Comparative Study of Vermicomposting: Apple Pomace Alone and in Combination with Wheat Straw and Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Components of Vermicomposting with Eisenia fetida
2.2. Experiment Design
2.3. Physicochemical Analysis
2.4. Microbiological Analysis
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results
3.1. Changes in pH, Moisture, Dry Matter, Organic Matter, and Minerals
3.2. Changes in N, P, K, Ca, and Mg Levels during Vermicomposting
3.3. Changes in the Levels of Heavy Metals during Vermicomposting
3.4. Microbial Quality of the Resulting Vermicomposts
3.5. Sensory Evaluation of the Resulting Vermicomposts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lim, S.L.; Wu, T.Y. Characterization of matured vermicompost derived from valorization of palm oil mill by product. J. Agric. Food Chem. 2016, 64, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Silpa, K.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development Series; World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; Urban Development Series; Knowledge Papers No. 15; World Bank: Washington, DC, USA, 2012; Available online: http://openknowledge.worldbank.org/handle/10986/17388 (accessed on 1 March 2012).
- Al-Suhaibani, N.; Selim, M.; Alderfasi, A.; El-Hendawy, S.; Selim, M. Comparative performance of integrated nutrient management between composted agricultural wastes, chemical fertilizers, and biofertilizers in improving soil quantitative and qualitative properties and crop yields under arid conditions. Agronomy 2020, 10, 1503. [Google Scholar] [CrossRef]
- Abdollahi Saadatlu, I.; Barzinpour, F.; Yaghoubi, S. A sustainable model for municipal solid waste system considering global warming potential impact: A case study. Comput. Ind. Eng. 2022, 169, 108127. [Google Scholar] [CrossRef]
- Eghbali, H.; Arkat, J.; Tavakkoli-Moghaddam, R. Sustainable supply chain network design for municipal solid waste management: A case study. J. Clean. Prod. 2022, 381, 135211. [Google Scholar] [CrossRef]
- Eurostat. Municipal Waste Statistics. 2024. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics (accessed on 8 February 2024).
- European Environment Agency (EEA). Municipal Waste Generation and Population, Serbia. 2024. Available online: https://www.eea.europa.eu/en (accessed on 1 April 2024).
- Kaur, T. Vermicomposting: An Effective Option for Recycling Organic Wastes. Organic Agriculture; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Paul, J.A.; Karmegam, N.; Thilagavathy, D. Municipal solid waste (MSW) vermicomposting with an epigeic earthworm Perionyx ceylanensis Mich. Bioresour. Technol. 2011, 102, 6769–6773. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.; Suthar, S. Vermistabilization of paper mill wastewater sludge using Eisenia fetida. Bioresour. Technol. 2013, 128, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Chekwube, E.M.; Erasmus, M. Mediators of biomass transformation—A focus on the enzyme composition of the vermicomposting process. Environ. Chall. 2023, 12, 100732. [Google Scholar] [CrossRef]
- Edwards, C.A.; Subler, S. Human pathogen reduction during vermicomposting. In Vermiculture Technology; Edwards, C.A., Arancon, N.Q., Sherman, R., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 2011; pp. 249–261. [Google Scholar]
- Ahmed, N.; Al-Mutairi, K.A. Earthworms Effect on Microbial Population and Soil Fertility as Well as Their Interaction with Agriculture Practices. Sustainability 2022, 14, 7803. [Google Scholar] [CrossRef]
- Zhi, W.; Li, L.; Dong, W.; Brown, W.; Kaye, J.; Steefel, C.; Williams, K.H. Distinct Source Water Chemistry Shapes Contrasting Concentration-Discharge Patterns. Water Resour. Res. 2019, 55, 4233–4251. [Google Scholar] [CrossRef]
- Enebe, M.C.; Erasmus, M. Vermicomposting technology—A perspective on vermicompost production technologies, limitations and prospects. J. Environ. Manag. 2023, 345, 118585. [Google Scholar] [CrossRef]
- Demir, Z. Effects of Vermicompost on Soil Physicochemical Properties and Lettuce (Lactuca sativa Var. Crispa) Yield in Greenhouse under Different Soil Water Regimes. Commun. Soil Sci. Plant Anal. 2019, 50, 2151–2168. [Google Scholar] [CrossRef]
- Zziwa, A.; Jjagwe, J.; Kizito, S.; Kabenge, I.; Komakech, A.J.; Kayondo, H. Nutrient Recovery from Pineapple Waste through Controlled Batch and Continuous Vermicomposting Systems. J. Environ. Manag. 2021, 279, 117814. [Google Scholar] [CrossRef]
