Silicon Improves Heat and Drought Stress Tolerance Associated with Antioxidant Enzyme Activity and Root Viability in Creeping Bentgrass (Agrostis stolonifera L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Culture and Stress Treatment
2.2. Ortho-Si Treatment
2.3. Measurements
2.3.1. Turf Quality
2.3.2. Photochemical Efficiency (PE)
2.3.3. Leaf Pigment Content
- Chl a (µg mL) = (11.24 × A661.6 nm) − (2.04 × A644.8 nm).
- Chl b (µg mL) = (20.13 × A644.8 nm) − (4.19 × A661.6 nm).
- Chl a + b (µg/mL) = (7.05 × A661.1 nm) + (18.09 × A644.8 nm).
- Carotenoids (µg mL) = [(1000 × A470 nm) − (1.90 × chl a − 63.14 × chl b)].
2.3.4. Leaf Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA)
2.3.5. Leaf Antioxidant Enzyme SOD, CAT, APX, and Peroxidase (POD) Activity
2.3.6. Leaf Si Concentration
2.3.7. Root Growth Characteristics and Viability Analysis
2.3.8. Experimental Design and Statistical Analysis
3. Results
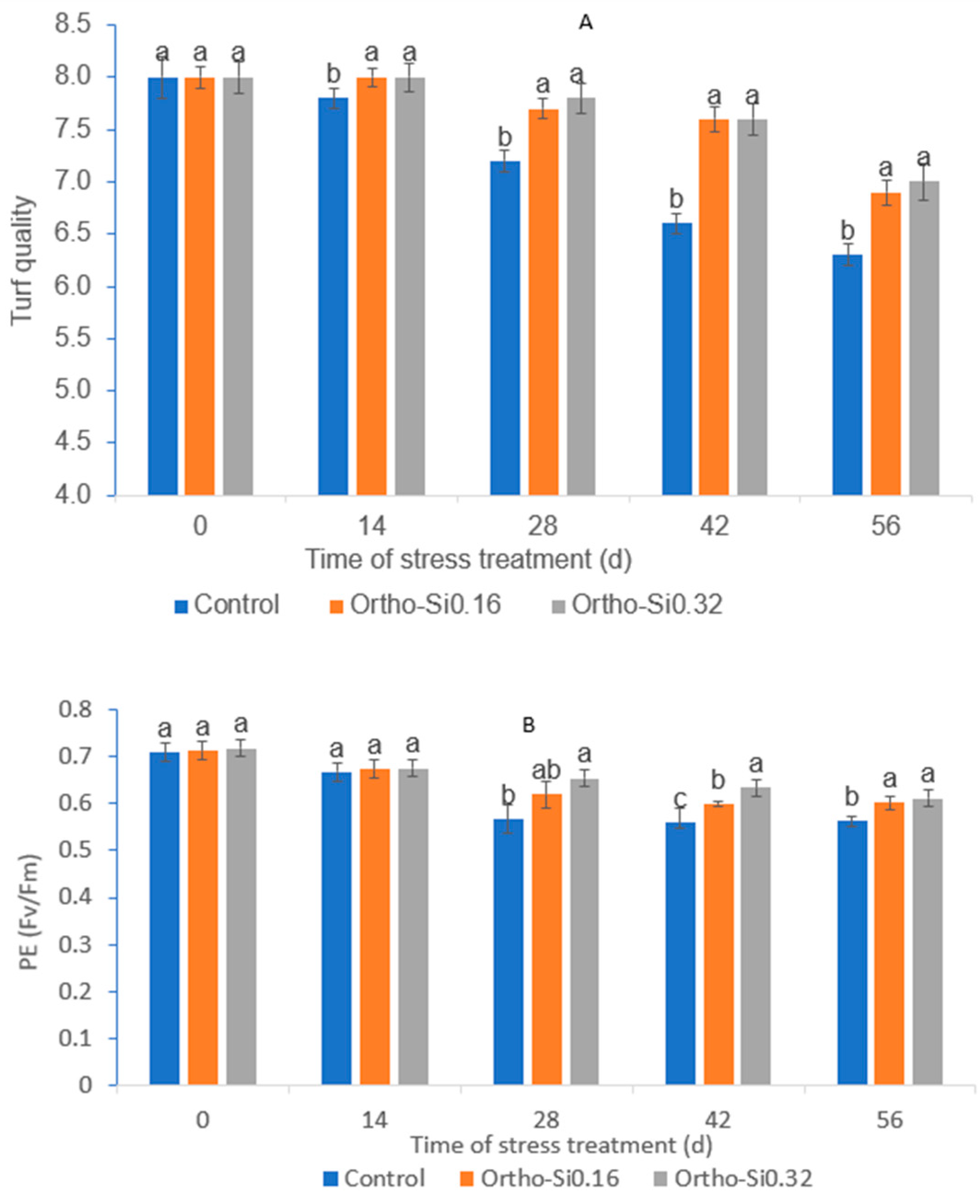
3.1. Turf Quality
3.2. Leaf Photochemical Efficiency (PE)
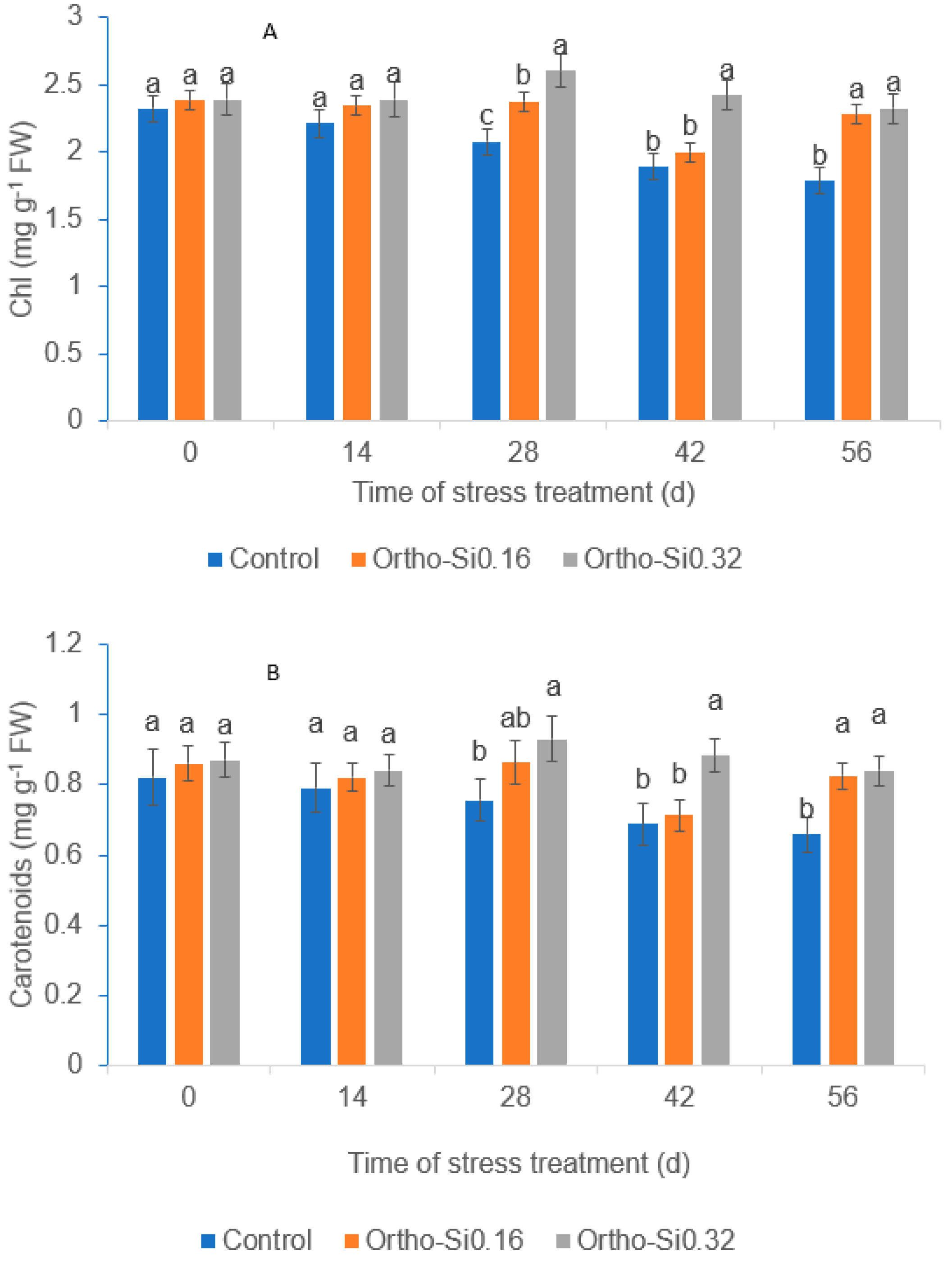
3.3. Leaf Chlorophyll Content
3.4. Leaf Carotenoids Content
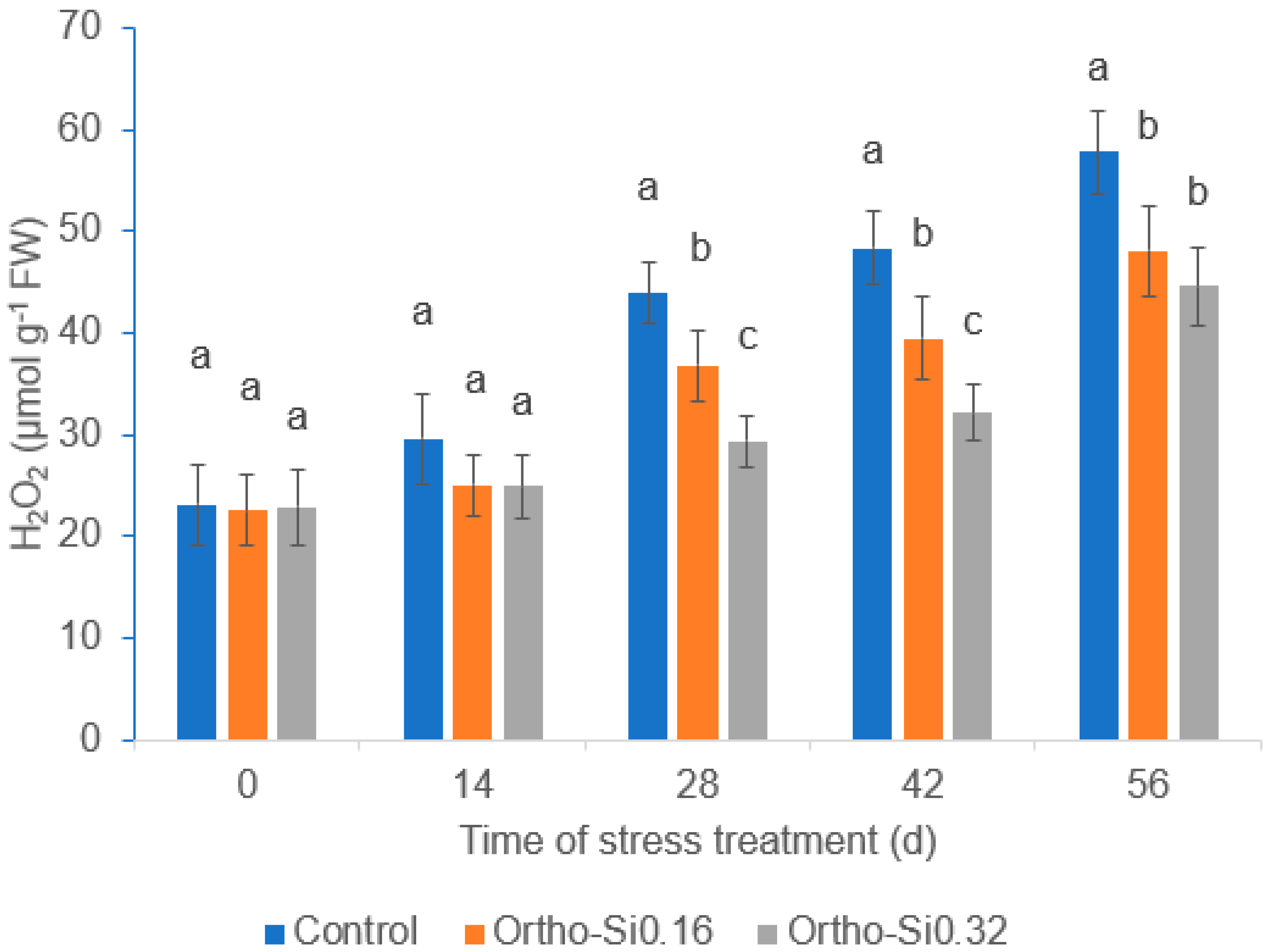
3.5. Leaf H2O2 and MDA Content
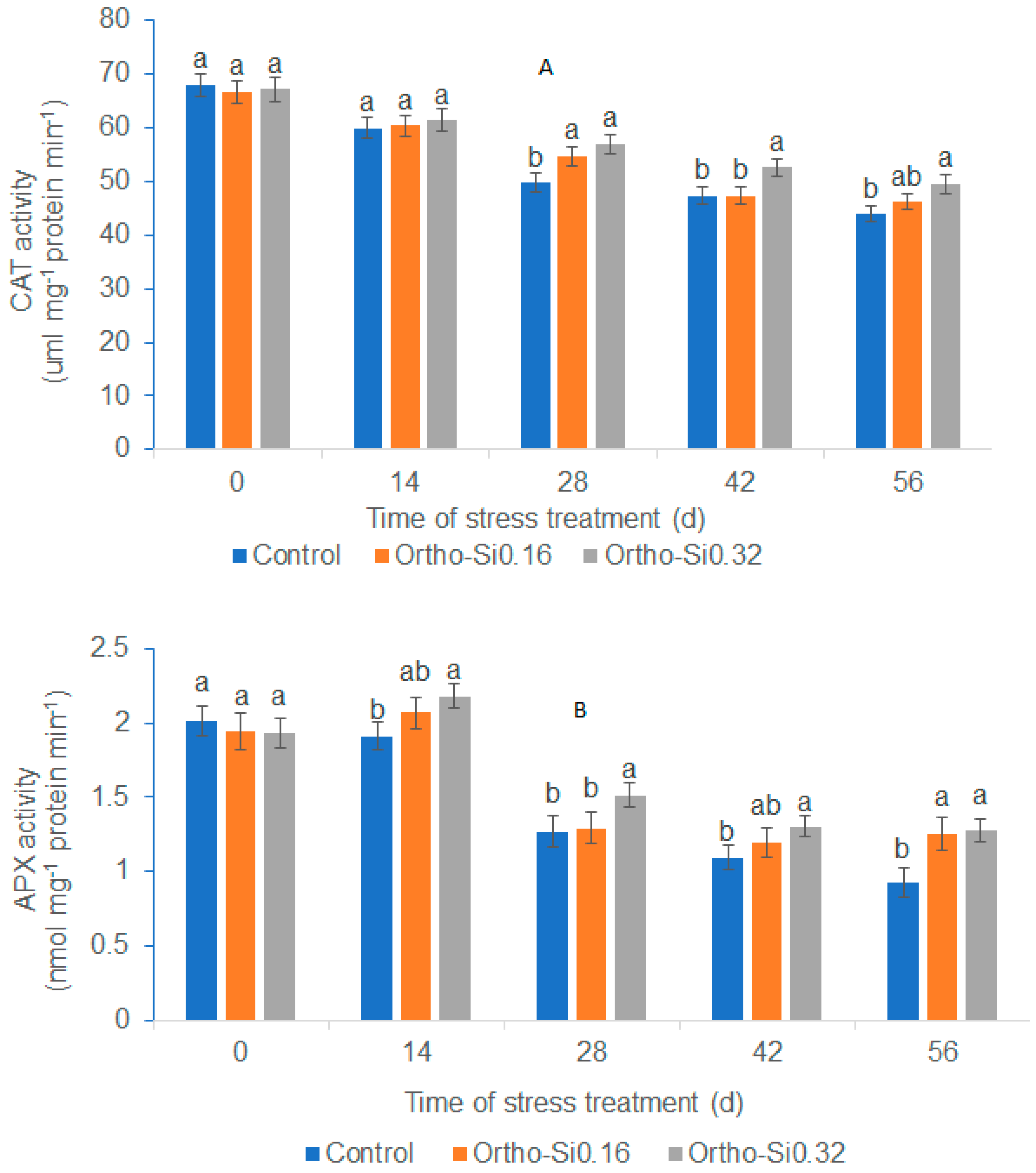
3.6. Leaf Antioxidant Enzyme Activity
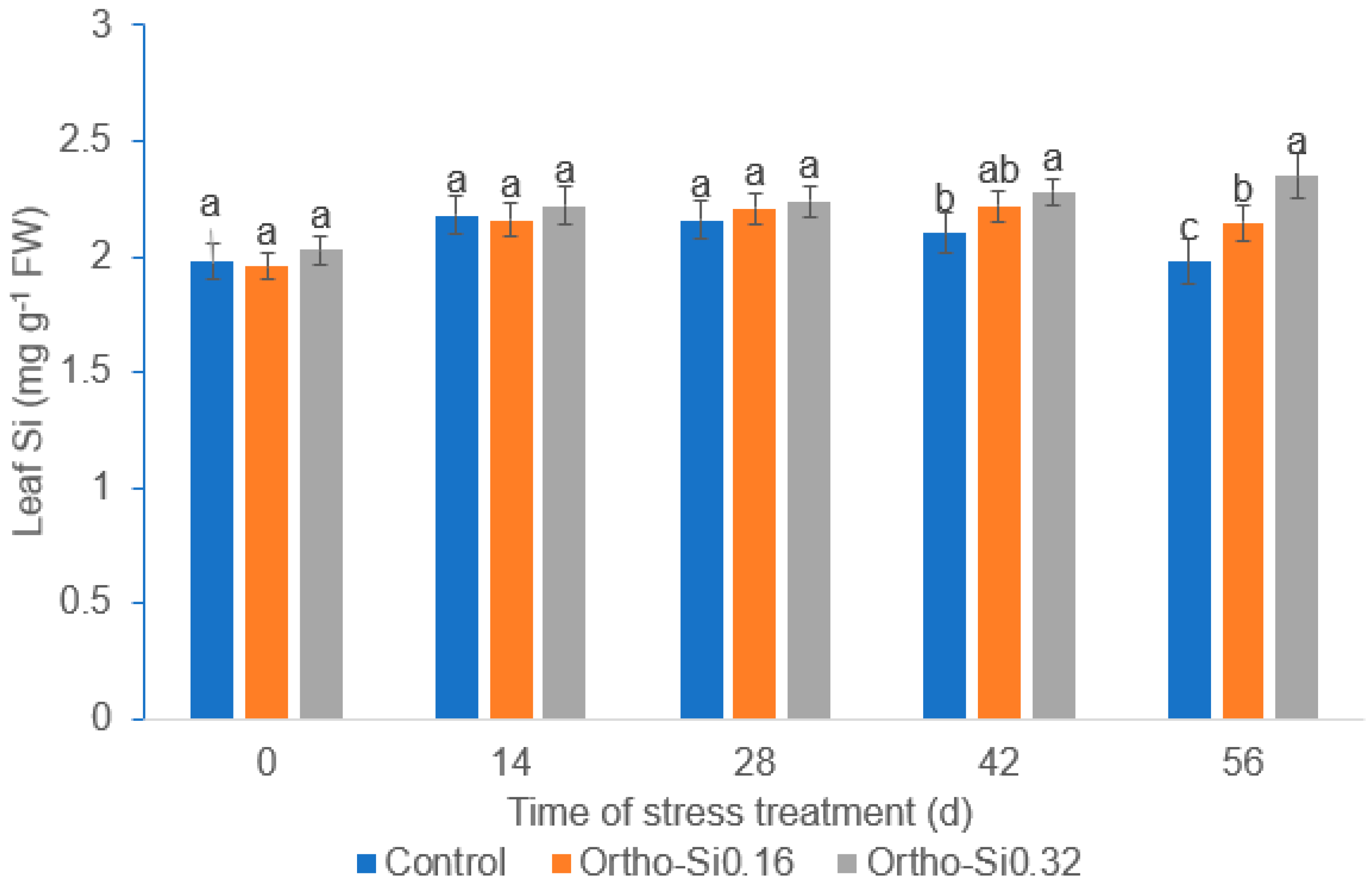
3.7. Leaf Si Content
3.8. Root Growth Characteristics, Biomass, and Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Taylor, Z.; Goatley, M.; Booth, J.; Brown, I.; Kosiarski, K. Seaweed extract-based biostimulant impacts on nitrate reductase activity and root viability of ultradwarf bermudagrass subjected to heat and drought stress. HortScience 2022, 57, 1328–1333. [Google Scholar] [CrossRef]
