Low-Cadmium Wheat Cultivars Limit the Enrichment, Transport and Accumulation of Cadmium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection and Preparation
2.3. Measurement of Sample Indicators
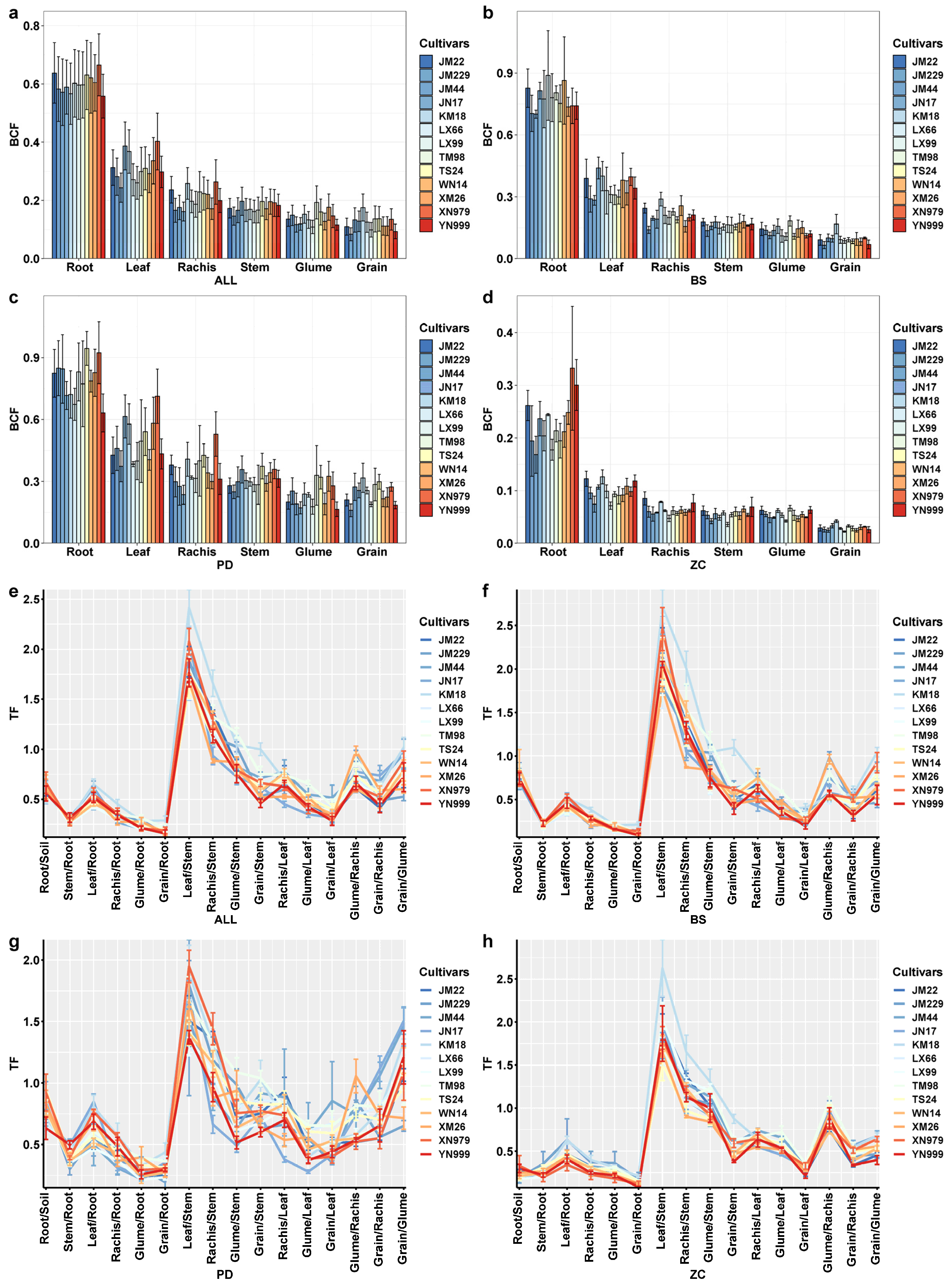
2.4. Bioaccumulation Factors (BCFs) and Transfer Factors (TFs)
2.5. Data Analysis
3. Results
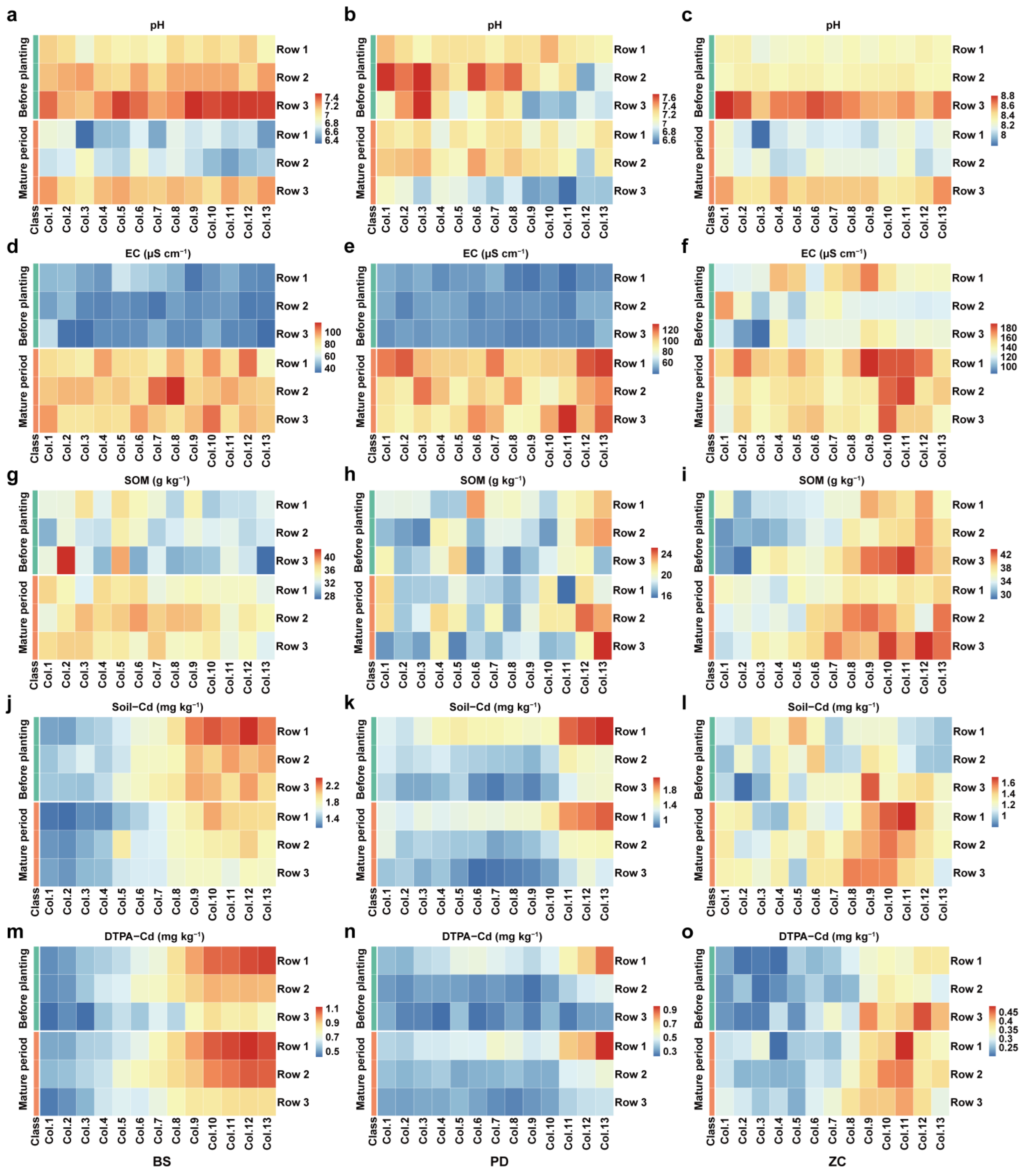
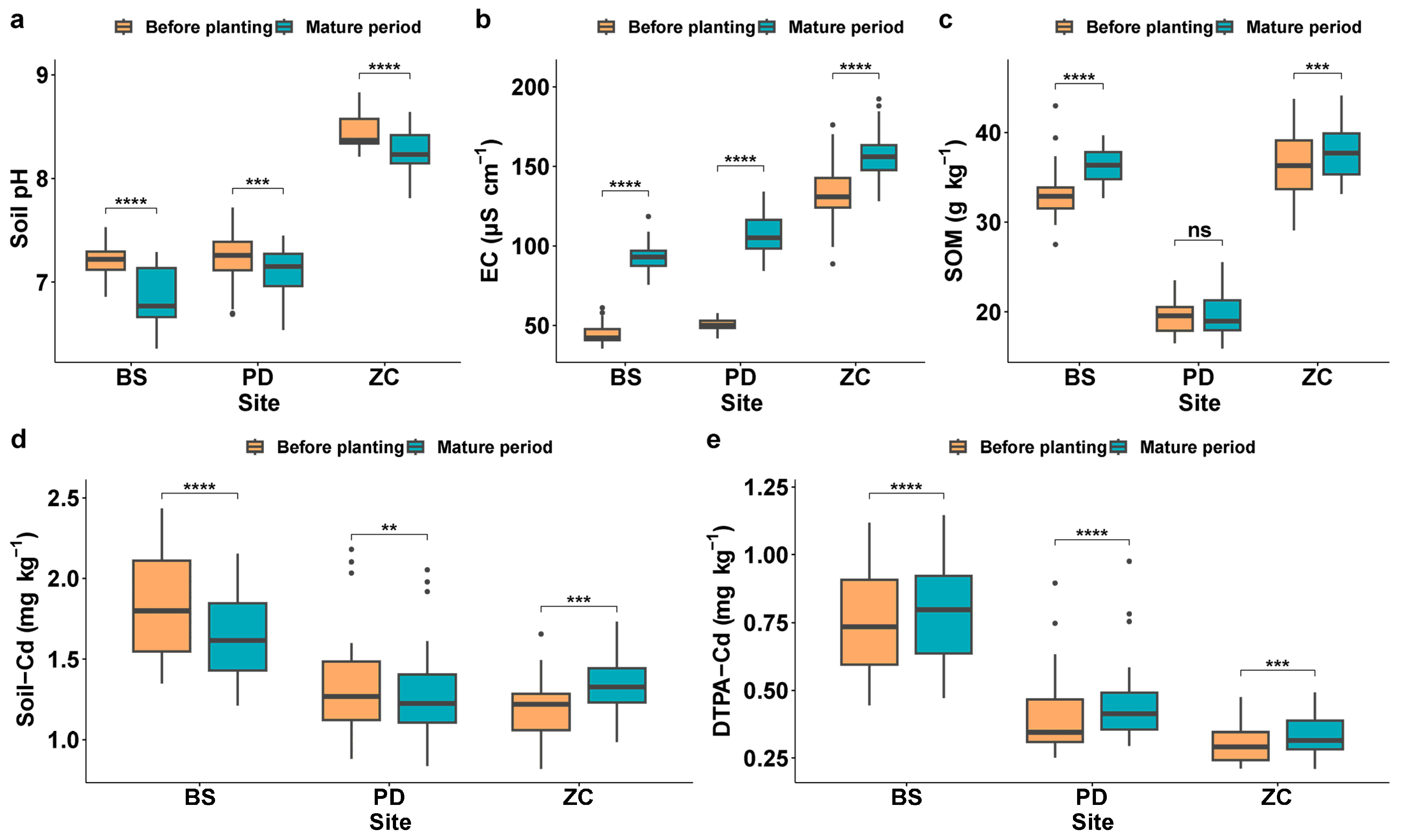
3.1. Differences in Soil Properties before and after Planting of LAWC-Cd Planting
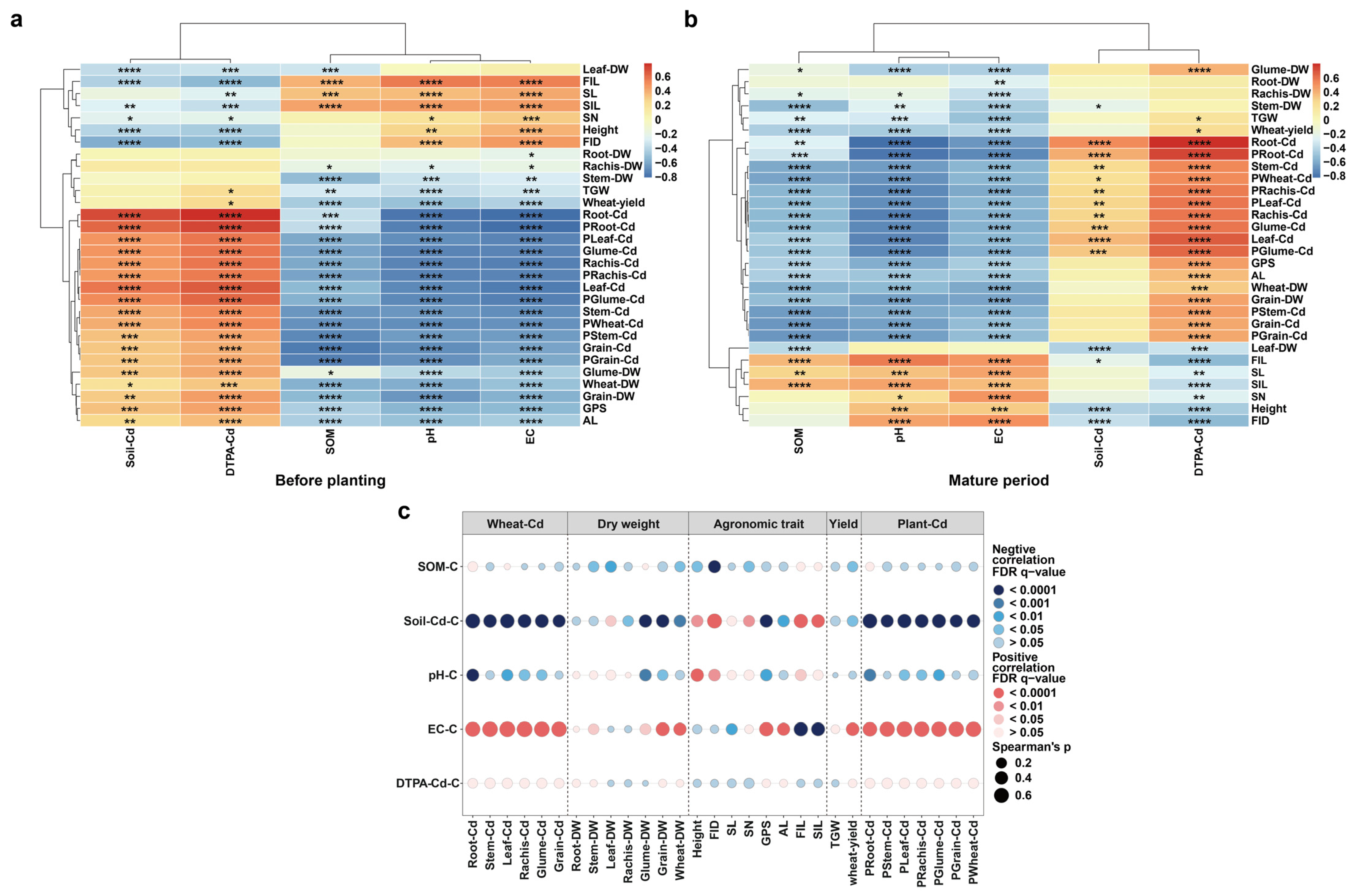
3.2. Influence of Soil Environmental Factors on Cd Content and Agronomic Traits in Wheat
3.3. Characteristics of Cd Enrichment, Transport, and Accumulation in Various Organs of Wheat
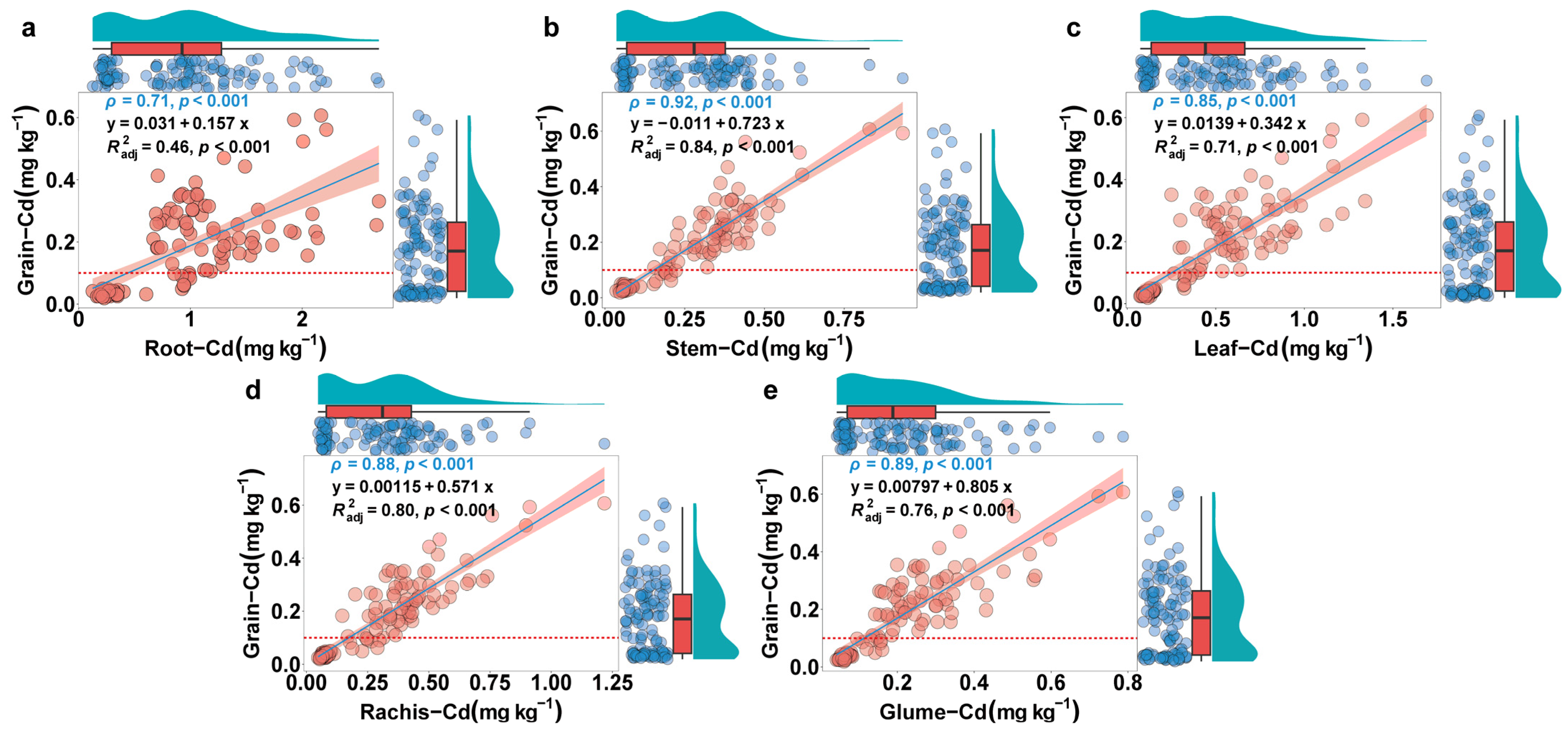
3.4. Correlation of the Cd Accumulation Capacity of Wheat Grain with Agronomic Traits, Yield, and Dry Weight
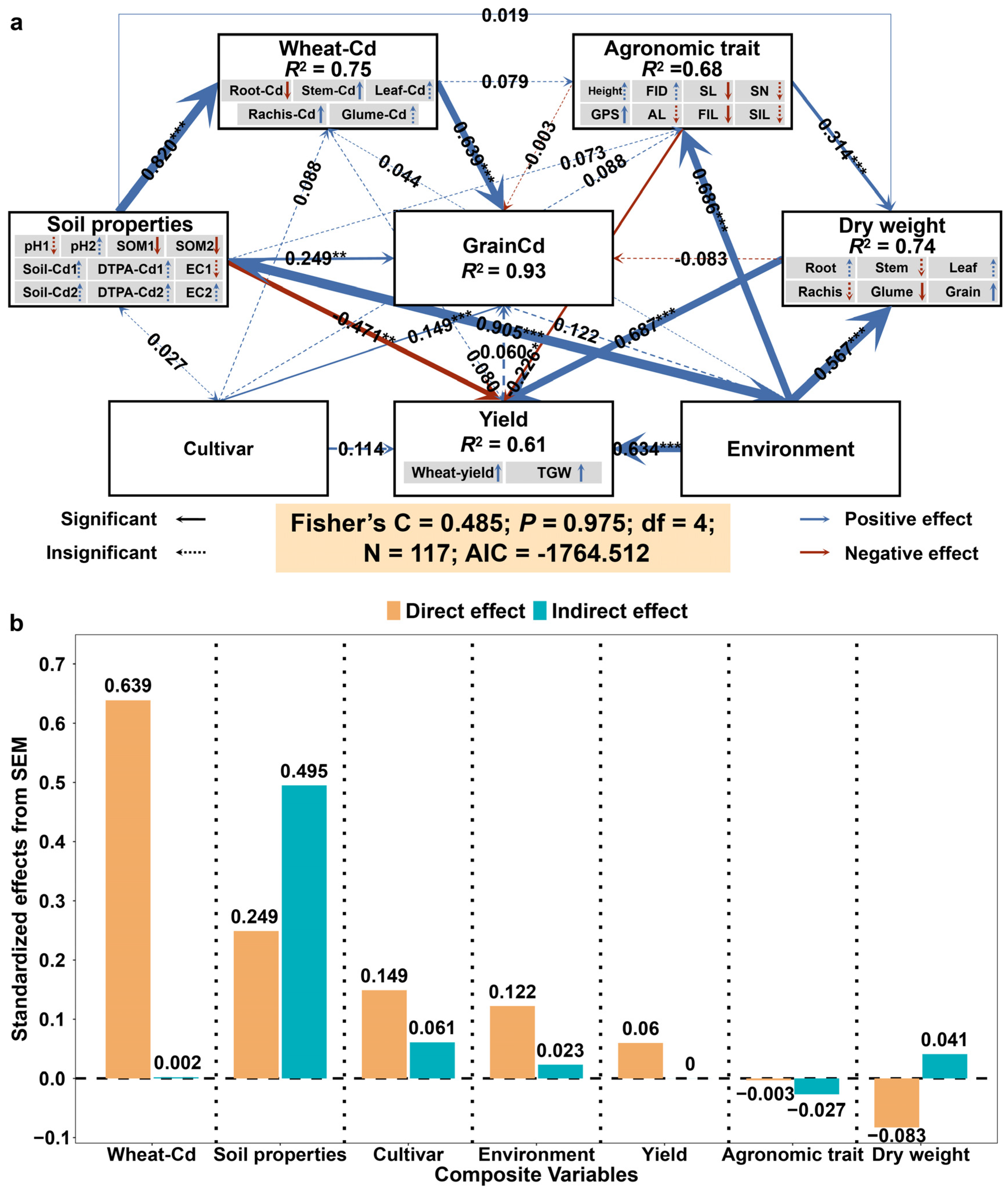
3.5. Modeling the Effect Role of Soil and Wheat Traits on Grain Cd Concentrations
4. Discussion
4.1. Effect of LAWC-Cds on Soil Properties before and after the Wheat Growth Period
4.2. Mechanisms Limiting Cd Enrichment, Transport and Accumulation in LAWC-Cds