- Santana, N.A.; Jacques, R.J.S.; Antoniolli, Z.I.; Martínez-Cordeiro, H.; Domínguez, J. Changes in the chemical and biological characteristics of grape marc vermicompost during a two-year production period. Appl. Soil Ecol. 2020, 154, 103587. [Google Scholar] [CrossRef]
- Domínguez, J.; Aira, M.; Kolbe, A.R.; Gómez-Brandón, M.; Pérez-Losada, M. Changes in the composition and function of bacterial communities during vermicomposting may explain beneficial properties of vermicompost. Sci. Rep. 2019, 9, 9657. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Garg, V.K. Management of food and vegetable processing waste spiked with buffalo waste using earthworms (Eisenia fetida). Environ. Sci. Pollut. Res. 2017, 24, 7829–7836. [Google Scholar] [CrossRef] [PubMed]
- Nejatzadeh, F. Data on growth and production of (Aloe vera L.) treated by different levels of vermicompost and nitrogen fertilizer. Data Brief 2019, 22, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Stojanova, M. Plant Nutrition; Academic Press: Skopje, North Macedonia, 2018; ISBN 978-608-231-237-8. [Google Scholar]
- Stojanova, M. Grapevine Nutrition; Academic Thought: Skopje, North Macedonia, 2023; ISBN 978-608-5019-06-9. [Google Scholar]
- Rehman, S.; De Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermicompost: Enhancing Plant Growth and Combating Abiotic and Biotic Stress. Agronomy 2023, 13, 1134. [Google Scholar] [CrossRef]
- Monroy, F.; Aira, M.; Domínguez, J. Reduction of total coliform numbers during vermicomposting is caused by short-term direct effects of earthworms on microorganisms and depends on the dose of application of pig slurry. Sci. Total Environ. 2009, 407, 5411–5416. [Google Scholar] [CrossRef] [PubMed]
- Hénault-Ethier, L.; Martin, J.V.; Gélinas, Y. Persistence of Escherichia coli in batch and continuous vermicomposting systems. Waste Manag. 2016, 56, 88–99. [Google Scholar] [CrossRef]
- Aira, M.; Gómez-Brandón, M.; González-Porto, P.; Domínguez, J. Selective reduction of the pathogenic load of cow manure in an industrial-scale continuous-feeding vermireactor. Bioresour. Technol. 2011, 102, 9633–9637. [Google Scholar] [CrossRef]
- Mu, J.; Li, X.; Jiao, J.; Ji, G.; Wu, J.; Hu, F.; Li, H. Biocontrol potential of vermicompost through antifungal volatiles produced by indigenous bacteria. Biol. Control 2017, 112, 49–54. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, M.; Jiang, L.; Chen, X.; Griffiths, B.S.; Li, H.; Hu, F. Vermicompost increases defense against root-knot nematode (Meloidogyne incognita) in tomato plants. Appl. Soil Ecol. 2016, 105, 177–186. [Google Scholar] [CrossRef]
- Magazin, N.; Milić, B.; Keserović, Z. Production and assortment of apple in Serbia. Plant Dr. 2022, 50, 411–426. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Shalini, R.; Gupta, D.K. Utilization of Pomace from Apple Processing Industries: A Review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- SRPS ISO 10390:2007; Soil Quality—Determination of pH. Institute for Standardization of Serbia: Belgrade, Serbia, 2007.
- SRPS ISO 5984:2013; Animal Feeding Stuffs—Determination of Crude Ash. Institute for Standardization of Serbia: Belgrade, Serbia, 2013.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis—Part 3. Chemical Methods; Sparks, D.L., Ed.; SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- SRPS ISO 10694:2005; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). Institute for Standardization of Serbia: Belgrade, Serbia, 2005.
- SRPS ISO 11047:2004; Soil Quality—Determination of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc—Flame and Electrothermal Atomic Absorption Spectrometric Methods. Institute for Standardization of Serbia: Belgrade, Serbia, 2004.
- SRPS EN ISO 4833-1:2014/A1:2022; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique—Amendment 1: Clarification of Scope. Institute for Standardization of Serbia: Belgrade, Serbia, 2022.
- SRPS ISO 16649-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. Institute for Standardization of Serbia: Belgrade, Serbia, 2008.
- SRPS ISO 15213:2011; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Sulfite-Reducing Bacteria Growing under Anaerobic Conditions. Institute for Standardization of Serbia: Belgrade, Serbia, 2011.
- SRPS EN ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. Institute for Standardization of Serbia: Belgrade, Serbia, 2017.
- SRPS ISO 11290-1: 2017; Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 1: Detection Method. Institute for Standardization of Serbia: Belgrade, Serbia, 2017.
- SRPS ISO 6658:2018; Sensory Analysis—Methodology—General Guidance. Institute for Standardization of Serbia: Belgrade, Serbia, 2018.
