Effects of Salt Water on Growth and Quality of Raphanus sativus L. and Physiological Responses against Salt Stress
Abstract
1. Introduction
2. Materials and Methods
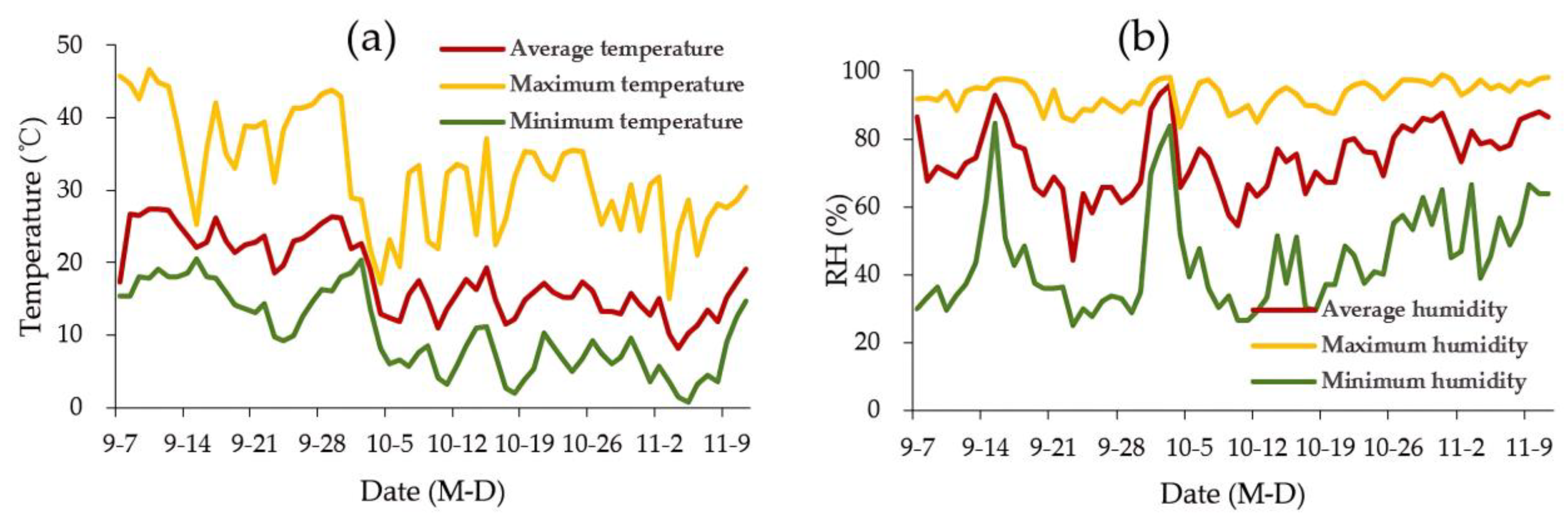
2.1. Temperature and Humidity in the Greenhouse
2.2. Experiment Design and Planting Plots
2.3. Measurement of Growth and Quality Parameters
2.3.1. Growth Indexes
2.3.2. Quality Indicators
2.3.3. Other Indicators
2.4. Data Collection and Analyses
3. Results
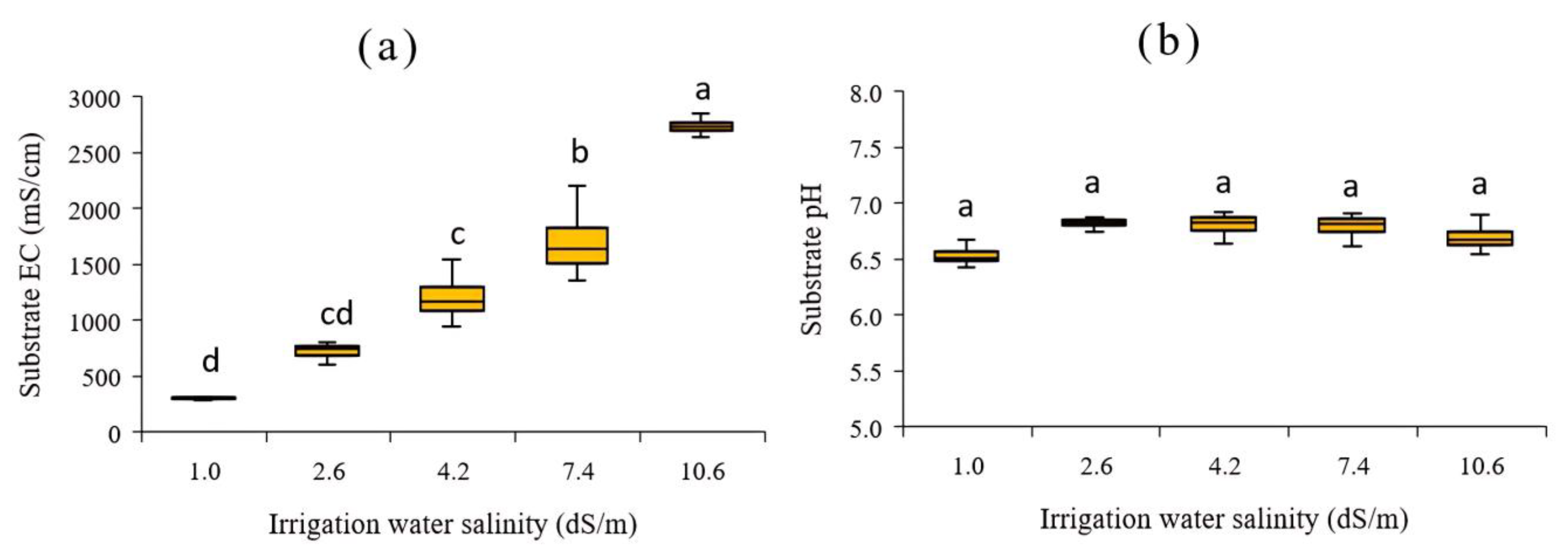
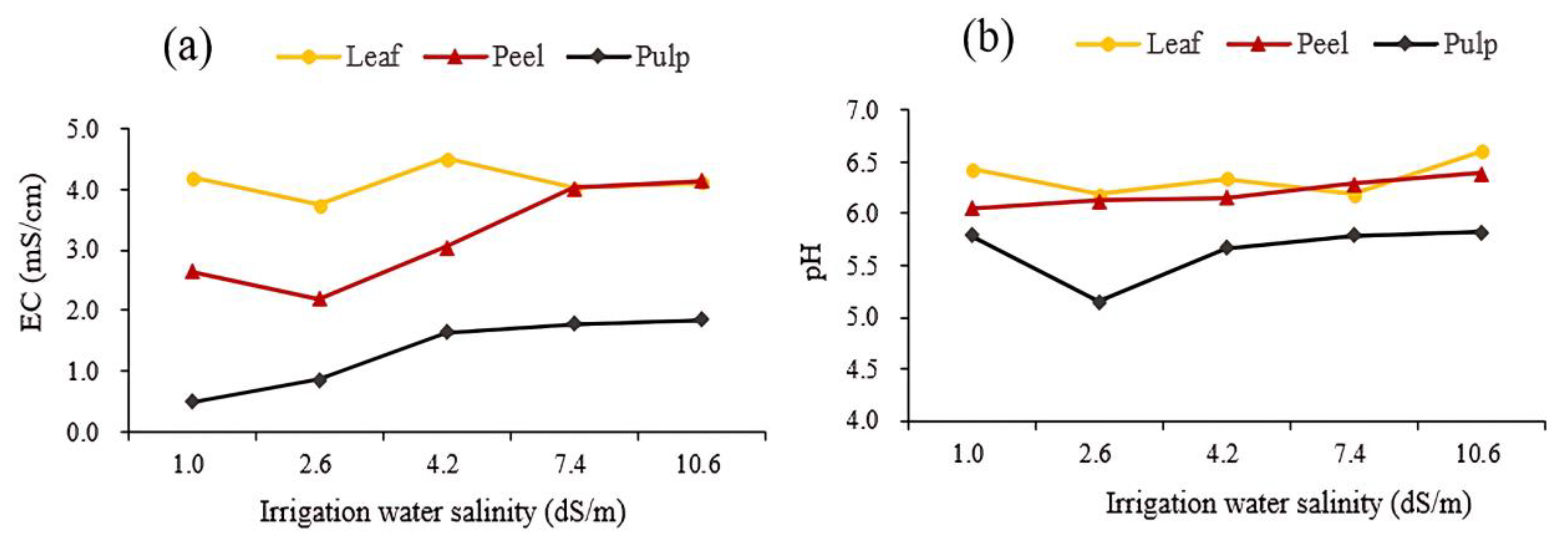
3.1. Effect of Different Salinity Levels in Irrigation Water on EC and pH Values of Substrate
3.2. Effect of Different Salinity Levels in Irrigation Water on the Growth of Fruit Radish
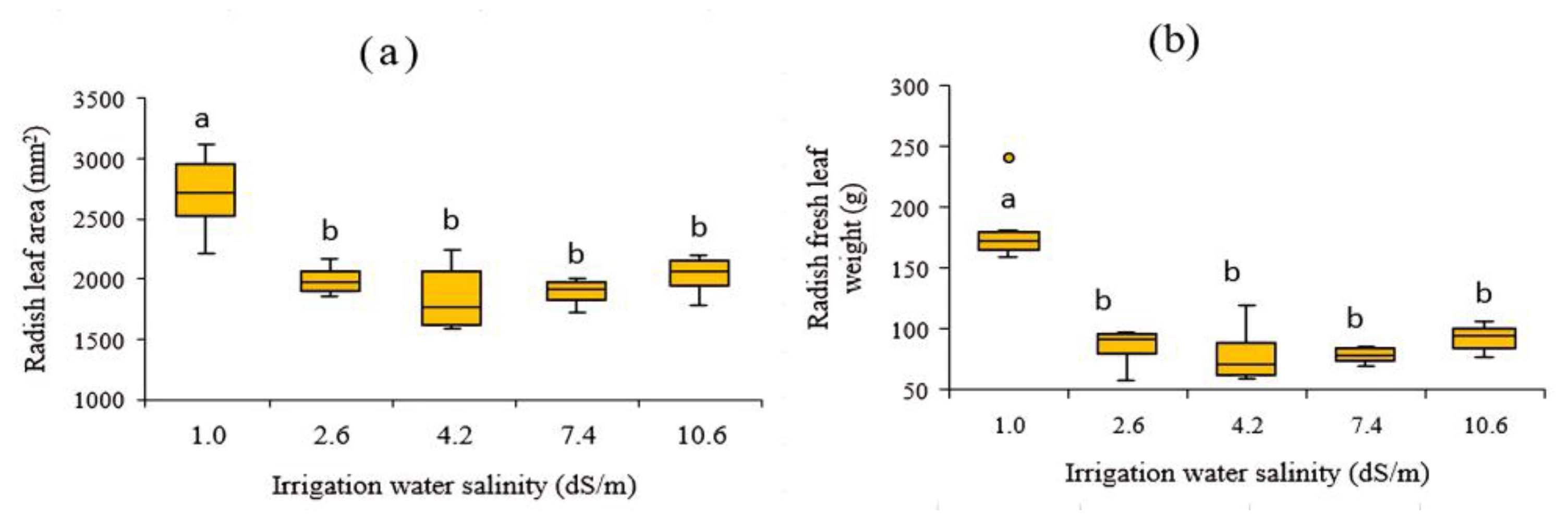
3.2.1. Effect of Different Salinity Levels in Irrigation Water on the Growth of Stems and Leaves
3.2.2. Effect of Different Salinity Levels in Irrigation Water on the Growth of Fleshy Roots of Fruit Radish
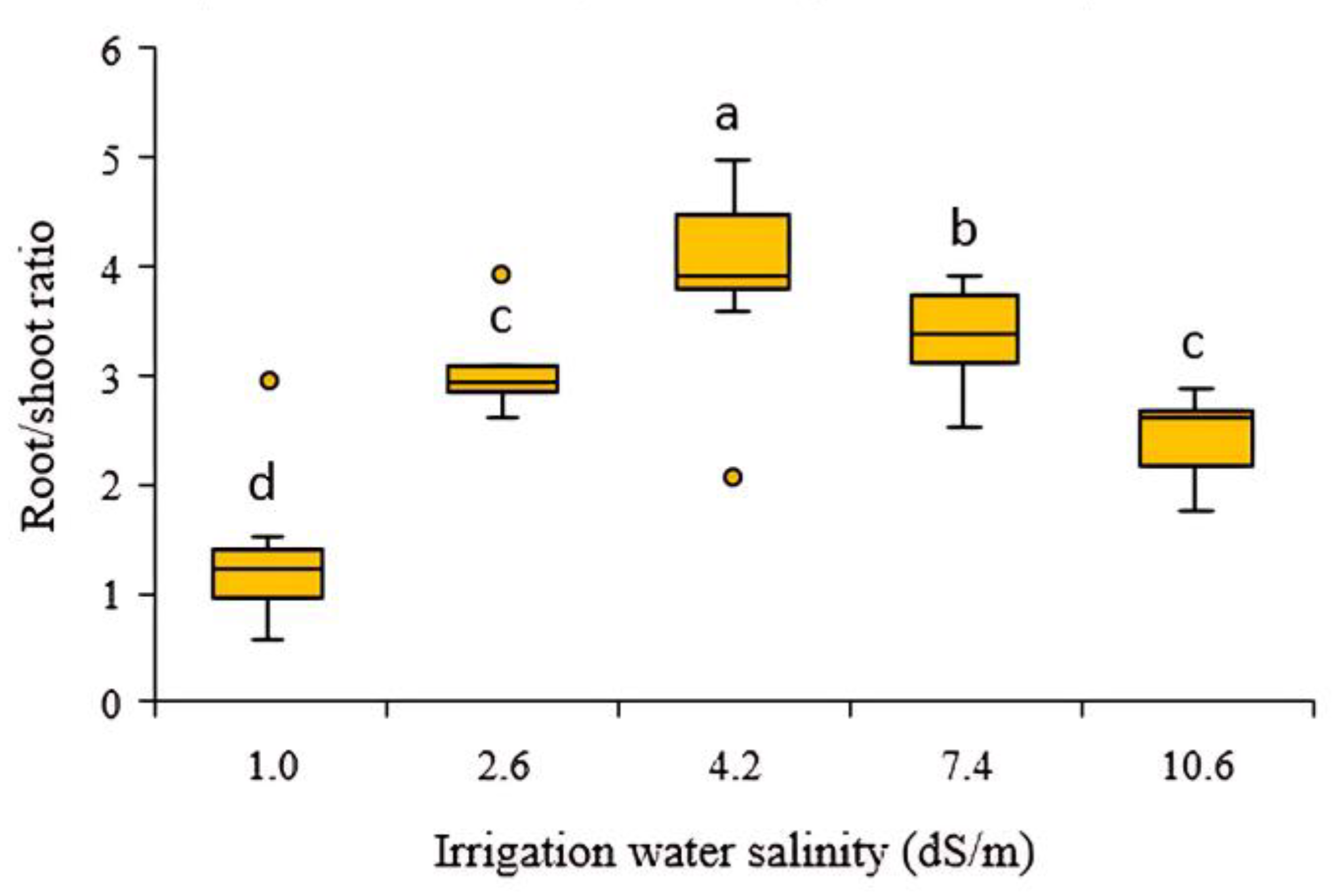
3.2.3. Effect of Different Salinity Levels in Irrigation Water on Root/Shoot Ratio of Fruit Radish
3.3. Effect of Different Salinity Levels in Irrigation Water on EC and pH Value of Fruit Radishes
3.4. Effect of Different Salinity Levels in Irrigation Water on Quality of Fruit Radish