4.3. Causal Relationships between Soil Properties, Wheat Growth Factors and Grain Cd Concentrations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev. Environ. Contam. Toxicol. 2016, 241, 73–137. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.A.; Rehman, M.Z.U.; Anwar-ul-Haq, M.; Rizwan, M.; Nawaz, R.; Shakoor, M.B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Ali, S. Effect of green and chemically synthesized titanium dioxide nanoparticles on cadmium accumulation in wheat grains and potential dietary health risk: A field investigation. J. Hazard. Mater. 2021, 415, 125585. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, S.; Cuypers, A.; Maringwa, J.; Smeets, K.; Horemans, N.; Lambrichts, I.; Van Kerkhove, E. Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology 2007, 236, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Tuffour, A.; Kosiba, A.A.; Peprah, F.A.; Gu, J.; Zhou, Y.; Shi, H.F. Cadmium-induced stress: A close look at the relationship between autophagy and apoptosis. Toxicol. Sci. 2023, 194, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Zhou, D.M. A meta-analysis on phenotypic variation in cadmium accumulation of rice and wheat: Implications for food cadmium risk control. Pedosphere 2019, 29, 545–553. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wu, W.Y.; Liu, F.; Liao, R.K.; Hu, Y.Q. Accumulation of heavy metals in soil-crop systems: A review for wheat and corn. Environ. Sci. Pollut. Res. 2017, 24, 15209–15225. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, S.B.; Guo, X.Y.; Ning, R.Y. Influencing factors of cadmium content in wheat grain: A meta-analysis and decision tree analysis. Environ. Sci. 2023, 44, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.Y.; Liao, X.Y.; Li, X.H.; Zheng, S.N.; Zhao, F.H. Phytoexclusion of heavy metals using low heavy metal accumulating cultivars: A green technology. J. Hazard. Mater. 2021, 413, 125427. [Google Scholar] [CrossRef]
- Yang, S.S.; Wu, P.; Jeyakumar, P.; Wang, H.L.; Zheng, X.; Liu, W.J.; Wang, L.K.; Li, X.F.; Ru, S.H. Technical solutions for minimizing wheat grain cadmium: A field study in North China. Sci. Total Environ. 2022, 818, 151791. [Google Scholar] [CrossRef]
- Zaid, I.U.; Zheng, X.; Li, X.F. Breeding low-cadmium wheat progress and perspectives. Agronomy 2018, 8, 249. [Google Scholar] [CrossRef]
- Sheng, M.L.; Fei, X.F.; Lou, Z.H.; Xiao, R.; Ren, Z.Q.; Lv, X.N. Processing toxic metal source proxies appropriately for better spatial heterogeneity source apportionment. Sci. Total Environ. 2023, 898, 165516. [Google Scholar] [CrossRef]