- Vendruscolo, F.; Albuquerque, P.M.; Streit, F. Apple Pomace: A Versatile Substrate for Biotechnological Applications. Crit. Rev. Biotech. 2008, 28, 1–12. [Google Scholar] [CrossRef]
- Pirmohammadi, R.; Rouzbehan, Y.; Rezayazdi, K.; Zahedifar, M. Chemical composition, digestibility and in situ degradability of dried and ensiled apple pomace and maize silage. Small Rumin. Res. 2006, 66, 150–155. [Google Scholar] [CrossRef]
- Mignard, P.; Beguería, S.; Giménez, R.; Font Forcada, C.; Reig, G.; Moreno, M.Á. Effect of Genetics and Climate on Apple Sugars and Organic Acids Profiles. Agronomy 2022, 12, 827. [Google Scholar] [CrossRef]
- Filipović, A.; Mandić, A.; Hadziabulic, A.; Johanis, H.; Stipanovic, A.; Brekalo, H. Characterization and Evaluation of Vermicomposting Materials. Ekológia 2023, 42, 101–107. [Google Scholar] [CrossRef]
- Lee, L.H.; Wu, T.Y.; Shak, K.P.Y.; Lim, S.L.; Ng, K.Y.; Nguyen, M.N.; Teoh, W.H. Sustainable approach to biotransform industrial sludge into organic fertilizer via vermicomposting: A mini-review. J. Chem. Technol. Biotechnol. 2018, 93, 925–935. [Google Scholar] [CrossRef]
- Oyege, I.; Balaji Bhaskar, M.S. Effects of Vermicompost on Soil and Plant Health and Promoting Sustainable Agriculture. Soil Syst. 2023, 7, 101. [Google Scholar] [CrossRef]
- Vyas, P.; Sharma, S.; Gupta, J. Vermicomposting with microbial amendment: Implications for bioremediation of industrial and agricultural waste. BioTechnologia 2022, 103, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gołebiewska, E.; Zawadzka, M.; Choińska, R.; Koronkiewicz, K.; Piasecka-Jóźwiak, K.; Bujak, M. Sustainable extraction of bioactive compound from apple pomace through lactic acid bacteria (LAB) fermentation. Sci. Rep. 2023, 13, 19310. [Google Scholar] [CrossRef]
- Jung, J.; Cavender, G.; Zhao, Y. Impingement drying for preparing dried apple pomace flour and its fortification in bakery and meat products. J. Food Sci. Technol. 2015, 52, 5568–5578. [Google Scholar] [CrossRef] [PubMed]
- Kara, C.; Doymaz, I. Effective moisture dif fusivity determin ation and math-ematical modelling of drying curves of apple pomace. Heat Mass Transf. 2015, 51, 983–989. [Google Scholar] [CrossRef]
- Singh, J.; Singh, S.; Vig, A.P.; Kaur, A. Environmental Influence of Soil toward Effective Vermicomposting. Earthworms—The Ecological Engineers of Soil; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Rulebook on the Conditions for Classification and Determination of the Quality of Plant Nutrition Products, Deviations in the Content of Nutrients, and the Minimum and Maximum Values of Permitted Deviations in the Content of Nutrients, and on the Content of Declaration and the Method of Marking Plant Nutrition Products “Official Gazette of the Republic of Serbia”. No. 30 from 31 March 2017, No. 31 from 27 April 2018. (In Serbian). Available online: https://www.esiweb.org/ (accessed on 22 May 2024).
- Pizzanelli, S.; Calucci, L.; Forte, C.; Borsacchi, S. Studies of Organic Matter in Composting, Vermicomposting, and Anaerobic Digestion by 13C Solid-State NMR Spectroscopy. Appl. Sci. 2023, 13, 2900. [Google Scholar] [CrossRef]
- Khatua, C.; Sengupta, S.; Krishna Balla, V.; Kundu, B.; Chakraborti, A.; Tripathi, S. Dynamics of organic matter decomposition during vermicomposting of banana stem waste using Eisenia fetida. Waste Manag. 2018, 79, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, R.M.; Arancon, N.Q.; Edwards, C.A.; Metzger, J.D. The influence of earthworm-processed pig manure on the growth and productivity of marigolds. Bioresour. Technol. 2002, 81, 103–108. [Google Scholar] [CrossRef]
- Gajalakshmi, S.; Abbasi, S.A. Solid Waste Management by Composting: State of the Art. Environ. Sci. Technol. 2008, 38, 311–400. [Google Scholar] [CrossRef]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus 2012, 4, 26. [Google Scholar] [CrossRef]
- Hsu, J.H.; Lo, S.L. Effect of composting on characterization and leaching of cooper, manganese and zinc from swinw manure. Environ. Pollut. 2001, 114, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schuchardt, F.; Sheng, F.; Zhang, R.; Cao, Z. Assessment of maturity of vineyard pruning compost by Fourier Transform Infrared Spectroscopy, biological and chemical analyses. Landbauforsch. Völkenrode 2004, 54, 163–169. [Google Scholar]
- Chaudhuri, P.S.; Pal, T.K.; Bhattacharjee, G.; Dey, S.K. Chemical changes during vermicomposting (Perionyx excavatus) of Kitchen waste. Trop. Ecol. 2000, 41, 107–110. [Google Scholar]
- Waseem, M.A.; Giraddi, R.S.; Math, K.K. Assessment of nutrients and micro flora in vermicompost enriched with various organics. J. Exp. Zool. India 2013, 16, 697–703. [Google Scholar]
- Ramnarain, Y.I.; Ansari, A.A.; Ori, L. Vermicomposting of different organic materials using the epigeic earthworm Eisenia foetida. Int. J. Recycl. Org. Waste Agric. 2019, 8, 23–36. [Google Scholar] [CrossRef]
- Karapantzou, I.; Mitropoulou, G.; Prapa, I.; Papanikolaou, D.; Charovas, V.; Kourkoutas, Y. Physicochemical Changes and Microbiome Associations during Vermicomposting of Winery Waste. Sustainability 2023, 15, 7484. [Google Scholar] [CrossRef]
- Nath, G.; Singh, K.; Sing, D. Chemical analysis of vermicomposts/vermiwash of different combinations of animal, agro and kitchen wastes. Aust. J. Basic Appl. Sci. 2009, 3, 3671–3676. [Google Scholar]
- Khwairakpam, M.; Bhargava, R. Vermitechnology for sewage sludge recycling. J. Hazard. Mater. 2009, 161, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, K.; Ranganathan, L. Chemical characterization of mono and polycultured soil wormcasts by tropical earthworms. Environ. Ecol. 2000, 18, 742–746. [Google Scholar]
- Nsiah-Gyambibi, R.; Essandoh, H.M.; Asiedu, N.Y.; Fei-Baffoe, B. Valorization of fecal sludge stabilization via vermicomposting in microcosm enriched substrates using organic soils for vermicompost production. Heliyon 2021, 7, e06422. [Google Scholar] [CrossRef]
- Khan, M.B.; Cui, X.; Jilani, G.; Lazzat, U.; Zehra, A.; Hamid, Y.; Hussain, B.; Tang, L.; Yang, X.; He, Z. Eisenia fetida and biochar synergistically alleviate the heavy metals content during valorization of biosolids via enhancing vermicompost quality. Sci. Total Environ. 2019, 684, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 22 May 2024).