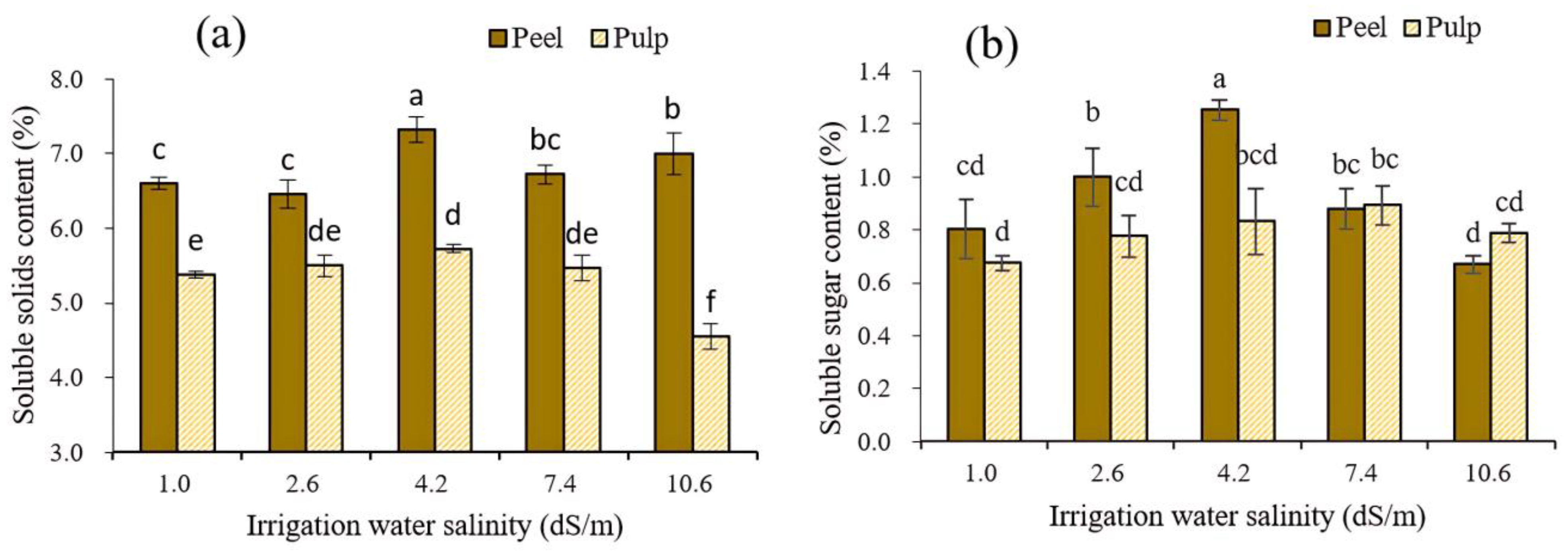
3.4.1. Effect on the Soluble Solids and Soluble Sugar Content
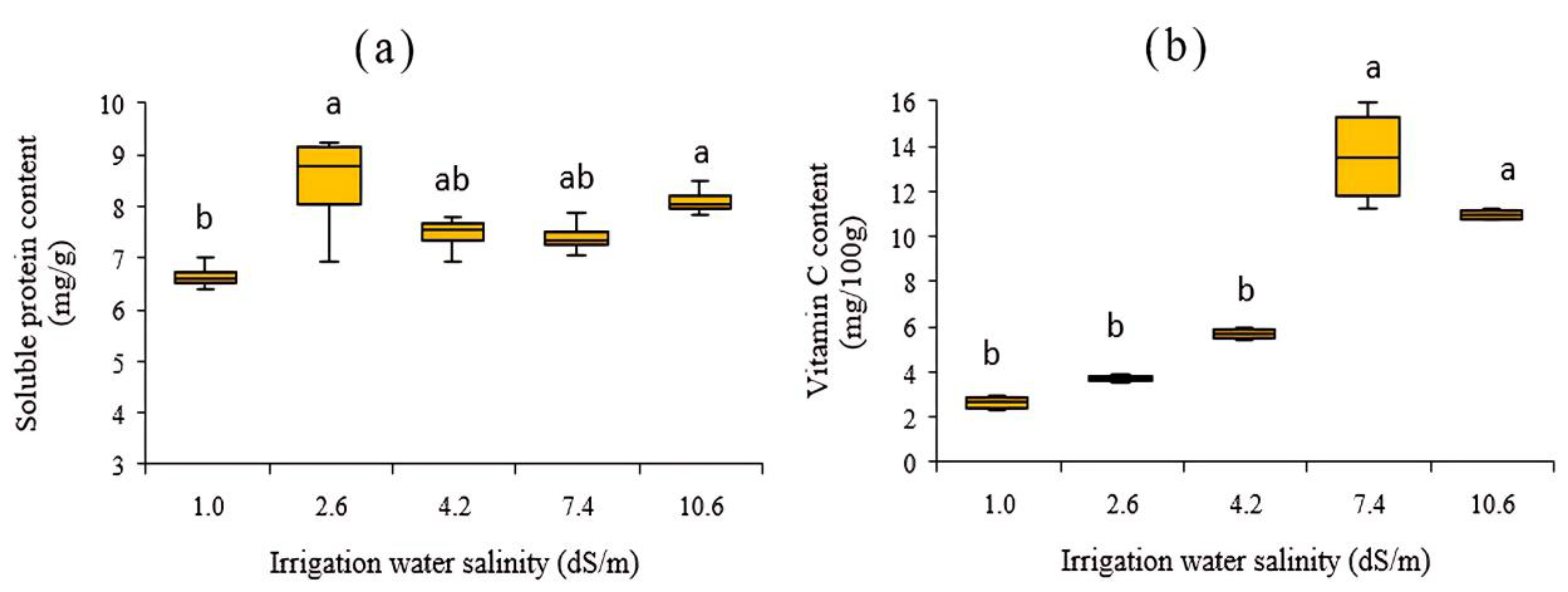
3.4.2. Effects on Soluble Protein and Vitamin C Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Metternicht, G.I.; Zinck, J.A. Remote sensing of soil salinity: Potentials and constraints. Remote Sens. Environ. 2003, 85, 1–20. [Google Scholar] [CrossRef]
- Yang, J.; Yao, R.; Wang, X.; Xie, W.; Zhang, X.; Zhu, W.; Zhang, L.; Sun, R. Research on salt-affected soils in China: History, status quo and prospect. Acta Pedol. Sin. 2022, 59, 10–27. [Google Scholar]
- Andrea, G.; Rami, M. The advantages of NF desalination of brackish water for sustainable irrigation: The case of the Arava Valley in Israel. Desalination Water Treat. 2009, 10, 101–107. [Google Scholar]
- Alomar, R.; Jena, D. Potassium and Brackish Water Irrigation Influence Electrical Conductivity, Potassium Dynamic, and Maize Yield in Coastal Saline Soil. Commun. Soil Sci. Plant Anal. 2024, 55, 723–734. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, P. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Abid, M.; Ghafoor, A.; Iqbal, M.M. Brackish Water for Irrigation: III Effects on Yields of Crops in Wheat-Rice Rotation and Properties of the Bhalike Soil Series. Asian J. Plant Sci. 2003, 2, 661. [Google Scholar]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 487808. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.; Zhou, G. Integrated approaches for increasing plant yield under salt stress. Front. Plant Sci. 2023, 14, 1215343. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; da Silva Messias, R.; Perin, E.C.; Borowski, J.M.; Bamberg, A.L.; Rombaldi, C.V. Mild salt stress improves strawberry fruit quality. LWT 2016, 73, 693–699. [Google Scholar] [CrossRef]
- Qi, L.; Cao, B.; Liu, W.; LI, Z.; Pang, Y.; Mao, P.; Guo, L. Effects of salt stress on seedlings growth, utilization and distribution characteristics of 15N and 13C in Robinia pseudoacacia. J. Shandong Univ. (Nat. Sci.) 2022, 3, 10–19. [Google Scholar]
- Ouyang, Z.; Tian, J.; Yan, X. Effects of Mineralization Degree of Irrigation Water on Yield, Fruit Quality, and Soil Microbial and Enzyme Activities of Cucumbers in Greenhouse Drip Irrigation. Horticulturae 2024, 10, 3390. [Google Scholar] [CrossRef]
- Akrami, M.; Arzani, A. Physiological alterations due to field salinity stress in melon (Cucumis melo L.). Acta Physiol. Plant. 2018, 40, 1–14. [Google Scholar] [CrossRef]
- Gong, Z.; Xin, J.; Shang, X.; Guo, X.M.; Wang, Y.P. Photosynthetic characteristics and stress tolerance physiology of Toona sinensis seedlings under saline-alkali stress. Acta Bot. Boreali-Occident. Sin. 2021, 41, 1199–1209. [Google Scholar]
- Yao, L.; Han, H. Effect of salt stress on growth and index of physiology and biochemical of Paphanus sativus L. var. radculus pers. North. Hortic. 2016, 13, 5–8. [Google Scholar]
- Šmídová, Z.; Izzo, R. Improvement of nutritional value of tomatoes under salt stress conditions. Czech J. Food Sci. 2009, 27, S138–S139. [Google Scholar] [CrossRef]
- Moles, T.M.; de Brito Francisco, R.; Mariotti, L.; Pompeiano, A.; Lupini, A.; Incrocci, L.; Carmassi, G.; Scartazza, A.; Pistelli, L.; Guglielminetti, L.; et al. Salinity in autumn-winter season and fruit quality of tomato landraces. Front. Plant Sci. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Gerald, S.; Hartmut, F.; Liu, Y.H. Peat substrates are a prerequisite for sustainable horticulture in Europe. Humic Acid 2002, 4, 38–42. [Google Scholar]
- Chen, K.; Liu, W.; Feng, D.; Zhu, H.; Zhang, J. Effects of soil water regulation on growth and yield of fruit radish under drip irrigation in greenhouse. J. Irrig. Drain. 2023, 12, 22–27. [Google Scholar]
