Variability of Root and Shoot Traits under PEG-Induced Drought Stress at an Early Vegetative Growth Stage of Soybean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Trait Measurements
2.3. Statistical Analysis
3. Results
3.1. Analysis of Variance
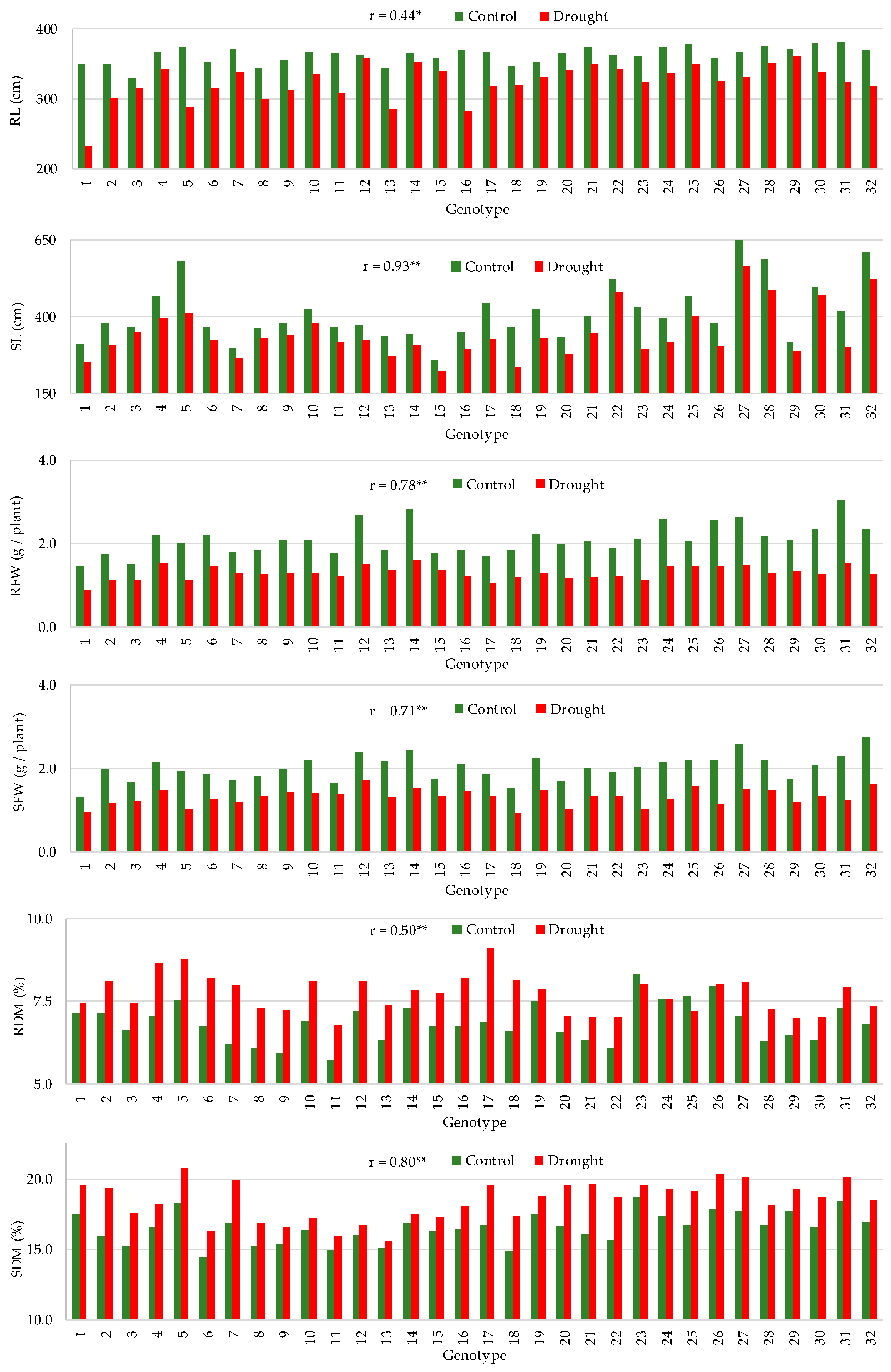
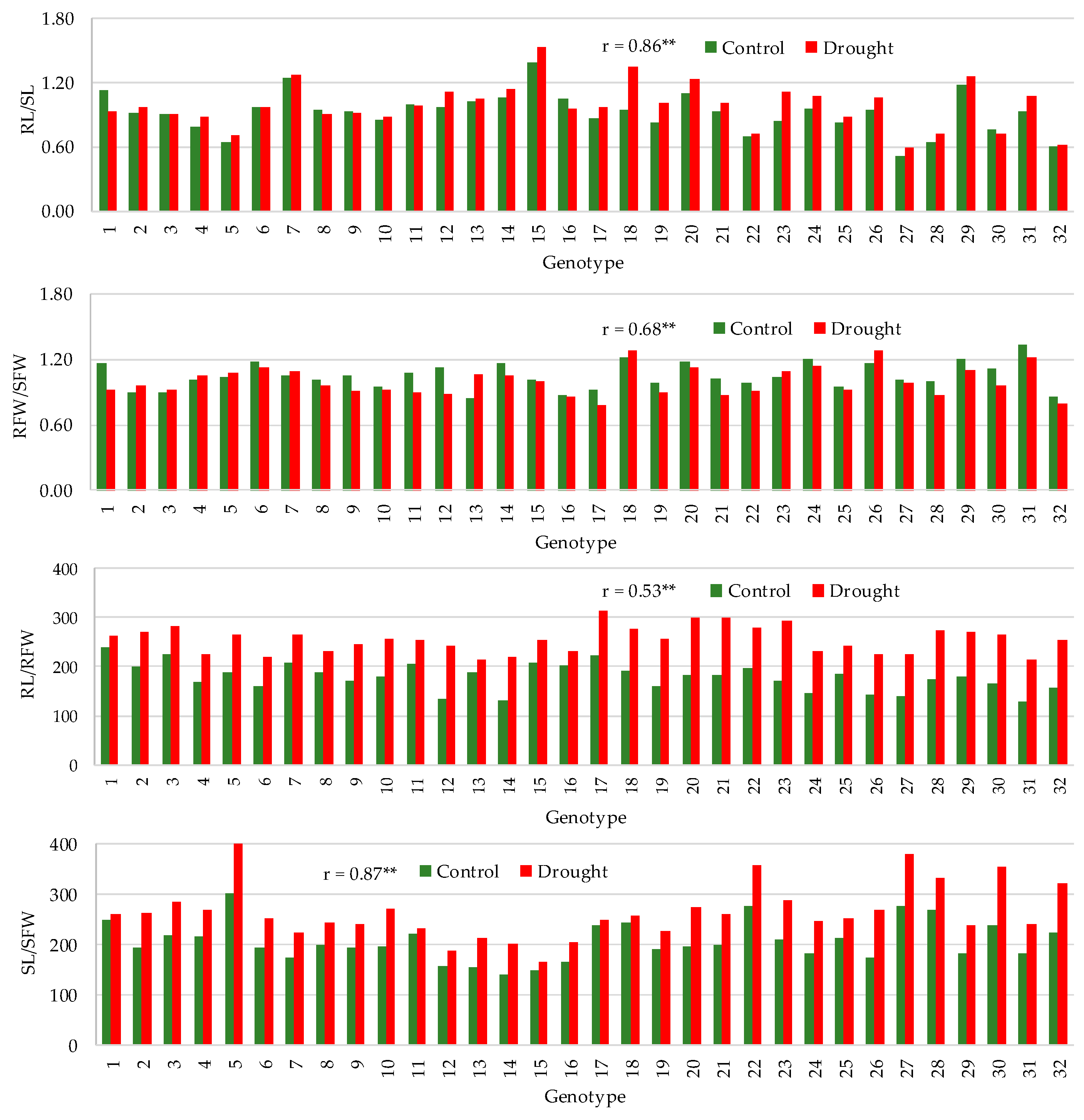
3.2. Effect of Drought on Trait Means
3.3. Correlation between Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rotundo, J.L.; Marshall, R.; McCormick, R.; Truong, S.K.; Styles, D.; Gerde, J.A.; Gonzalez-Escobar, E.; Carmo-Silva, E.; Janes-Bassett, V.; Logue, J.; et al. European soybean to benefit people and the environment. Sci. Rep. 2004, 14, 7612. [Google Scholar] [CrossRef]
- Messina, M.J. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999, 70, 439S–450S. [Google Scholar] [CrossRef]
- Donau Soja. The Non-GM Multicrop Report, Enga, Donau Soja and ProTerra, Edition 02, February 2024. Available online: https://www.donausoja.org/news/market-report/ (accessed on 2 April 2024).
