Carbon and Limestone Levels in Agricultural Plots
Abstract
1. Introduction
2. Materials and Methods
3. Results
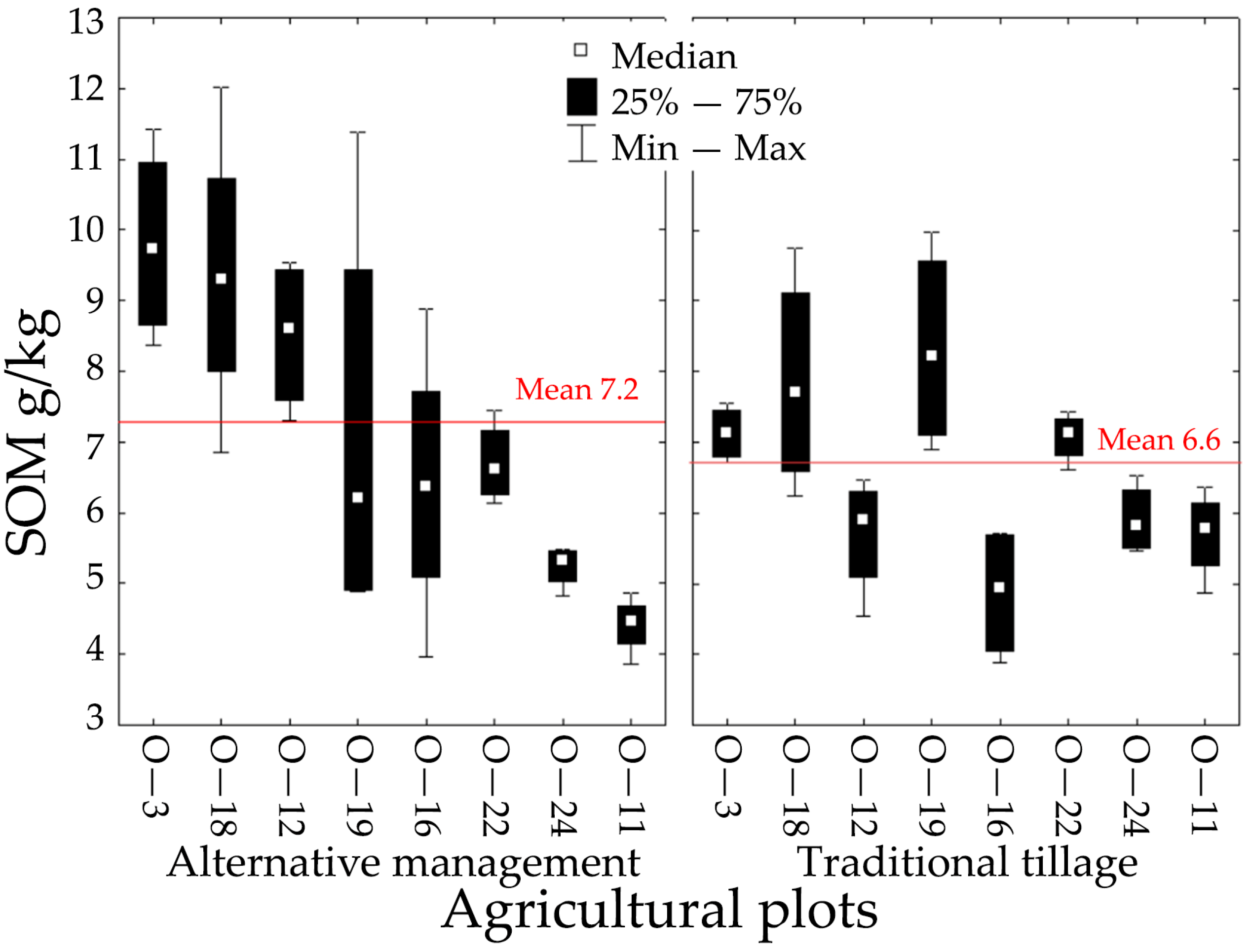
3.1. Soil Organic Matter Comparing Tillage and Alternative Management
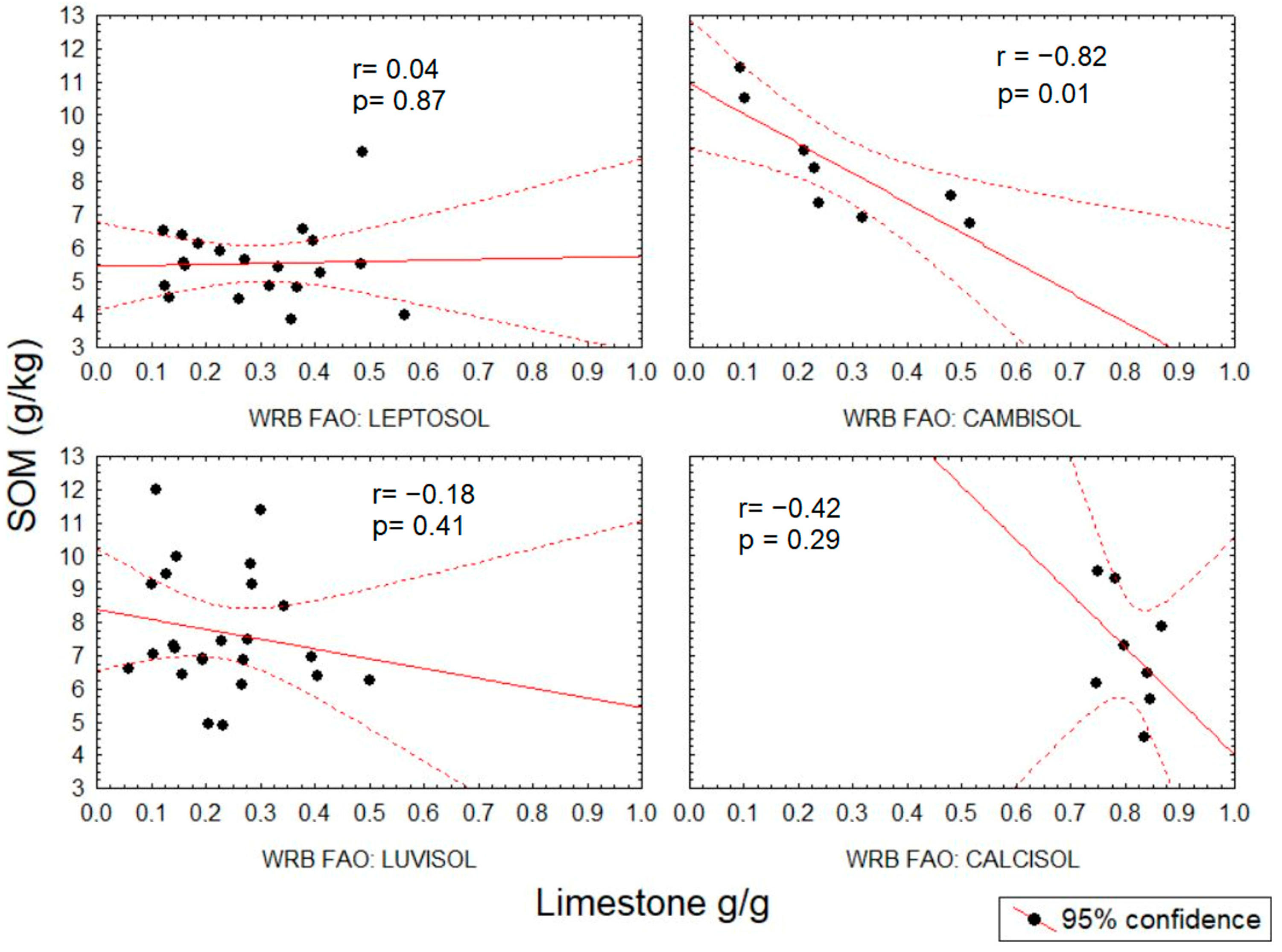
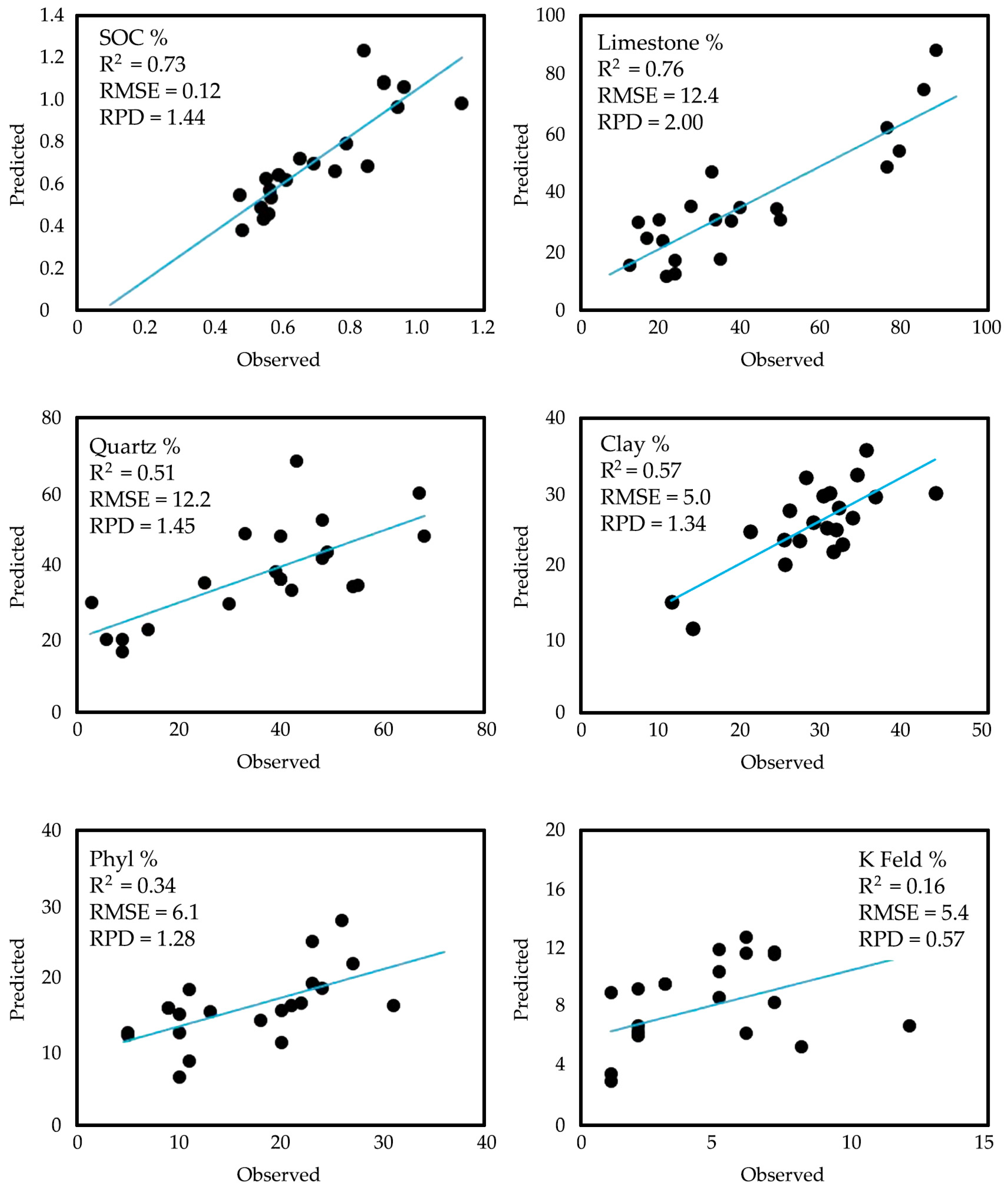
3.2. Prediction of Soil Characteristics Based on Spectral Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montanarella, L.; Pennock, D.J.; McKenzie, N.; Badraoui, M.; Chude, V.; Baptista, I.; Mamo, T.; Yemefack, M.; Singh Aulakh, M.; Yagi, K.; et al. World’s soils are under threat. SOIL 2016, 2, 79–82. [Google Scholar] [CrossRef]
- Gómez, J. Sustainability using cover crops in Mediterranean tree crops, olives and vines—Challenges and current knowledge. Hung. Geogr. Bull. 2017, 66, 13–28. [Google Scholar] [CrossRef]
- Gómez, J.A.; Llewellyn, C.; Basch, G.; Sutton, P.B.; Dyson, J.S.; Jones, C.A. The effects of cover crops and conventional tillage on soil and runoff loss in vineyards and olive groves in several Mediterranean countries. Soil Use Manag. 2011, 27, 502–514. [Google Scholar] [CrossRef]
- Cécillon, L.; Barthès, B.G.; Gomez, C.; Ertlen, D.; Génot, V.; Hedde, M.; Stevens, A.; Brun, J.J. Assessment and monitoring of soil quality using near-infrared reflectance spectroscopy (NIRS). Eur. J. Soil Sci. 2009, 60, 770–784. [Google Scholar] [CrossRef]
- Dematte, J.A.M.; Campos, R.C.; Alves, M.C.; Fiorio, P.R.; Nanni, M.R. Visible-NIR reflectance: A new approach on soil evaluation. Geoderma 2004, 121, 95–112. [Google Scholar] [CrossRef]