- Suthar, S.; Sajwan, P.; Kumar, K. Vermiremediation of heavy metals in wastewater sludge from paper and pulp industry using earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2014, 109, 177–184. [Google Scholar] [CrossRef]
- Yadav, A.; Garg, V.K. Feasibility of nutrient recovery from industrial sludge by vermicomposting technology. J. Hazard. Mater. 2009, 168, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, P.; Kaushik, C.P.; Garg, V.K. Vermicomposting of sugar industry waste (press-mud) mixed with cow dung employing an epigeic earthworm Eisenia fetida. Waste Manag. Res. 2010, 28, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.; Karmegam, N. Vermistabilisation of pressmud using Perionyx ceylanensis Mich. Bioresour. Technol. 2010, 101, 8464–8468. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S. Pilot-scale vermireactors for sewage sludge stabilization and metal remediation process: Comparison with small-scale vermireactors. Ecol. Eng. 2010, 36, 703–712. [Google Scholar] [CrossRef]
- Singh, K.A.; Vig, A.P.; Rup, P.J. Role of Eisenie foetida in rapid recycling of nutrients from bio sludge of beverage industry. Ecotoxicol. Environ. Saf. 2010, 73, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Usman, B.; Ahmad, A.; Jibrin, N.A.; Gaya, E.A.; Jibrin, M. Bioaccumulation and Human Health Risk of Heavy Metals from Pesticides in Some Crops Grown in Plateau State, Nigeria. Biol. Life Sci. Forum. 2021, 4, 12. [Google Scholar] [CrossRef]
- Vanisree, C.R.; Singh Sankhla, M.; Singh, P.; Jadhav, E.B.; Kumar Verma, R.; Kant Awasthi, K.; Awasthi, G.; Nagar, V. Heavy Metal Contamination of Food Crops: Transportation via Food Chain, Human Consumption, Toxicity and Management Strategies. Environmental Impact and Remediation of Heavy Metals; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Đukić, D.; Jemcev, V.T.; Mandić, L. The factors that regulate the composition of communities soil microorganisms. In Sanitary Microbiology of Soil; Faculty of Agronomy, Čačak: Čačak, Serbia, 2011; pp. 235–238. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Martínez-Cordeiro, H.; Domínguez, J. Changes in the nutrient dynamics and microbiological properties of grape Marc in a continuous-feeding vermicomposting system. Waste Manag. 2021, 135, 1–10. [Google Scholar] [CrossRef]
- Soobhany, N. Preliminary evaluation of pathogenic bacteria loading on organic Municipal Solid Waste compost and vermicompost. J. Environ. Manag. 2018, 206, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Hait, S.; Tare, V. Optimizing vermistabilization of waste activated sludge using vermicompost as bulking material. Waste Manag. 2011, 31, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Swati, A.; Hait, S.A. Comprehensive Review of the Fate of Pathogens during Vermicomposting of Organic Wastes. J. Environ. Qual. 2017, 47, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Karimi, H.; Mokhtari, M.; Salehi, F. Changes in microbial pathogen dynamics during vermicomposting mixture of cow manure–organic solid waste and cow manure–sewage sludge. Int. J. Recycl. Org. Waste Agric. 2017, 6, 57–61. [Google Scholar] [CrossRef]
- Du, Y.; Zou, W.; Zhang, K.; Ye, G.; Yang, J. Advances and applications of clostridium co-culture systems in biotechnology. Front. Microbiol. 2020, 11, 2842. [Google Scholar] [CrossRef] [PubMed]
- Harindintwali, J.D.; Wang, F.; Yang, W.; Zhou, J.; Muhoza, B.; Mugabowindekwe, M.; Yu, X. Harnessing the power of cellulolytic nitrogen-fixing bacteria for biovalorization of lignocellulosic biomass. Ind. Crops Prod. 2022, 186, 115235. [Google Scholar] [CrossRef]
- Anandyawati, A.; Muktamar, Z.; Prameswari, W.; Amrullah, A.H.K.; Anggoro, A. Comparison of the quality of animal manure compost conventional methods with vermicompost animal manure from Lumbricus rubellus. Intl. J. Agric. Technol. 2023, 19, 1435–1446. Available online: http://www.ijat-aatsea.com (accessed on 2 August 2023).
