Comparison of Oil Extraction and Density Extraction Method to Extract Microplastics for Typical Agricultural Soils in China
Abstract
1. Introduction
2. Materials and Methods
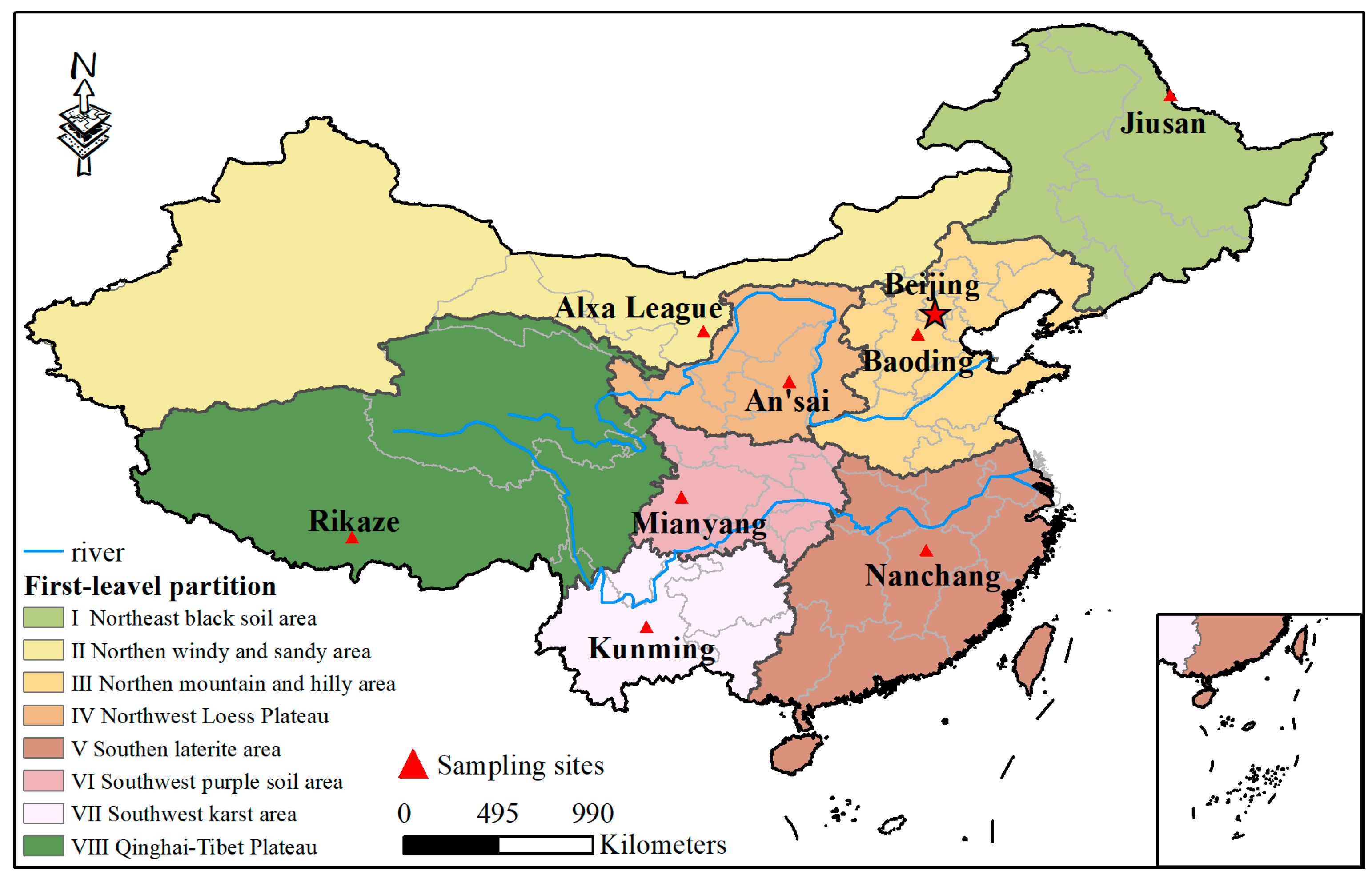
2.1. Soil Sample
| Total Nitrogen Content/g·kg−1 | Total Phosphorus Content/g·kg−1 | Available Phosphorus/mg·kg−1 | Organic Matter Content/g·kg−1 | pH | Ammonium Nitrogen/mg·kg−1 | Nitrate Nitrogen/mg·kg−1 | Soil Bulk Density/g·cm−3 | Soil Types | Main Crops | Area/106 km2 | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Northwest black soil area(I) | 1.35 | 0.7 | 5.55 | 16.4 | 4.86 | 11.54 | 8.45 | 1.31 | Black soil | Wheat | 1.09 | The research group determined |
| Northern windy and sandy area (II) | 0.31 | 0.52 | 61.06 | 9.65 | 7.67 | 0.32 | 5.57 | 1.46 | Weathered sandstone, desert sand | Corn | 2.39 | Wang [43] Molatudi [44] |
| Northern mountain and hilly area (III) | 0.87 | 0.72 | 58.3 | 19.3 | 7.79 | 29.14 | 23.77 | 1.36 | Brown soil | Wheat | 0.81 | Xue [45] Li et al. [46] |