- Bai, L.Y.; Huang, X.M.; Li, Z.L.; Li, S.S.; Lv, C.; Zhang, K.; Dai, J.L. Stability and adaptability of wheat cultivars with low cadmium accumulation based on farmland trials. Eur. J. Agron. 2023, 144, 126764. [Google Scholar] [CrossRef]
- Fu, Y.C.; Zhu, X.L.; Yuan, C.; Xie, X.L.; Li, P.X.; Zhu, W.; Li, H.L.; Liu, D.H. Cadmium absorption and enrichment in wheat and its cadmium pollution prediction: Research progress. Chinese Agric. Sci. Bull. 2020, 36, 37–41. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T. Role of rhizosphere mechanisms in Cd uptake by various wheat cultivars. Plant Soil 2008, 312, 195–205. [Google Scholar] [CrossRef]
- Huang, J.W.; Fan, G.P.; Liu, C.; Zhou, D.M. Predicting soil available cadmium by machine learning based on soil properties. J. Hazard. Mater. 2023, 460, 132327. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Y.; Ding, S.; Huang, X.M.; Chen, X.G.; Chen, Y.H.; Cao, X.Y.; Wang, X.R.; Yu, X.J.; Dai, J.L. Prediction of the cadmium content in grains of low-accumulating wheat cultivars and soil cadmium threshold for safe production. J. Clean. Prod. 2023, 417, 138081. [Google Scholar] [CrossRef]
- Nguyen, C.; Roucou, A.; Grignon, G.; Cornu, J.; Méléard, B. Efficient models for predicting durum wheat grain Cd conformity using soil variables and cultivars. J. Hazard. Mater. 2021, 401, 123131. [Google Scholar] [CrossRef]