- Domínguez, J.J.; Edwards, C.A. Biology and ecology of earthworms species used for vermicomposting. In Vermiculture Technology: Earthworms, Organic Waste and Environmental Management; Edwards, C.A., Arancon, N.Q., Sherman, R.L., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 27–40. [Google Scholar]
- Riwandi, D.; Muktamar, Z.; Hasanudin, A.; Allsari, V. The quality of vermicompost from various sources composted with earthworm Perionyx excavates. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2nd International Conference on Organic Agriculture in the Tropics (ORGATROP), Virtually, 28–29 October 2021; IOP Publishing Ltd.: Bristol, UK, 2022; Volume 1005, p. 012006. [Google Scholar] [CrossRef]
- López, E.; Sainz, J. Gestión de Residuos Orgánicos de Uso Agrícola. Santiago de Compostela; Servizo de Publicación e Intercambio Científico: Galicia, Spain, 2011. [Google Scholar]
- Castillo-González, E.; Giraldi-Díaz, M.R.; De Medina-Salas, L.; Sánchez-Castillo, M.P. Pre-Composting and Vermicomposting of Pineapple (Ananas Comosus) and Vegetable Waste. Appl. Sci. 2019, 9, 3564. [Google Scholar] [CrossRef]
- Hanc, A.; Chadimova, Z. Nutrient recovery from apple pomace waste by vermicomposting technology. Bioresour. Technol. 2014, 168, 240–244. [Google Scholar] [CrossRef]
- Garg, V.K.; Suthar, S.; Yadav, A. Management of food industry waste employing vermicomposting technology. Bioresour. Technol. 2012, 126, 437–443. [Google Scholar] [CrossRef]



| Experiment/ Vermicomposting Materials | Days * | Physicochemical Properties, ± SD | ||||
|---|---|---|---|---|---|---|
| pH | Dry Matter, % | Moisture, % | Organic Matter, % | Minerals % | ||
| Experiment 1 Apple pomace, 60% + cattle manure, 40% | 0 | 5.41 Aa ± 0.28 | 60.93 Aa ± 0.06 | 39.07 Aa ± 0.06 | 77.16 Aa ± 0.76 | 22.84 Aa ± 0.76 |
| 60 | 6.91 Ba ± 0.13 | 61.20 Ba ± 0.20 | 38.80 Ba ± 0.20 | 76.20 Ba ± 1.25 | 23.80 Ba ± 1.25 | |
| 150 | 7.88 Ca ± 0.08 | 62.40 Ca ± 0.40 | 37.60 Ca ± 0.40 | 74.19 Ca ± 1.04 | 25.81 Ca ± 1.03 | |
| 240 | 7.63 Ca ± 0.15 | 64.30 Da ± 0.36 | 35.70 Da ± 0.35 | 58.44 Da ± 0.52 | 41.56 Da ± 0.75 | |
| Experiment 2 Wheat straw, 60% + cattle manure, 40% | 0 | 7.70 Ab ± 0.05 | 61.43 Ab ± 0.67 | 38.57 Ab ± 0.67 | 58.83 Ab ± 0.61 | 41.17 Ab ± 0.61 |
| 60 | 7.22 Ab ± 0.13 | 61.60 Aa ± 0.53 | 38.40 Aa ± 0.53 | 58.67 Ab ± 0.59 | 41.33 Ab ± 0.49 | |
| 150 | 8.46 Bb ± 0.06 | 63.16 Bb ± 0.36 | 36.84 Bb ± 0.46 | 57.35 Bb ± 0.75 | 42.65 Bb ± 0.75 | |