- Leone, A.P.; Viscarra-Rossel, R.A.; Amenta, P.; Buondonno, A. Prediction of Soil Properties with PLSR and vis-NIR Spectroscopy: Application to Mediterranean Soils from Southern Italy. Curr. Anal. Chem. 2012, 8, 283–299. [Google Scholar] [CrossRef]
- Gomez, C.; Rossel, R.A.V.; McBratney, A.B. Soil organic carbon prediction by hyperspectral remote sensing and field vis-NIR spectroscopy: An Australian case study. Geoderma 2008, 146, 403–411. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Banin, A. Near-Infrared Analysis as a Rapid Method to Simultaneously Evaluate Several Soil Properties. Soil Sci. Soc. Am. J. 1995, 59, 364–372. [Google Scholar] [CrossRef]
- Brown, D.J.; Shepherd, K.D.; Walsh, M.G.; Mays, M.D.; Reinsch, T.G. Global soil characterization with VNIR diffuse reflectance spectroscopy. Geoderma 2006, 132, 273–290. [Google Scholar] [CrossRef]
- Daniel, K.W.; Tripathi, N.K.; Honda, K. Artificial neural network analysis of laboratory and in situ spectra for the estimation of macronutrients in soils of Lop Buri (Thailand). Aust. J. Soil Res. 2003, 41, 47–59. [Google Scholar] [CrossRef]
- Baumgardner, M.F.; Silva, L.F.; Biehl, L.L.; Stoner, E.R. Reflectance Properties of Soils. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1986; Volume 38, pp. 1–44. [Google Scholar] [CrossRef]
- Clark, R.N. Spectroscopy of rocks and minerals and principles of spectroscopy. In Remote Sensing for the Earth Sciences: Manual of Remote Sensing; Rencz, N., Ed.; Johng Wiley & Sons: New York, NY, USA, 1999; Volume 3. [Google Scholar]