- Yu, G.F.; Jiang, X.; Sun, L.; Wang, F.; Bian, Y.R. A review for effect of organic substances on the availability of cadmium in soils. Acta Ecol. Sin. 2002, 22, 770–776. [Google Scholar]
- Cieśliński, G.; Van Rees, K.C.J.; Szmigielska, A.M.; Krishnamurti, G.S.R.; Huang, P.M. Low-molecular-weight organic acids in rhizosphere soils of durum wheat and their effect on cadmium bioaccumulation. Plant Soil 1998, 203, 109–117. [Google Scholar] [CrossRef]
- Liu, N.; Huang, X.M.; Sun, L.M.; Li, S.S.; Chen, Y.H.; Cao, X.Y.; Wang, W.X.; Dai, J.L.; Rinnan, R. Screening stably low cadmium and moderately high micronutrients wheat cultivars under three different agricultural environments of China. Chemosphere 2020, 241, 125065. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-Ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Z.G. Recent advances in minimizing cadmium accumulation in wheat. Toxics 2022, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Huang, Y.J.; Wang, Y.P.; Shen, A.L.; Gao, J.W. Research progress on the influencing factors of cadmium accumulation in wheat. J. Zhejiang Agric. Sci. 2022, 63, 2508–2511. [Google Scholar] [CrossRef]