- Wang, X.; Song, S.; Wang, X.; Liu, J.; Dong, S. Transcriptomic and metabolomic analysis of seedling-stage soybean responses to peg-simulated drought stress. Int. J. Mol. Sci. 2022, 23, 6869. [Google Scholar] [CrossRef]
- Clement, M.; Lambert, A.; Herouart, D.; Boncompagni, E. Identification of new up-regulated genes under drought stress in soybean nodules. Gene 2008, 426, 15–22. [Google Scholar] [CrossRef]
- Arya, H.; Singh, M.B.; Bhalla, B.L. Towards developing drought-smart soybeans. Front. Plant Sci. 2021, 12, 750664. [Google Scholar] [CrossRef]
- Narayanan, S.; Fallen, B. Evaluation of soybean plant introductions for traits that can improve emergence under varied soil moisture levels. Agronomy 2019, 9, 118. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wang, X.; Li, Y.; Li, Y.; Gou, Z.; Zhao, X.; Hong, H.; Ren, H.; Qi, X.; et al. Identification of genes for drought resistance and prediction of gene candidates in soybean seedlings based on linkage and association mapping. Crop J. 2022, 10, 830–839. [Google Scholar] [CrossRef]
- Demicheli, J.; Sabljic, I.; Beguy, G.; Ploschuk, E.; Sahrawy, M.; Serrato, A.J.; Pagano, E.A. Improving drought tolerance in soybean by classical breeding leads to physiological adjustments of photosynthesis and stomata functioning. Plant Stress 2023, 10, 100275. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Dong, S. Screening and identification of drought tolerance of spring soybean at seedling stage under climate change. Front. Sustain. Food Syst. 2022, 6, 988319. [Google Scholar] [CrossRef]
- Umburanas, R.C.; Cordeschi Donegá, V.; Mendes de Queiroz, V.; Fontana, D.C.; Bampi, D.; Dourado-Neto, D.; Reichardt, K. Root attributes and seedling biomass of old and modern soybean cultivars under water deficit. Emir. J. Food Agric. 2019, 31, 688–696. [Google Scholar]
- Rasheed, A.; Mahmood, A.; Maqbool, R.; Albaqami, M.; Sher, A.; Sattar, A.; Bakhsh, G.; Nawaz, M.; Umair Hassan, M.; Al-Yahyai, R.; et al. Key insights to develop drought-resilient soybean: A review. J. King Saud Univ. Sci. 2022, 34, 102089. [Google Scholar] [CrossRef]
- Dayoub, E.; Lamichhane, J.R.; Schoving, C.; Debaeke, P.; Maury, P. Early-stage phenotyping of root traits provides insights into the drought tolerance level of soybean cultivars. Agronomy 2021, 11, 188. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, S.; Yang, W.; Li, B.; Lan, C.; Zhang, J.; Yuan, L.; Wang, Y.; Xie, Q.; Han, J.; et al. Multi-omic dissection of the drought resistance traits of soybean landrace LX. Plant Cell Environ. 2021, 44, 1379–1398. [Google Scholar] [CrossRef]
- Pierik, R.; Testerink, C. The art of being flexible: How to escape from shade, salt, and drought. Plant Physiol. 2014, 166, 5–22. [Google Scholar] [CrossRef]