- Van Der Meer, F. Spectral reflectance of carbonate mineral mixtures and bidirectional reflectance theory: Quantitative analysis techniques for application in remote sensing. Remote Sens. Rev. 1995, 13, 67–94. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Irons, J.R.; Epema, G.F. Soil reflectance. In Remote Sensing for the Earth Sciences: Manual of Remote Sensing; Rencz, N., Ed.; John Wiley & Sons: New York, NY, USA, 1999; Volume 3. [Google Scholar]
- CSIC. Cartografía Edafológica y Capacidad de Uso del Suelo de la Subregión de Madrid, 1:100,000. 1975-990. Instituto de Edafología y Biología Vegetal del Consejo Superior de Investigaciones Científicas, Madrid, 1990. Available online: https://datos.comunidad.madrid/catalogo/dataset/spacmsuelosfao1990 (accessed on 15 March 2024).
- Gee, G.W.; Bauder, J.W. Particle-size Analysis. In Methods of Soil Analysis; in SSSA Book Series; American Society of Agronomy, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- FAO. Standard Operating Procedure for Soil Bulk Density, Cylinder Method; FAO: Rome, Italy, 2023.
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. III. Simultaneous determination of a set of reference intensities. J. Appl. Crystallogr. 1975, 8, 17–19. [Google Scholar] [CrossRef]
- Schultz, L.G. Quantitative Interpretation of Mineralogical Composition from X-ray and Chemical Data for the Pierre Shale; Geological Survey: Washington, DC, USA, 1964. [Google Scholar]