| 240 | 7.95 Cb ± 0.13 | 66.77 Cb ± 0.25 | 33.23 Cb ± 0.25 | 50.27 Cb ± 1.10 | 49.73 Cb ± 1.11 | |
| Experiment 3 Apple pomace, 80% + cattle manure, 10% + wheat straw, 10% | 0 | 5.91 Aa ± 0.12 | 60.63 Aa ± 0.25 | 39.37 Aa ± 0.25 | 66.96 Ac ± 0.21 | 33.04 Ac ± 0.21 |
| 60 | 6.81 Bc ± 0.10 | 60.86 Ab ± 0.12 | 39.14 Ab ± 0.12 | 66.10 Ac ± 0.26 | 33.90 Ac ± 0.26 | |
| 150 | 8.33 Cb ± 0.40 | 61.46 Ba ± 0.38 | 38.54 Ba ± 0.58 | 60.15 Bc ± 0.43 | 39.85 Bc ± 0.44 | |
| 240 | 7.36 Dc ± 0.15 | 63.26 Cc ± 0.55 | 36.74 Bc ± 0.55 | 49.07 Cc ± 0.91 | 50.93 Cc ± 1.68 | |
| Experiment 4 Apple pomace, 100% | 0 | 4.73 Ac ± 0.13 | 58.38 Ac ± 0.54 | 41.62 Ac ± 0.54 | 61.83 Ad ± 0.57 | 38.17 Ad ± 0.56 |
| 60 | 7.90 Bd ± 0.10 | 57.70 Bc ± 5.74 | 42.30 Ac ± 5.73 | 60.21 Bd ± 1.08 | 39.79 Bd ± 1.08 | |
| 150 | 7.23 Cc ± 0.15 | 62.33 Cc ± 0.68 | 37.67 Bc ± 0.17 | 48.93 Cd ± 0.83 | 51.07 Cd ± 0.87 | |
| 240 | 7.12 Cd ± 0.13 | 66.93 Db ± 0.85 | 33.07 Cb ± 0.85 | 40.17 Dd ± 0.95 | 59.83 Dd ± 0.95 | |
| Experiment/Vermicomposting Materials | Days * | ± SD) | ||||
|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | ||
| Experiment 1 Apple pomace, 60% + cattle manure, 40% | 0 | 2.31 Aa ± 0.01 | 0.87 Aa ± 0.02 | 0.47 Aa ± 0.03 | 4.32 Aa ± 0.03 | 1.07 Aa ± 0.03 |
| 60 | 2.29 Aa ± 0.01 | 0.89 Aa ± 0.01 | 0.52 Aa ± 0.03 | 4.41 Aa ± 0.04 | 1.08 Aa ± 0.02 | |
| 150 | 2.27 Aa ± 0.01 | 0.95 Aa ± 0.03 | 0.58 Ba ± 0.03 | 5.14 Ba ± 0.04 | 1.23 Ba ± 0.03 | |
| 240 | 2.83 Ba ± 0.02 | 1.33 Ba ± 0.03 | 0.69 Ca ± 0.47 | 5.38 Ba ± 0.03 | 1.44 Ba ± 0.06 | |
| Experiment 2 Wheat straw, 60% + cattle manure, 40% | 0 | 0.85 Ab ± 0.03 | 0.72 Ab ± 0.03 | 0.62 Ab ± 0.03 | 3.44 Ab ± 0.04 | 1.18 Ab ± 0.03 |
| 60 | 0.95 Ab ± 0.01 | 0.76 Ab ± 0.01 | 0.67 Bb ± 0.03 | 3.51 Ab ± 0.01 | 1.20 Ab ± 0.02 | |
| 150 | 1.72 Bb ± 0.03 | 0.88 Bb ± 0.02 | 0.72 Cb ± 0.03 | 3.85 Bb ± 0.05 | 1.46 Bb ± 0.04 | |
| 240 | 2.21 Cb ± 0.04 | 0.96 Cb ± 0.03 | 0.97 Db ± 0.02 | 4.27 Cb ± 0.03 | 1.78 Cb ± 0.03 | |
| Experiment 3 Apple pomace, 80% + cattle manure, 10% + wheat straw, 10% | 0 | 1.79 Ac ± 0.01 | 0.75 Aa ± 0.00 | 0.64 Ab ± 0.04 | 3.73 Ac ± 0.03 | 1.08 Aa ± 0.03 |
| 60 | 1.82 Ac ± 0.01 | 0.78 Aa ± 0.03 | 0.66 Ab ± 0.05 | 3.82 Ac ± 0.03 | 1.17 Ab ± 0.03 | |
| 150 | 1.88 Ab ± 0.02 | 0.96 Bb ± 0.01 | 0.74 Ab ± 0.04 | 4.06 Bc ± 0.04 | 1.42 Bb ± 0.03 | |
| 240 | 2.14 Bb ± 0.04 | 1.07 Ca ± 0.03 | 0.96 Cb ± 0.02 | 4.22 Bb ± 0.03 | 1.86 Cc ± 0.02 | |