- Chapman, N.; Miller, A.J.; Lindsey, K.; Whalley, W.R. Roots, water, and nutrient acquisition: Let’s get physical. Trends Plant Sci. 2012, 17, 701–710. [Google Scholar] [CrossRef]
- Fuentealba, M.P.; Zhang, J.; Kenworthy, K.E.; Erickson, J.E.; Kruse, J.; Trenholm, L.E. Root development and profile characteristics of bermudagrass and zoysiagrass. Hortscience 2015, 50, 1429–1434. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Nie, Y.; Bai, S.H.; Zhou, L.; Shao, J.; Cheng, W.; Wang, J.; Hu, F.; Fu, Y. Drought-induced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef]
- Thu, N.B.A.; Nguyen, Q.T.; Hoang, X.L.T.; Thao, N.P.; Tran, L.S.P. Evaluation of drought tolerance of the Vietnamese soybean cultivars provides potential resources for soybean production and genetic engineering. BioMed Res. Int. 2014, 2014, 809736. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Gholamhossieni, M. Quantification of soybean seed germination response to seed deterioration under PEG-induced water stress using hydrotime concept. Acta Physiol. Plant. 2018, 40, 126. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Deshmukh, S.N.; Kolhe, P.N.; Kale, M.R.; Varne, M.D.; Pawar, K. Evaluation of drought effect on soybean genotypes mediated through PEG-6000 (Polyethylene Glycol). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 726–734. [Google Scholar] [CrossRef]
- Kakati, J.P.; Fallen, B.; Bridges, W.; Narayanan, S. Characterization of a soybean (Glycine max L. Merr.) population for germination and seedling root traits under water stress. Agronomy 2022, 12, 1944. [Google Scholar] [CrossRef]
- Boote, K.J. Improving soybean cultivars for adaptation to climate change and climate variability. In Crop Adaptation to Climate Change; Yadav, S.S., Redden, R.J., Hatfield, J.L., Lotze-Campen, H., Hall, E.A., Eds.; John Wiley & Sons Ltd.: West Sussex, UK, 2011; pp. 370–395. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhao, W.; Hou, X.; Dong, S. Current views of drought research: Experimental methods, adaptation mechanisms and regulatory strategies. Front. Plant Sci. 2024, 15, 1371895. [Google Scholar] [CrossRef]
- Zhang, L.J.; Fan, J.J.; Ruan, Y.Y. Application of polyethylene glycol in the study of plant osmotic stress physiology. Plant Physiol. Commun. 2004, 40, 361–368. [Google Scholar]
- Guan, Z.; Wang, L.; Duan, L.; Zhou, Z.; Zhang, F.; Wang, Y. Effects of PEG simulated drought stress on seed germination of Abutilon theophrasti medicus. Seed 2022, 41, 66–70. [Google Scholar] [CrossRef]
- Michel, B.E. Evaluation of the water potentials of solutions of polyethylene glycol 8000. Plant Physiol. 1983, 72, 66–70. [Google Scholar] [CrossRef]