- van der Marei, H.W. Quantitative analysis of clay minerals and their admixtures. Contrib. Mineral. Petrol. 1966, 12, 96–138. [Google Scholar] [CrossRef]
- FAO. A Primer on Soil Analysis Using Visible and Near-Infrared (Vis-NIR) and Mid-Infrared (MIR) Spectroscopy; Revision; FAO: Rome, Italy, 2024. [CrossRef]
- Shepherd, K.D.; Walsh, M.G. Development of reflectance spectral libraries for characterization of soil properties. Soil Sci. Soc. Am. J. 2002, 66, 988–998. [Google Scholar] [CrossRef]
- Allison, L.E. Organic Carbon. In Methods of Soil Analysis: Part 2. Chemical and Microbiological Properties. Agronomy Monograph; Black, C.A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1973; pp. 1367–1376. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B.; Chen, H.J. A comparative study of various tests of normality. J. Am. Stat. Assoc. 1968, 63, 1343–1372. [Google Scholar] [CrossRef]
- Rossel, R.A.V. ParLeS: Software for chemometric analysis of spectroscopic data. Chemom. Intell. Lab. Syst. 2008, 90, 72–83. [Google Scholar] [CrossRef]
- Reeves, J.B.; McCarty, G.W.; Reeves, V.B. Mid-infrared diffuse reflectance spectroscopy for the quantitative analysis of agricultural soils. J. Agric. Food Chem. 2001, 49, 766–772. [Google Scholar] [CrossRef]
- Wold, H. Systems Analysis by Partial Least Squares, IIASA Collaborative Paper; IIASA: Laxenburg, Austria, 1983; Available online: http://pure.iiasa.ac.at/2336/ (accessed on 20 March 2024).