| Experiment 4 Apple pomace, 100% | 0 | 1.52 Ad ± 0.03 | 0.51 Ac ± 0.01 | 0.38 Ac ± 0.03 | 3.28 Ad ± 0.03 | 1.39 Ac ± 0.14 |
| 60 | 1.57 Ad ± 0.02 | 0.79 Ba ± 0.01 | 0.42 Ac ± 0.03 | 3.42 Aa ± 0.03 | 1.43 Ac ± 0.07 | |
| 150 | 1.85 Bb ± 0.02 | 0.84 Bb ± 0.01 | 0.68 Bc ± 0.03 | 3.60 Bd ± 0.01 | 1.40 Ab ± 0.02 | |
| 240 | 1.97 Cc ± 0.02 | 0.98 Cb ± 0.03 | 0.93 Cb ± 0.03 | 3.89 Cc ± 0.02 | 1.64 Bd± 0.03 | |
| Experiment/Vermicomposting Materials | Days * | ± SD | |||
|---|---|---|---|---|---|
| Zn | Cu | Cd | Pb | ||
| Experiment 1 Apple pomace, 60% + cattle manure, 40% | 0 | 74.58 Aa ± 0.42 | 26.17 Aa ± 0.15 | 0.28 Aa ± 0.02 | 3.04 Aa ± 0.05 |
| 60 | 77.03 Ba ± 0.85 | 25.23 Aa ± 0.32 | 0.35 Ba ± 0.01 | 3.15 Aa ± 0.05 | |
| 150 | 98.18 Ca ± 0.55 | 28.28 Ba ± 0.20 | 0.37 Ba ± 0.01 | 3.57 Ba ± 0.75 | |
| 240 | 112.22 Da ± 1.49 | 38.12 Ca ± 0.13 | 0.39 Ba ± 0.01 | 5.18 Ca ± 0.03 | |
| Experiment 2 Wheat straw, 60% + cattle manure, 40% | 0 | 70.23 Ab ± 0.15 | 19.73 Ab ± 0.25 | 0.21 Ab ± 0.01 | 5.13 Ab ± 0.03 |
| 60 | 75.37 Bb ± 0.47 | 20.55 Ab ± 0.13 | 0.26 Ab ± 0.01 | 5.50 Bb ± 0.01 | |
| 150 | 91.30 Cb ± 0.20 | 23.32 Bb ± 0.12 | 0.31 Bb ± 0.01 | 7.92 Cb ± 0.01 | |
| 240 | 99.60 Db ± 0.26 | 25.55 Cb ± 0.09 | 0.40 Ca ± 0.01 | 11.57 Db ± 0.57 | |
| Experiment 3 Apple pomace, 80% + cattle manure, 10% + wheat straw, 10% | 0 | 83.48 Ac ± 0.48 | 21.53 Ac ± 0.33 | 0.32 Ac ± 0.00 | 5.50 Ac ± 0.01 |
| 60 | 84.25 Ba ± 0.25 | 21.38 Ac ± 0.40 | 0.35 Ba ± 0.00 | 6.42 Bc ± 0.03 | |
| 150 | 99.31 Cb ± 0.71 | 25.60 Bc ± 0.52 | 0.37 Ca ± 0.00 | 9.22 Cc ± 0.03 | |
| 240 | 102.05 Dc ± 0.22 | 30.43 Cc ± 0.21 | 0.42 Db ± 0.00 | 11.74 Db ± 0.07 | |
| Experiment 4 Apple pomace, 100% | 0 | 80.03 Ad ± 0.06 | 20.14 Ac ± 0.12 | 0.35 Ad ± 0.01 | 5.88 Ad ± 0.03 |
| 60 | 80.85 Ac ± 0.30 | 20.74 Ab ± 0.05 | 0.38 Bc ± 0.00 | 6.32 Bd ± 0.11 | |
| 150 | 91.66 Bc ± 1.16 | 23.37 Bc ± 0.37 | 0.41 Cc ± 0.01 | 8.34 Cd ± 0.04 | |
| 240 | 92.17 Bd ± 0.08 | 25.17 Cb ± 0.31 | 0.45 Dc ± 0.01 | 9.80 Dc ± 0.01 | |
| Experiment/Vermicomposting Materials | Days * | ± SD) | ||||
|---|---|---|---|---|---|---|
| Aerobic Mesophilic Bacteria | Salmonella sp. | Listeria monocytogenes | Escherichia coli | Sulfite-Reducing Clostridia | ||
| Experiment 1 Apple pomace, 60% + cattle manure, 40% | 0 | 1.9 × 109 ± 0.82 aA | nd | nd | 2.8 × 103 ± 0.95 aA | 1.0 × 103 ± 0.91 aA |
| 60 | 5.3 × 108 ± 4.00 bA | nd | nd | 1.7 × 103 ± 0.75 aA | <102 bA | |