- Liu, Y.K.; Lu, M.; Tao, Q.; Luo, J.P.; Li, J.X.; Guo, X.Y.; Liang, Y.C.; Yang, X.E.; Li, T.Q. A comparative study of root cadmium radial transport in seedlings of two wheat (Triticum aestivum L.) genotypes differing in grain cadmium accumulation. Environ. Pollut. 2020, 266, 115235. [Google Scholar] [CrossRef]
- Feng, Y.J.; Li, T.X.; Pu, Y.; Zhang, X.Z. Characteristics of cadmium accumulation and distribution in different organs of wheat with different cadmium—Accumulating type. Acta Agron. Sin. 2022, 48, 1761–1770. [Google Scholar]
- Shi, G.L.; Li, D.J.; Wang, Y.F.; Liu, C.H.; Hu, Z.B.; Lou, L.Q.; Rengel, Z.; Cai, Q.S. Accumulation and distribution of arsenic and cadmium in winter wheat (Triticum aestivum L.) at different developmental stages. Sci. Total Environ. 2019, 667, 532–539. [Google Scholar] [CrossRef]
- Mo, X.H.; Wang, M.K.; Zeng, H.; Wang, J.J. Rhizosheath: Distinct features and environmental functions. Geoderma 2023, 435, 116500. [Google Scholar] [CrossRef]
- Pande, P.M.; Azarbad, H.; Tremblay, J.; St-Arnaud, M.; Yergeau, E. Metatranscriptomic response of the wheat holobiont to decreasing soil water content. ISME Commun. 2023, 3, 30. [Google Scholar] [CrossRef]
- GB/T 23739-2009; Soil Quality-Analysis of Available Lead and Cadmium Contents in Soils-Atomic Absorption Spectrometry. Standards Press of China: Beijing, China, 2009.