- Chen, Y. Plant Physiology Experiment Guidance; China Agriculture Press: Beijing, China, 2022. [Google Scholar]
- Saqib, M.; Akhtar, J.; Qureshi, R.H.; Aslam, M.; Nawaz, S. Effect of salinity and sodicity on growth and ionic relations of different wheat genotypes. Pak. J. Soil Sci. 2000, 18, 1. [Google Scholar]
- Watanabe, K.; Takaragawa, H.; Ueno, M.; Kawamitsu, Y. Changes in agronomic and physiological traits of sugarcane grown with saline irrigation water. Agronomy 2020, 10, 722. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D.S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Chartzoulakis, K.S.; Therios, I.N.; Misopolinos, N.D.; Noitsakis, B.I. Growth, ion content and photosynthetic performance of salt-stressed kiwifruit plants. Irrig. Sci. 1995, 16, 23–28. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, W.; Liu, T.; Liu, B.; Wang, Y. Effects of salt stress on the leaf growth and physiological indexes of sugar beet. J. Hebei Agric. Sci. 2011, 15, 11–14. [Google Scholar]
- Shi, Y.; Ma, J.; Li, X. Effect of NaCl forces of different density on electrical conductivity and proline content of sorghum seedling. J. Shanxi Agric. Sci. 2007, 11, 28–30. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zang, Y. Current Progress of Na+ Coping Strategies in the Roots of Glycophytic Plants. Plant Physiol. J. 2011, 47, 1025–1032. [Google Scholar]