| 150 | 2.5 × 107 ± 2.75 cA | nd | nd | 3.8 × 102 ± 3.62 bA | <102 bA | |
| 240 | 2.9 × 105 ± 2.00 dA | nd | nd | nd | <102 bA | |
| Experiment 2 Wheat straw, 60% + cattle manure, 40% | 0 | 1.1 × 109 ± 1.21 aA | nd | nd | 2.2 × 105 ± 0.79 aB | 3.0 × 103 ± 1.05 aB |
| 60 | 6.5 × 107 ± 0.92 bB | nd | nd | 1.3 × 102 ± 0.96 bB | <102 bA | |
| 150 | 2.4 × 107 ± 2.07 cA | nd | nd | <102 cB | <102 bA | |
| 240 | 1.8 × 105 ± 0.29 dB | nd | nd | nd | <102 bA | |
| Experiment 3 Apple pomace, 80% + cattle manure, 10% + wheat straw, 10% | 0 | 1.0 × 109 ± 2.31 aA | nd | nd | 6.2 × 103 ± 0.99 aC | <102 C |
| 60 | 7.8 × 107 ± 1.14 bC | nd | nd | 3.0 × 102 ± 1.30 bC | <102 B | |
| 150 | 2.0 × 107 ± 0.89 cB | nd | nd | <102 cB | <102 B | |
| 240 | 1.2 × 105 ± 0.37 dC | nd | nd | nd | <102 B | |
| Experiment 4 Apple pomace, 100% | 0 | 5.0 × 107 ± 1.19 aB | nd | nd | 6.0 × 103 ± 2.07 aC | <10 D |
| 60 | 3.5 × 107 ± 0.60 bD | nd | nd | 5.2 × 102 ± 1.76 bC | <10 C | |
| 150 | 2.2 × 106 ± 3.15 cC | nd | nd | nd | <10 C | |
| 240 | 3.3 × 104 ± 1.10 dD | nd | nd | nd | <10 C | |
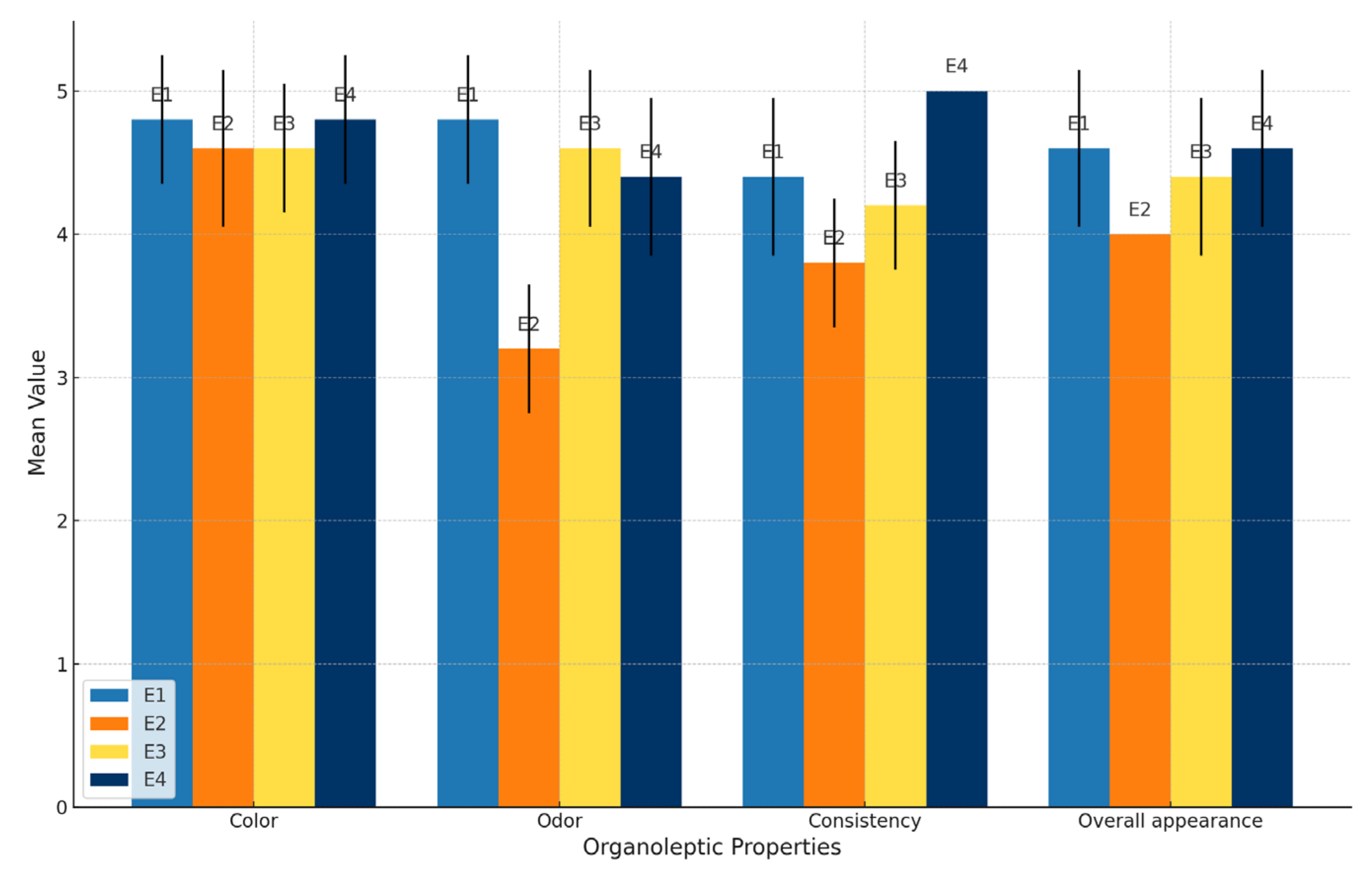
| Organoleptic Properties | E1 | E2 | E3 | E4 |
|---|---|---|---|---|
| ± SD | ± SD | ± SD | ± SD | |
| Color | 4.8 a ± 0.45 | 4.6 b ± 0.55 | 4.6 b ± 0.45 | 4.8 a ± 0.45 |
| Odor | 4.8 a ± 0.45 | 3.2 b ± 0.45 | 4.6 c ± 0.55 | 4.4 d ± 0.55 |
| Consistency | 4.4 a ± 0.55 | 3.8 b ± 0.45 | 4.2 c ± 0.45 | 5 d ± 0.0 |
| Overall appearance | 4.6 a ± 0.55 | 4 b ± 0.0 | 4.4 c ± 0.55 | 4.6 a ± 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kureljušić, J.M.; Vesković Moračanin, S.M.; Đukić, D.A.; Mandić, L.; Đurović, V.; Kureljušić, B.I.; Stojanova, M.T. Comparative Study of Vermicomposting: Apple Pomace Alone and in Combination with Wheat Straw and Manure. Agronomy 2024, 14, 1189. https://doi.org/10.3390/agronomy14061189
Kureljušić JM, Vesković Moračanin SM, Đukić DA, Mandić L, Đurović V, Kureljušić BI, Stojanova MT. Comparative Study of Vermicomposting: Apple Pomace Alone and in Combination with Wheat Straw and Manure. Agronomy. 2024; 14(6):1189. https://doi.org/10.3390/agronomy14061189
Chicago/Turabian StyleKureljušić, Jasna M., Slavica M. Vesković Moračanin, Dragutin A. Đukić, Leka Mandić, Vesna Đurović, Branislav I. Kureljušić, and Marina T. Stojanova. 2024. "Comparative Study of Vermicomposting: Apple Pomace Alone and in Combination with Wheat Straw and Manure" Agronomy 14, no. 6: 1189. https://doi.org/10.3390/agronomy14061189