- NY/T 1121.6-2006; Soil Testing Part 6: Method for Determination of Soil Organic Matter. China Agriculture Press: Beijing, China, 2006.
- HJ 802-2016; Soil Quality-Determination of Conductivity-Electrode Method. China Environmental Science Press: Beijing, China, 2016.
- HJ 962-2018; Soil-Determination of pH-Potentiometry. National Environmental Protection Standard of the People’s Republic: Beijing, China, 2018.
- Pan, G.X.; Andrew, C.C.; Albert, L.P. Modeling transfer and partitioning of potentially toxic pollutants in soil crop system for human food security. Chin. J. Appl. Ecol. 2002, 13, 854–858. [Google Scholar] [CrossRef]
- Liu, P.K.; Li, L.P.; Ippolito, J.A.; Xing, W.Q.; Wang, Y.L.; Wang, Y.L.; Cheng, Y.X.; Qiu, K.Y. Heavy metal distribution in wheat plant components following foliar Cd application. Chemosphere 2023, 322, 138177. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.J.; Liu, X.; Zhou, S.R. Asynchrony among species and functional groups and temporal stability under perturbations: Patterns and consequences. J. Ecol. 2020, 108, 2038–2046. [Google Scholar] [CrossRef]
- Tian, P.; Liu, S.E.; Zhao, X.C.; Sun, Z.L.; Yao, X.; Niu, S.L.; Crowther, T.W.; Wang, Q.K. Past climate conditions predict the influence of nitrogen enrichment on the temperature sensitivity of soil respiration. Commun. Earth Environ. 2021, 2, 251. [Google Scholar] [CrossRef]
- Shi, Y.F.; Shi, S.H.; Huang, X.M. The application of structural equation modeling in ecology based on R. Chin. J. Ecol. 2022, 41, 1015–1023. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality-Risk Control Standard for Soil Contamination Ofagricultural Land. China Environmental Science Press: Beijing, China, 2018.
- GB 2762-2022; National Standards for Food Safety-Limits of Contaminants in Food. Standards Press of China: Beijing, China, 2022.
- Affholder, M.C.; Moazzami, A.A.; Weih, M.; Kirchmann, H.; Herrmann, A.M. Cadmium reduction in spring wheat: Root exudate composition affects Cd partitioning between roots and shoots. J. Soil Sci. Plant Nutr. 2023, 23, 3537–3547. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zhou, R.X.; Teng, L.D.; Chen, H.B.; Li, M.; Wang, L.; Zhran, M.; Cao, F.B. Genotypic variation in grain cadmium concentration in wheat: Insights into soil pollution, agronomic characteristics, and rhizosphere microbial communities. Environ. Pollut. 2024, 340, 122792. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Z.B.; Li, S.J.; Deng, N.X.; Mei, P. Effects of root exudates on the activation and remediation of cadmium ion in contaminated soils. Environ. Sci. Pollut. Res. 2020, 27, 2926–2934. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ur-Rehman, M.; Bani Mfarrej, M.F.; Usman, M.; Azhar, M.; Rizwan, M.; Alharby, H.F.; Bamagoos, A.A.; Alshamrani, R.; Ahmad, Z. Exogenous application of low and high molecular weight organic acids differentially affected the uptake of cadmium in wheat-rice cropping system in alkaline calcareous soil. Environ. Pollut. 2023, 329, 121682. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Wang, M.; Zhao, Z.Q.; Li, X.Y.; Han, Y.; Chen, S.B. Alleviation of cadmium phytotoxicity to wheat is associated with Cd re-distribution in soil aggregates as affected by amendments. RSC Adv. 2018, 8, 17426–17434. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P.Y.; Ye, J.P.; Zhang, G.M.; Cai, Y.J. Simultaneous in-situ remediation and fertilization of Cd-contaminated weak-alkaline farmland for wheat production. J. Environ. Manag. 2019, 250, 109528. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Abedi, T.; Mojiri, A. Cadmium uptake by wheat (Triticum aestivum L.): An overview. Plants 2020, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Qi, J.; Fu, T.W.; Chen, Y.J.; Qiu, X.Y. Novel mechanisms of cadmium tolerance and Cd-induced fungal stress in wheat: Transcriptomic and metagenomic insights. Ecotoxicol. Environ. Saf. 2023, 256, 114842. [Google Scholar] [CrossRef]