- Janik, L.J.; Merry, R.H.; Forrester, S.T.; Lanyon, D.M.; Rawson, A. Rapid Prediction of Soil Water Retention using Mid Infrared Spectroscopy. Soil Sci. Soc. Am. J. 2007, 71, 507–514. [Google Scholar] [CrossRef]
- Dunn, B.W.; Beecher, H.G.; Batten, G.D.; Ciavarella, S. The potential of near-infrared reflectance spectroscopy for soil analysis—A case study from the Riverine Plain of south-eastern Australia. Aust. J. Exp. Agric. 2002, 42, 607–614. [Google Scholar] [CrossRef]
- International Union of Soil Sciences Working Group World Reference Base. Reference Base for Soil Resources (WRB) 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. In World Soil Resources Report; No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Krishnan, P.; Alexander, J.D.; Butler, B.J.; Hummel, J.W. Reflectance Technique for Predicting Soil Organic Matter. Soil Sci. Soc. Am. J. 1980, 44, 1282–1285. [Google Scholar] [CrossRef]
- Bartholomeus, H.M.; Schaepman, M.E.; Kooistra, L.; Stevens, A.; Hoogmoed, W.B.; Spaargaren, O.S.P. Spectral reflectance based indices for soil organic carbon quantification. Geoderma 2008, 145, 28–36. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Wu, X.; Tian, Q.; Ji, J.; Qin, Z. Possibilities of reflectance spectroscopy for the assessment of contaminant elements in suburban soils. Appl. Geochem. 2005, 20, 1051–1059. [Google Scholar] [CrossRef]
- Murray, I. Aspects of interpretation of NIR spectra. In Analytical Application of Spectroscopy; Creaser, C.S., Davies, A.M.C., Eds.; Royal Society of Chemistry: London, UK, 1988; pp. 9–21. [Google Scholar]
- Jiménez-González, M.A.; López-Romano, H.; Carral, P.; Álvarez-González, A.M.; Herranz-Luque, J.E.; Sastre-Rodríguez, B.E.; García-Díaz, A.; Muñoz-Organero, G.; Marques, M.J. Ten-Year Impact of Cover Crops on Soil Organic Matter Quantity and Quality in Semi-Arid Vineyards. Land 2023, 12, 2143. [Google Scholar] [CrossRef]
- Cambou, A.; Allory, V.; Cardinael, R.; Vieira, L.C.; Barthès, B.G. Comparison of soil organic carbon stocks predicted using visible and near infrared reflectance (VNIR) spectra acquired in situ vs. on sieved dried samples: Synthesis of different studies. Soil Secur. 2021, 5, 100024. [Google Scholar] [CrossRef]
- Farifteh, J.; Farshad, A.; George, R.J. Assessing salt-affected soils using remote sensing, solute modelling, and geophysics. Geoderma 2006, 130, 191–206. [Google Scholar] [CrossRef]
- Brunet, D.; Barthès, B.G.; Chotte, J.L.; Feller, C. Determination of carbon and nitrogen contents in Alfisols, Oxisols and Ultisols from Africa and Brazil using NIRS analysis: Effects of sample grinding and set heterogeneity. Geoderma 2007, 139, 106–117. [Google Scholar] [CrossRef]