- Ning, L.H.; Du, W.K.; Song, H.N.; Shao, H.B.; Qi, W.C.; Sheteiwy, M.S.; Yu, D.Y. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Environ. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdel-Fattah, G.G.; Holford, P.; Korany, S.M.; Alsherif, E.A.; AbdElgawad, H.; Ulhassan, Z.; Jośko, I.; Ali, B.; Sheteiwy, M.S. Funneliformis constrictum modulates polyamine metabolism to enhance tolerance of Zea mays L. to salinity. Microbiol. Res. 2023, 266, 12725. [Google Scholar]
- Oliveira, C.E.D.S.; Zoz, T.; Seron, C.D.C.; Boleta, E.H.M.; Lima, B.H.D.; Souza, L.R.R.; Pedeinho, D.R.; Matias, R.; Lopes, C.d.S.; Neto, S.S.d.O.; et al. Can saline irrigation improve the quality of tomato fruits? Agron. J. 2022, 114, 900–914. [Google Scholar] [CrossRef]
- Hong, H.T.K.; Trang, P.T.H.; Ho, T.T.; Dang, J.; Sato, R.; Yoshida, K.; Silaguntsuti, P.; Agarie, S. Reproductive growth characteristics of Mesembryanthemum crystallinum L. in High-Salinity stress conditions. Sci. Hortic. 2024, 331, 113172. [Google Scholar] [CrossRef]
- Gugliuzza, G.; Gentile, C.; Scuderi, D.; Palazzolo, E.; Farina, V. Effects of salt stress on growth of Quercus ilex L. seedlings. Open Agric. 2023, 8, 20220211. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Gao, B.; Song, J.; Liu, J.P.; Sui, N.; Fan, H.; Wang, B. Effects of salt stress on photosynthesis and ion accumulation patterns of Suaeda salsa under different habitats. Chin. J. Plant Ecol. 2010, 34, 671–677. [Google Scholar]
- Li, Y.; Chu, Y.; Yao, K.; Shi, C.; Deng, X.; Lin, J. Response of sugar metabolism in the cotyledons and roots of Ricinus communis subjected to salt stress. Plant Biol. 2023, 25, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Zhao, L.; Zhou, M.; Zhang, L.; Wang, G.; Jia, J. Effects of salt stress on nutritional quality of orange-heading Chinese cabbage seedlings. Pak. J. Bot. 2023, 55, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, W.; Huang, W.; Biao, A.; Lin, S.; Wang, Y.; Yan, S.; Zeng, S. Salt stress affects the fruit quality of Lycium ruthenicum Murr. Ind. Crops Prod. 2023, 193, 116240. [Google Scholar] [CrossRef]
- Nawaz, K.; Hussain, K.; Majeed, A.; Khan, F.; Afghan, S.; Ali, K. Fatality of salt stress to plants, morphological, physiological and biochemical aspects. Afr. J. Biotechnol. 2010, 9, 5475–5480. [Google Scholar]

| Irrigation Water Salinity (dS/m) | Ion Concentration (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| Na+ | Ca2+ | Mg2+ | K+ | Cl− | HCO3− | SO42− | |