- Chang, Z.; Liu, Y.; Dong, H.; Teng, K.; Hao, L.; Zhang, X. Effects of cytokinin and nitrogen on drought tolerance of creeping bentgrass. PLoS ONE 2016, 11, e0154005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ervin, E.H.; Liu, Y.; Hu, G.; Shang, C.; Fukao, T.; Alpuerto, J. Differential responses of antioxidants, abscisic acid, and auxin to deficit irrigation in two perennial ryegrass cultivars contrasting in drought tolerance. J. Am. Soc. Hort. Sci. 2015, 140, 562–572. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2005. [Google Scholar]
- Guertal, E.A.; Datnoff, L.E. Silicon in turfgrass: A review. Crop Sci. 2021, 61, 3861–3876. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakpv, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Kaczynski, P.; Pietkun, M.; Lozowicka, B. Evaluation of titanium and silicon role in mitigation of fungicides toxicicity in whaet expressed at the level of biochemical and antioxidant profile. Chemsphere 2022, 308, 136284. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.; Mur, A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Zellner, W.; Datnoff, L. Silicon as a biostimulant in agriculture. In Biostimulants for Sustainable Crop Production; Rouphael, Y., Jardin, P.D., Brown, P., De Pascale, S., Colla, G., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenic variation in the Silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Uriarte, R.F.; Shew, H.D.; Bowman, D.C. Effects of soluble silica on brown patch and dollar sport of creeping bentgrass. J. Plant Nutr. 2004, 27, 325–339. [Google Scholar] [CrossRef]
- Merewitz, E.B.; Liu, S. Improvement in heat tolerance of creeping bentgrass with melatonin, rutin, and silicon. J. Am. Soc. Hort. Sci. 2019, 144, 141–148. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Ann. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic stress and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Eneji, A.E.; Inanaga, S.; Muranaka, S.; Li, J.; Hattori, T.; An, P.; Tsuji, W. Growth and nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J. Plant Nutr. 2008, 31, 355–365. [Google Scholar] [CrossRef]
- Bae, E.J.; Hong, A.R.; Choi, S.M.; Lee, K.S.; Park, Y.B. Silicon pretreatment alleviates drought stress and increases antioxidative activity in Kentucky bluegrass. Int. Turfgrass Soc. Res. J. 2017, 13, 591–600. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Q.; Ervin, E.H.; Yang, Z.; Zhang, X. Physiological mechanisms of enhancing salt stress tolerance of perennial ryegrass by 24-epibrassinolide. Front. Plant Sci. 2017, 8, 1017. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.E.; Zhang, X.; Chalmers, D.R. Response of photosynthesis and superoxide dismutase to silica applied to creeping bentgrass grown under two fertility levels. J. Plant Nutr. 1999, 22, 1763–1773. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. The functional role of silicon in plant biology: Consistent alleviation of abiotic stress with silicon addition: A meta-analysis. Funct. Ecol. 2016, 30, 1340–1357. [Google Scholar] [CrossRef]
- Agari, S.; Hanaoka, N.; Ueno, O.; Miyazaki, A.; Kubota, F.; Agata, W.; Kaufman, P.B. Effects of silicon on tolerance to water deficit and heat stress in rice plants (Oryza sativa L.), monitored by electrolyte leakage. Plant Prod. Sci. 1998, 2, 96–103. [Google Scholar] [CrossRef]
- Morris, K. A Guide to NTEP Turfgrass Quality Ratings. 2022. Available online: https://www.ntep.org/reports/ratings.htm#:~:text=How%20is%20Turfgrass%20Quality%20Evaluated,an%20absolutely%20outstanding%20treatment%20plot (accessed on 10 January 2020).