- Morgan, C.L.S.; Waiser, T.H.; Brown, D.J.; Hallmark, C.T. Simulated in situ characterization of soil organic and inorganic carbon with visible near-infrared diffuse reflectance spectroscopy. Geoderma 2009, 151, 249–256. [Google Scholar] [CrossRef]
- Brown, D.J.; Bricklemyer, R.S.; Miller, P.R. Validation requirements for diffuse reflectance soil characterization models with a case study of VNIR soil C prediction in Montana. Geoderma 2005, 129, 251–267. [Google Scholar] [CrossRef]
- Summers, D.; Lewis, M.; Ostendorf, B.; Chittleborough, D. Visible near-infrared reflectance spectroscopy as a predictive indicator of soil properties. Ecol. Indic. 2011, 11, 123–131. [Google Scholar] [CrossRef]
- Bilgili, A.V.; van Es, H.M.; Akbas, F.; Durak, A.; Hively, W.D. Visible-near infrared reflectance spectroscopy for assessment of soil properties in a semi-arid area of Turkey. J. Arid Environ. 2010, 74, 229–238. [Google Scholar] [CrossRef]
- Khayamim, F.; Wetterlind, J.; Khademi, H.; Robertson, A.H.J.; Cano, A.F.; Stenberg, B. Using Visible and near Infrared Spectroscopy to Estimate Carbonates and Gypsum in Soils in Arid and Subhumid Regions of Isfahan, Iran. J. Near Infrared Spectrosc. 2015, 23, 155–165. [Google Scholar] [CrossRef]
- Vrieling, A. Satellite remote sensing for water erosion assessment: A review. Catena 2006, 65, 2–18. [Google Scholar] [CrossRef]
- Escadafal, R. Remote sensing of arid soil surface color with Landsat thematic mapper. Adv. Space Res. 1989, 9, 159–163. [Google Scholar] [CrossRef]

| Location Site | Management and History of Plots | Coordinates |
|---|---|---|
| O-11A | 7 yr without tillage. Spontaneous vegetation. Supplementary irrigation | X: 462910,60 Y: 4457309,26 |
| O-11B | Traditional tillage | X: 462891,19 Y: 4457338,22 |
| O-12A | 15 yr without tillage, very scarce spontaneous veg. | X: 474610,20 Y: 4440945,56 |
| O-12B | Traditional tillage | X: 474577,02 Y: 4440961,21 |
| O-16A | 6 yr without tillage. Spontaneous veg. soil amended with fertilizers or organic matter | X: 469401,64 Y: 4470425,69 |
| O-16B | Traditional tillage | X: 469433,79 Y: 4470398,92 |
| O-18A | 6 yr without tillage. Spontaneous veg. mowed 2–4 times/yr | X: 470004,44 Y: 4470247,88 |
| O-18B | Traditional tillage | X: 470038,42 Y: 4470255,51 |
| O-19A | 6 yr without tillage. Spontaneous vegetation | X: 470143,77 Y: 4470065,28 |
| O-19B | Traditional tillage | X: 470176,83 Y: 4470054,05 |
| O-22A | 7 yr without tillage. Spontaneous vegetation | X: 468746,05 Y:4456035,20 |