| 1.0 | 53 | 54.4 | 45.2 | 5.5 | 104.2 | 311.1 | 44.3 |
| 2.6 | 431.4 | 60.1 | 46.1 | 10 | 683.9 | 316 | 62.6 |
| 4.2 | 809.8 | 65.8 | 47 | 14.5 | 1263.6 | 320.9 | 80.9 |
| 7.4 | 1566.6 | 77.2 | 48.8 | 23.5 | 2423 | 330.7 | 117.5 |
| 10.6 | 2323.4 | 88.6 | 50.6 | 32.5 | 3582.4 | 340.5 | 154.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Liu, M.; Xu, H.; Feng, D.; Sun, X. Effects of Salt Water on Growth and Quality of Raphanus sativus L. and Physiological Responses against Salt Stress. Agronomy 2024, 14, 1190. https://doi.org/10.3390/agronomy14061190
Zhu H, Liu M, Xu H, Feng D, Sun X. Effects of Salt Water on Growth and Quality of Raphanus sativus L. and Physiological Responses against Salt Stress. Agronomy. 2024; 14(6):1190. https://doi.org/10.3390/agronomy14061190
Chicago/Turabian StyleZhu, Haiyan, Mingyu Liu, Haoyi Xu, Di Feng, and Xiaoan Sun. 2024. "Effects of Salt Water on Growth and Quality of Raphanus sativus L. and Physiological Responses against Salt Stress" Agronomy 14, no. 6: 1190. https://doi.org/10.3390/agronomy14061190
APA StyleZhu, H., Liu, M., Xu, H., Feng, D., & Sun, X. (2024). Effects of Salt Water on Growth and Quality of Raphanus sativus L. and Physiological Responses against Salt Stress. Agronomy, 14(6), 1190. https://doi.org/10.3390/agronomy14061190