- Bernt, E.; Bergmeyer, H. Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Hodges, D.M.; Deiong, J.M.; Forney, C.F.; Prange, R. Improving the thiobarbituric acid-recative–substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Kochhar, S.; Kochhar, V.K.; Khanduja, S.D. Changes in the pattern of isoperoxidases during maturation of grape berries cv Glulabi as affected by ethephon (2-chloroethyl) phosphonic acid. Am. J. Enol. Viticul. 1979, 30, 275–277. [Google Scholar] [CrossRef]
- Elliott, C.I.; Snyder, G.H. Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J. Agric. Food Chem. 1991, 39, 1118–1119. [Google Scholar] [CrossRef]
- Kraska, J.E.; Breitenbeck, G.A. Simple, rubust method for quantifying silicon in plant tissue. Commun. Soil Sci. Plant Anal. 2010, 41, 2075–2085. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Pilbeam, D.J.; Inal, A. Influence of Silicon on sunflower cultivars under drought stress. Commun. Soil Sci. Plant Anal. 2008, 39, 1885–1903. [Google Scholar] [CrossRef]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Bagci, E.G.; Coban, S. Influence of Silicon on antioixdant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J. Plant Interact. 2007, 2, 105–113. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Shi, Y. Silicon improves seed germination and allevaites oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol. Biochem. 2011, 78, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bazem, F.A.; Bernards, M.A. Hydrogen peroxide is required for poly(phenolic) domain formation during wound-induced suberization. J. Agric. Food Chem. 2002, 50, 1009–1015. [Google Scholar]
- Shi, Y. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solonum lycopersicum L. Front. Plant Sci. 2016, 7, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Redmond, C.T.; Potter, D.A. Silicon fertilization does not enhance creeping bentgrass resistance to cutworms and white crubs. Appl. Turfgrass Sci. 2006, 3, 1–6. [Google Scholar] [CrossRef]
- Zhang, Q.; Fry, J.; Lowe, K.; Tisserat, N. Evaluation of calcium silicate for brown patch and dollar spot suppression on turfgrasses. Crop. Sci. 2006, 46, 1635–1643. [Google Scholar] [CrossRef]
- Raven, J.A. The transport and function of silicon in plants. Biol. Rev. 1983, 58, 179–207. [Google Scholar] [CrossRef]

| Treatment | Rate | Biomass | Length | SA | Diameter | Volume | Viability |
|---|---|---|---|---|---|---|---|
| (mL m−2) | (g/pot) | (cm cm3) | (cm2 cm−3) | (mm) | (cm3 dm−3) | (A490 g−1 FW) | |
| Control | 0 | 1.12b | 23.2b | 1.30b | 0.175a | 5.95b | 0.56b |
| Ortho-Si | 0.16 | 1.10b | 25.6b | 1.37b | 0.172a | 5.78b | 0.69ab |
| Ortho-Si | 0.32 | 1.71a | 36.8a | 2.04a | 0.176a | 9.00a | 1.06a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Goatley, M.; Wang, K.; Goddard, B.; Harvey, R.; Brown, I.; Kosiarski, K. Silicon Improves Heat and Drought Stress Tolerance Associated with Antioxidant Enzyme Activity and Root Viability in Creeping Bentgrass (Agrostis stolonifera L.). Agronomy 2024, 14, 1176. https://doi.org/10.3390/agronomy14061176
Zhang X, Goatley M, Wang K, Goddard B, Harvey R, Brown I, Kosiarski K. Silicon Improves Heat and Drought Stress Tolerance Associated with Antioxidant Enzyme Activity and Root Viability in Creeping Bentgrass (Agrostis stolonifera L.). Agronomy. 2024; 14(6):1176. https://doi.org/10.3390/agronomy14061176
Chicago/Turabian StyleZhang, Xunzhong, Mike Goatley, Kehua Wang, Ben Goddard, Rose Harvey, Isabel Brown, and Kelly Kosiarski. 2024. "Silicon Improves Heat and Drought Stress Tolerance Associated with Antioxidant Enzyme Activity and Root Viability in Creeping Bentgrass (Agrostis stolonifera L.)" Agronomy 14, no. 6: 1176. https://doi.org/10.3390/agronomy14061176