| O-22B | Traditional tillage | X: 468522,19 Y: 4455989,51 |
| O-24A | 5 yr without tillage. Very scarce spontaneous vegetation, abundant stones, and no need for mowing. | X: 463752,42 Y: 4457488,32 |
| O-24B | Traditional tillage | X: 463744,95 Y: 4457527,20 |
| O-3A | Time unknown. Shallow-depth tillage and incorporation of wood pruning residues | X: 468841,07 Y: 4451822,38 |
| O-3B | Traditional tillage | X: 468779,75 Y: 4451721,48 |
| Title 1 | O-11 | O-12 | O-16 | O-18 | O-19 | O-22 | O-24 | O-3 |
|---|---|---|---|---|---|---|---|---|
| Phyllosil. | 9% | 10% | 24% | 25% | 23% | 23% | 19% | 12% |
| % | 8.6–15.6 | 9.2–10.2 | 19.3–26.7 | 20.6–26.1 | 20.5–24.3 | 21.6–25.4 | 17.1–21.7 | 7–19.5 |
| Quartz | 50.8% | 7.5% | 42.8% | 39.9% | 43.8% | 44.0% | 39.4% | 46.9% |
| % | 48.1–54.8 | 4.4–9.2 | 30.9–57 | 26.9–49.5 | 39.6–49.1 | 34.8–54.8 | 31.7–45.4 | 41.7–66.1 |
| K-Feldsp. | 6.8% | 1.2% | 6.6% | 4.8% | 8.4% | 9.0% | 6.5% | 5.2% |
| % | 3–13.8 | 0.8–1.7 | 3.5–11.6 | 2.8–6.9 | 5.6–10.3 | 6.5–12.3 | 3.9–7.5 | 2.8–7.2 |
| Limestone | 24.4% | 81.7% | 44.3% | 23.9% | 21.8% | 19.3% | 25.9% | 23.4% |
| % | 14.5–29.5 | 76.6–84.3 | 38.8–52.6 | 11.8–36.9 | 17–28.2 | 12.4–26.7 | 16.1–38.8 | 15.6–39.9 |
| Bulk dens | 1.44 | 1.19 | 1.55 | 1.47 | 1.51 | 1.44 | 1.49 | 1.32 |
| g cm−3 | 1.3–1.5 | 1.2–1.2 | 1.5–1.6 | 1.4–1.5 | 1.5–1.5 | 1.3–1.6 | 1.3–1.6 | 1.2–1.4 |
| Sand | 39.4 | 37.3 | 51.5 | 28.4 | 42.5 | 41.2 | 32.0 | 44.4 |
| % | 36.6–42 | 34.7–39.6 | 37.5–61 | 25.4–32.7 | 30.8–50.2 | 39.6–47.3 | 29.9–34.2 | 42.5–48 |
| Silt | 33.3 | 30.6 | 31.0 | 59.4 | 31.5 | 26.4 | 36.3 | 30.9 |
| % | 30.3–36.3 | 27.2–31.6 | 19.8–41.2 | 55.9–61.3 | 26.1–38.3 | 23.4–28.2 | 35.7–37.1 | 28.3–33.5 |
| Clay | 27.3 | 33.2 | 19.7 | 12.5 | 26.8 | 32.3 | 30.2 | 24.1 |
| % | 25.9–29.6 | 30–33.9 | 18.5–21.2 | 10.3–13.9 | 23.3–30.8 | 24.6–37.5 | 28.6–34.5 | 22.7–24.7 |
| Texture | Loam | Clay loam | Sandy loam | Silt loam | Loam | Clay loam | Clay loam | Loam |
| Effect | Wilks Value | F | Effect df | Error df | p |
|---|---|---|---|---|---|
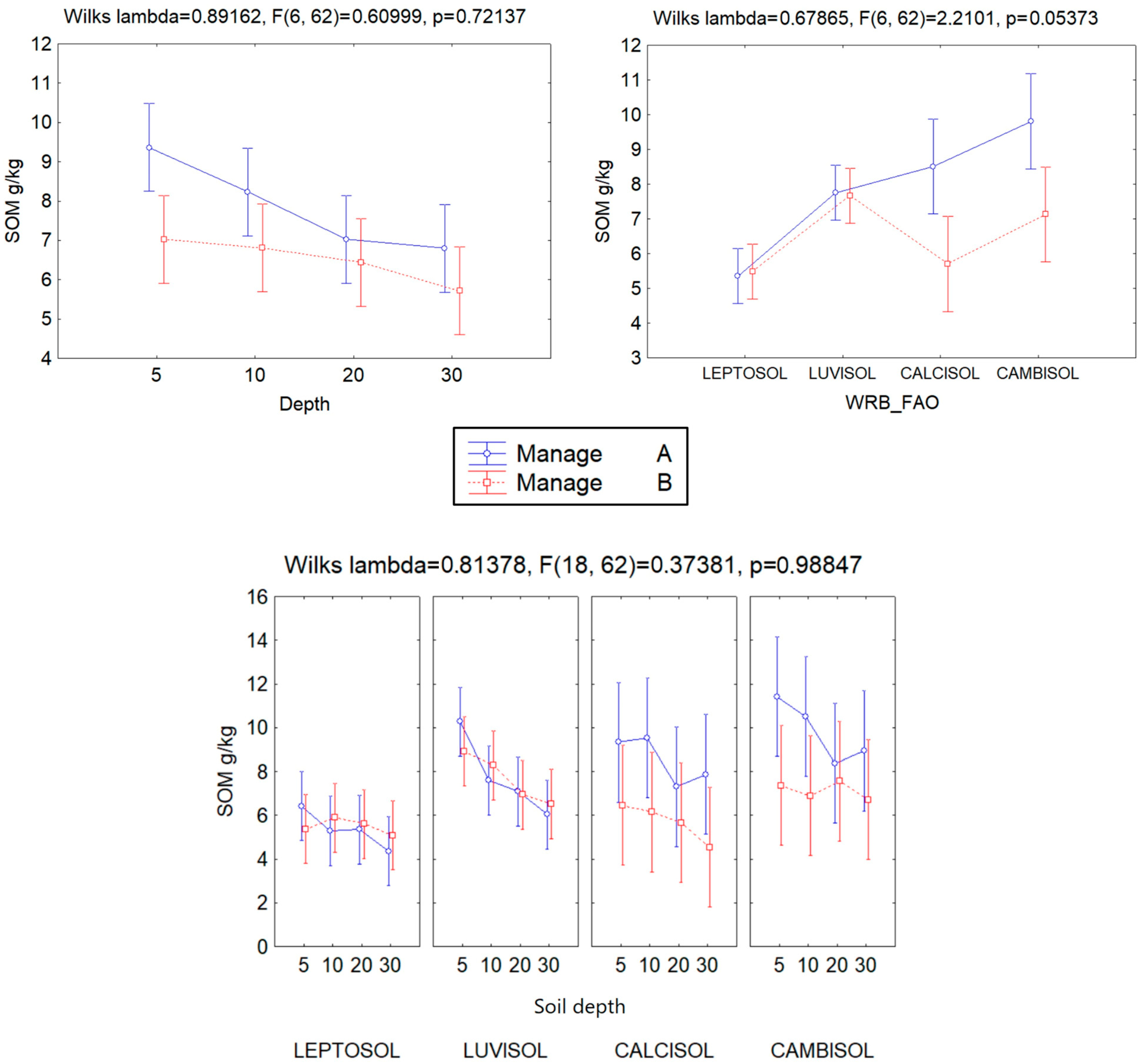
| Management | 0.691 | 6.93 | 2 | 31 | 0.003 * |
| Depth | 0.631 | 2.67 | 6 | 62 | 0.023 * |
| FAO soil | 0.189 | 13.41 | 6 | 62 | 0.000 * |
| Manage & FAO soil | 0.679 | 2.21 | 6 | 62 | 0.05 * |
| Manage & depth | 0.892 | 0.61 | 6 | 62 | 0.72 |
| Depth & FAO soil | 0.580 | 1.08 | 18 | 62 | 0.40 |
| Manage & depth & FAO soil | 0.814 | 0.37 | 18 | 62 | 0.99 |
| PLRS Calibration Set | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Median | R2 | RMSE | RPD | N Out | Var Y |
| SOC | 0.71 | 0.23 | 0.67 | 0.79 | 0.09 | 2.05 | 2 | 85% |