- SAS Institute. Statistical Analysis Software (SAS) User’s Guide Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Hallauer, A.R.; Carena, M.J.; Filho, J.B.M. Quantitative Genetics in Maize Breeding; Springer: New York, NY, USA, 2010. [Google Scholar]
- Neumann, P.M. Coping mechanisms for crop plants in drought-prone environments. Ann. Bot. 2008, 101, 901–907. [Google Scholar] [CrossRef]
- Zhan, A.; Schneider, H.; Lynch, J.P. Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol. 2015, 168, 1603–1615. [Google Scholar] [CrossRef]
- Lynch, J.P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef]

| Code | Genotype | MG | Country of Origin | Breeding Company | Code | Genotype | MG | Genotype Status | Breeding Company |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Zlata | 0–I | Croatia | UniZg | 17 | Buga | 0 | Croatia | UniZg |
| 2 | Ružica | 0–I | Croatia | UniZg | 18 | Gabriela | 0 | Croatia | UniZg |
| 3 | Zagrebčanka | I | Croatia | UniZg | 19 | Sanda | 0 | Croatia | AIO |
| 4 | Pedro | 0–I | Italy | ERSA | 20 | Sonja | 0 | Croatia | AIO |
| 5 | Bahia | 0–I | Italy | ERSA | 21 | Toma | 0 | Croatia | AIO |
| 6 | Ascasubi | I | Italy | ERSA | 22 | Ema | 00–0 | Croatia | AIO |
| 7 | Ika | 0–I | Croatia | AIO | 23 | Korana | 00–0 | Croatia | AIO |
| 8 | OS Zora | 0–I | Croatia | AIO | 24 | Lucija | 0 | Croatia | AIO |
| 9 | Tena | 0–I | Croatia | AIO | 25 | OS-1 | 0 | Croatia | AIO |
| 10 | Sara | 0–I | Croatia | AIO | 26 | OS-2 | 00–0 | Croatia | AIO |
| 11 | Seka | I | Croatia | AIO | 27 | OS-4 | 00–0 | Croatia | AIO |
| 12 | Tisa | I | Croatia | AIO | 28 | OS-5 | 0 | Croatia | AIO |
| 13 | OS-3 | 0–I | Croatia | AIO | 29 | OS-6 | 0 | Croatia | AIO |
| 14 | OS-8 | 0–I | Croatia | AIO | 30 | OS-7 | 00–0 | Croatia | AIO |
| 15 | DH 5170 | I | Canada | UniG | 31 | Merkur | 00 | Serbia | IFVC |
| 16 | Galina | 0 | Serbia | IFVC | 32 | Xonia | 00 | Italy | ERSA |
| Trait | Treatment | ANOVA across Treatments | ANOVA by Treatment | |||
|---|---|---|---|---|---|---|
| Genotype (G) | Treatment (T) | G × T | Genotype | h2 | ||
| RL | Control | ** | ** | ** | ** | 0.53 |
| Drought | ** | 0.83 | ||||
| SL | Control | ** | ** | ** | ** | 0.97 |
| Drought | ** | 0.96 | ||||
| RFW | Control | ** | ** | ** | ** | 0.85 |
| Drought | ** | 0.68 | ||||
| SFW | Control | ** | ** | ** | ** | 0.84 |
| Drought | ** | 0.80 | ||||
| RDM | Control | ** | ** | * | ** | 0.67 |
| Drought | ** | 0.68 | ||||
| SDM | Control | ** | ** | NS | ** | 0.76 |
| Drought | ** | 0.80 | ||||
| RL/SL | Control | ** | ** | ** | ** | 0.94 |
| Drought | ** | 0.96 | ||||
| RFW/SFW | Control | ** | ** | NS | ** | 0.72 |
| Drought | ** | 0.78 | ||||
| RL/RFW | Control | ** | ** | NS | ** | 0.80 |
| Drought | ** | 0.56 | ||||
| SL/SFW | Control | ** | ** | ** | ** | 0.92 |