- Rezapour, S.; Atashpaz, B.; Moghaddam, S.S.; Kalavrouziotis, I.K.; Damalas, C.A. Cadmium accumulation, translocation factor, and health risk potential in a wastewater-irrigated soil-wheat (Triticum aestivum L.) system. Chemosphere 2019, 231, 579–587. [Google Scholar] [CrossRef]
- Perrier, F.; Yan, B.; Candaudap, F.; Pokrovsky, O.S.; Gourdain, E.; Meleard, B.; Bussière, S.; Coriou, C.; Robert, T.; Nguyen, C.; et al. Variability in grain cadmium concentration among durum wheat cultivars: Impact of aboveground biomass partitioning. Plant Soil 2016, 404, 307–320. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Zhou, H.; Shao, L.L.; Wang, H.R.; Zhang, Y.B.; Zhu, T.; Ma, L.T.; Ding, Q.; Ma, L.J. Root characteristics critical for cadmium tolerance and reduced accumulation in wheat (Triticum aestivum L.). J. Environ. Manag. 2022, 305, 114365. [Google Scholar] [CrossRef]
- Zhang, L.X.; Gao, C.; Chen, C.; Zhang, W.W.; Huang, X.Y.; Zhao, F.J. Overexpression of rice OsHMA3 in wheat greatly decreases cadmium accumulation in wheat grains. Environ. Sci. Technol. 2020, 54, 10100–10108. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.P.; Chen, J.F.; Zhou, T.; Zhang, T.Y.; Shen, D.D.; Feng, Y.N.; Guan, P.F.; Huang, S.M.; Zhou, Z.F.; Huang, J.Y.; et al. Multiomics reveals an essential role of long-distance translocation in regulating plant cadmium resistance and grain accumulation in allohexaploid wheat (Triticum aestivum). J. Exp. Bot. 2022, 73, 7516–7537. [Google Scholar] [CrossRef]
- Shi, G.L.; Zhu, S.; Bai, S.N.; Xia, Y.; Lou, L.Q.; Cai, Q.S. The transportation and accumulation of arsenic, cadmium, and phosphorus in 12 wheat cultivars and their relationships with each other. J. Hazard. Mater. 2015, 299, 94–102. [Google Scholar] [CrossRef]
- Zhang, L.G.; Zhang, C.; Du, B.Y.; Lu, B.X.; Zhou, D.M.; Zhou, J.; Zhou, J. Effects of node restriction on cadmium accumulation in eight Chinese wheat (Triticum turgidum) cultivars. Sci. Total Environ. 2020, 725, 138358. [Google Scholar] [CrossRef]
- Yan, B.F.; Nguyen, C.; Pokrovsky, O.S.; Candaudap, F.; Coriou, C.; Bussière, S.; Robert, T.; Cornu, J.Y. Contribution of remobilization to the loading of cadmium in durum wheat grains: Impact of post-anthesis nitrogen supply. Plant Soil 2018, 424, 591–606. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xu, Y.M.; Liang, X.F.; Wang, L.; Sun, Y.B.; Huang, Q.Q.; Qin, X.; Zhao, L.J. Soil application of manganese sulfate could reduce wheat Cd accumulation in Cd contaminated soil by the modulation of the key tissues and ionomic of wheat. Sci. Total Environ. 2021, 770, 145328. [Google Scholar] [CrossRef] [PubMed]
- Li, L.P.; Zhang, Y.Q.; Ippolito, J.A.; Xing, W.Q.; Qiu, K.Y.; Wang, Y.L. Cadmium foliar application affects wheat Cd, Cu, Pb and Zn accumulation. Environ. Pollut. 2020, 262, 114329. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Xie, P.; Yang, J.; Lin, L.; Zhang, K.; Zhang, H.Z. Evaluating the contributions of leaf organ to wheat grain cadmium at the filling stage. Sci. Total Environ. 2022, 833, 155217. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Ding, S.; Li, X.; Ning, C.; Liu, H.; Sun, M.; Liu, D.; Zhang, K.; Li, S.; Yu, X.; et al. Low-Cadmium Wheat Cultivars Limit the Enrichment, Transport and Accumulation of Cadmium. Agronomy 2024, 14, 1191. https://doi.org/10.3390/agronomy14061191
Bai L, Ding S, Li X, Ning C, Liu H, Sun M, Liu D, Zhang K, Li S, Yu X, et al. Low-Cadmium Wheat Cultivars Limit the Enrichment, Transport and Accumulation of Cadmium. Agronomy. 2024; 14(6):1191. https://doi.org/10.3390/agronomy14061191
Chicago/Turabian StyleBai, Liyong, Suo Ding, Xiaoli Li, Chuanli Ning, He Liu, Mei Sun, Dongmei Liu, Ke Zhang, Shuangshuang Li, Xiaojing Yu, and et al. 2024. "Low-Cadmium Wheat Cultivars Limit the Enrichment, Transport and Accumulation of Cadmium" Agronomy 14, no. 6: 1191. https://doi.org/10.3390/agronomy14061191