| Limestone | 28.8 | 19.5 | 23 | 0.70 | 11.1 | 1.75 | 1 | 87% |
| Clay | 24.7 | 7.7 | 24.9 | 0.61 | 4.8 | 1.60 | 1 | 79% |
| Quartz | 41.5 | 15.3 | 45 | 0.56 | 10.2 | 1.50 | 1 | 75% |
| Phyllosilicate | 19.2 | 7.8 | 21 | 0.35 | 7.3 | 1.26 | 2 | 34% |
| K Feldspar | 8.9 | 7.7 | 7 | 0.14 | 7.1 | 1.09 | 1 | 20% |
| Plagioclase | 2.9 | 2.7 | 2 | 0.32 | 2.2 | 1.17 | 2 | 62% |
| PLRS validation set | ||||||||
| Mean | SD | Median | R2 | RMSE | RPD | |||
| SOC | 0.71 | 0.18 | 0.65 | 0.73 | 0.12 | 1.44 | ||
| Limestone | 40.3 | 24.8 | 33 | 0.76 | 12.4 | 2.00 | ||
| Clay | 27.9 | 7.0 | 29.5 | 0.57 | 5.0 | 1.34 | ||
| Quartz | 36.3 | 19.2 | 40 | 0.51 | 13.2 | 1.45 | ||
| Phyllosilicate | 16.6 | 7.7 | 18 | 0.34 | 6.1 | 1.28 | ||
| K Feldspar | 4.4 | 2.9 | 5 | 0.16 | 5.4 | 0.57 | ||
| Plagioclase | 3.4 | 4.9 | 2 | 0.03 | 4.9 | 0.78 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herranz-Luque, J.E.; Gonzalez-Canales, J.; Martín-Sanz, J.P.; Antón, O.; Moreno-Delafuente, A.; Navas-Vázquez, M.J.; Ramos-Nieto, R.; Bienes, R.; García-Díaz, A.; Marques, M.J.; et al. Monitoring Land Management Practices Using Vis–NIR Spectroscopy Provides Insights into Predicting Soil Organic Carbon and Limestone Levels in Agricultural Plots. Agronomy 2024, 14, 1150. https://doi.org/10.3390/agronomy14061150
Herranz-Luque JE, Gonzalez-Canales J, Martín-Sanz JP, Antón O, Moreno-Delafuente A, Navas-Vázquez MJ, Ramos-Nieto R, Bienes R, García-Díaz A, Marques MJ, et al. Monitoring Land Management Practices Using Vis–NIR Spectroscopy Provides Insights into Predicting Soil Organic Carbon and Limestone Levels in Agricultural Plots. Agronomy. 2024; 14(6):1150. https://doi.org/10.3390/agronomy14061150
Chicago/Turabian StyleHerranz-Luque, Juan E., Javier Gonzalez-Canales, Juan P. Martín-Sanz, Omar Antón, Ana Moreno-Delafuente, Mariela J. Navas-Vázquez, Rubén Ramos-Nieto, Ramón Bienes, Andrés García-Díaz, Maria Jose Marques, and et al. 2024. "Monitoring Land Management Practices Using Vis–NIR Spectroscopy Provides Insights into Predicting Soil Organic Carbon and Limestone Levels in Agricultural Plots" Agronomy 14, no. 6: 1150. https://doi.org/10.3390/agronomy14061150
APA StyleHerranz-Luque, J. E., Gonzalez-Canales, J., Martín-Sanz, J. P., Antón, O., Moreno-Delafuente, A., Navas-Vázquez, M. J., Ramos-Nieto, R., Bienes, R., García-Díaz, A., Marques, M. J., & Sastre, B. (2024). Monitoring Land Management Practices Using Vis–NIR Spectroscopy Provides Insights into Predicting Soil Organic Carbon and Limestone Levels in Agricultural Plots. Agronomy, 14(6), 1150. https://doi.org/10.3390/agronomy14061150