| Drought | ** | 0.94 | ||||
| Absolute Units | Change (% of Control) | |||||||
|---|---|---|---|---|---|---|---|---|
| Trait | Treatment | Mean | Min | Max | CV (%) | Mean | Min | Max |
| RL (mm) | Control | 362 | 329 | 381 | 3.3 | −11 | −1 | −34 |
| Drought | 324 | 231 | 359 | 8.3 | ||||
| SL (mm) | Control | 415 | 258 | 717 | 24.1 | −17 | −5 | −35 |
| Drought | 345 | 222 | 567 | 24.2 | ||||
| RFW (g/plant) | Control | 2.10 | 1.45 | 3.04 | 17.9 | −38 | −24 | −50 |
| Drought | 1.29 | 0.88 | 1.59 | 12.5 | ||||
| SFW (g/plant) | Control | 2.02 | 1.29 | 2.74 | 15.4 | −34 | −16 | −50 |
| Drought | 1.31 | 0.92 | 1.71 | 14.7 | ||||
| RDM (%) | Control | 6.85 | 5.71 | 8.34 | 8.9 | 13 | −6 | 33 |
| Drought | 7.72 | 6.77 | 9.12 | 7.4 | ||||
| SDM (%) | Control | 16.6 | 14.5 | 18.7 | 6.6 | 11 | 3 | 22 |
| Drought | 18.4 | 15.6 | 20.8 | 7.6 | ||||
| RL/SL | Control | 0.92 | 0.51 | 1.39 | 20.3 | 8 | −17 | 43 |
| Drought | 0.98 | 0.59 | 1.53 | 21.4 | ||||
| RFW/SFW | Control | 1.05 | 0.85 | 1.33 | 11.5 | −4 | −21 | 25 |
| Drought | 1.00 | 0.78 | 1.28 | 13.0 | ||||
| RL/RFW | Control | 179 | 129 | 241 | 15.7 | 45 | 9 | 79 |
| Drought | 255 | 214 | 314 | 10.6 | ||||
| SL/SFW | Control | 208 | 142 | 301 | 18.9 | 28 | 5 | 55 |
| Drought | 265 | 166 | 402 | 20.4 | ||||
| Trait | Treatment | SL (mm) | RFW (g/Plant) | SFW (g) | RDM (%) | SDM (%) |
|---|---|---|---|---|---|---|
| RL (mm) | Control | 0.37 * | 0.49 ** | 0.38 * | 0.41 * | 0.49 ** |
| Drought | 0.23 | 0.57 ** | 0.43 * | 0.42 * | 0.48 ** | |
| SL (mm) | Control | 0.33 | 0.59 ** | 0.28 | 0.60 ** | |
| Drought | 0.16 | 0.49 ** | 0.08 | 0.61 ** | ||
| RFW (g/plant) | Control | 0.75 ** | 0.94 ** | 0.79 ** | ||
| Drought | 0.57 ** | 0.88 ** | 0.49 ** | |||
| SFW (g/plant) | Control | 0.71 ** | 0.95 ** | |||
| Drought | 0.43 * | 0.86 ** | ||||
| RDM (%) | Control | 0.80 ** | ||||
| Drought | 0.41 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukan, M.; Kereša, S.; Pejić, I.; Sudarić, A.; Lovrić, A.; Šarčević, H. Variability of Root and Shoot Traits under PEG-Induced Drought Stress at an Early Vegetative Growth Stage of Soybean. Agronomy 2024, 14, 1188. https://doi.org/10.3390/agronomy14061188
Bukan M, Kereša S, Pejić I, Sudarić A, Lovrić A, Šarčević H. Variability of Root and Shoot Traits under PEG-Induced Drought Stress at an Early Vegetative Growth Stage of Soybean. Agronomy. 2024; 14(6):1188. https://doi.org/10.3390/agronomy14061188
Chicago/Turabian StyleBukan, Miroslav, Snježana Kereša, Ivan Pejić, Aleksandra Sudarić, Ana Lovrić, and Hrvoje Šarčević. 2024. "Variability of Root and Shoot Traits under PEG-Induced Drought Stress at an Early Vegetative Growth Stage of Soybean" Agronomy 14, no. 6: 1188. https://doi.org/10.3390/agronomy14061188