| Northwest loess plateau (IV) | 0.83 | 0.8 | 16.6 | 7.53 | 8.14 | 2.69 | 12.6 | 1.2 | Loess soil | Wheat; corn | 0.56 | The research group determined |
| Southwest red soil area (V) | 1.5 | 0.15 | 12.5 | 13.1 | 4.97 | 3.95 | 7.25 | 1.37 | Laterite | Rice | 1.27 | The research group determined |
| Southwest purple soil area (VI) | 0.81 | 0.84 | 9.02 | 8.75 | 8.2 | 3.17 | 17.69 | 1.34 | Purple soil | Tea | 0.51 | The research group determined |
| Southwest karst area (VII) | 1.11 | 0.68 | 2.53 | 15.37 | 5.58 | 26.92 | 8.9 | 1.28 | Laterite, Grey soil | Barley | 0.7 | Meng et al. [47] Zheng [48] |
| Qinghai–Tibet plateau (VIII) | 2.79 | 0.84 | 24.8 | 28.89 | 7.76 | 1.03 | 7.89 | 1.13 | Brown soil | Barley | 2.24 | Liu et al. [49] |
2.2. Microplastic Preparation
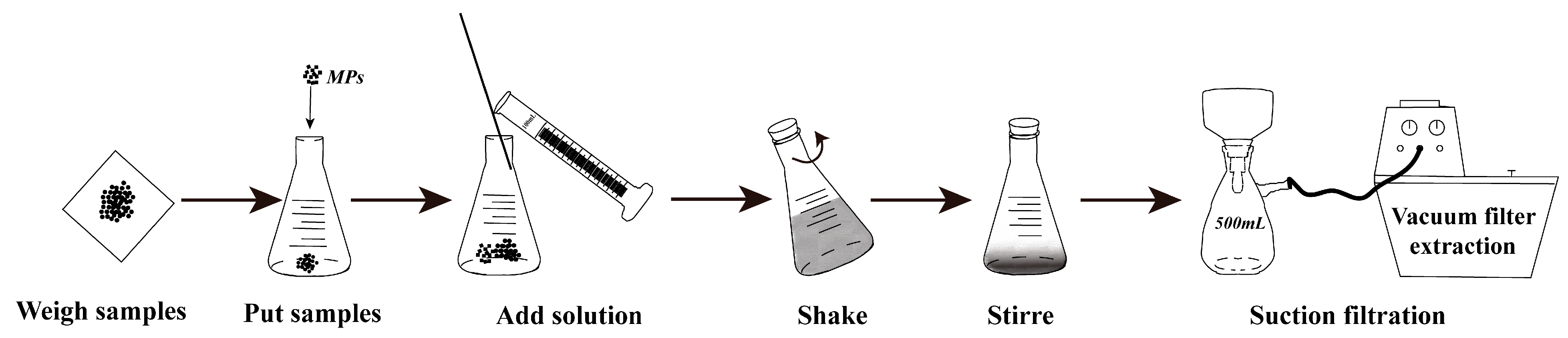
2.3. Extraction and Analysis of Microplastics
2.4. Quality Assurance and Quality Control
3. Results and Discussion
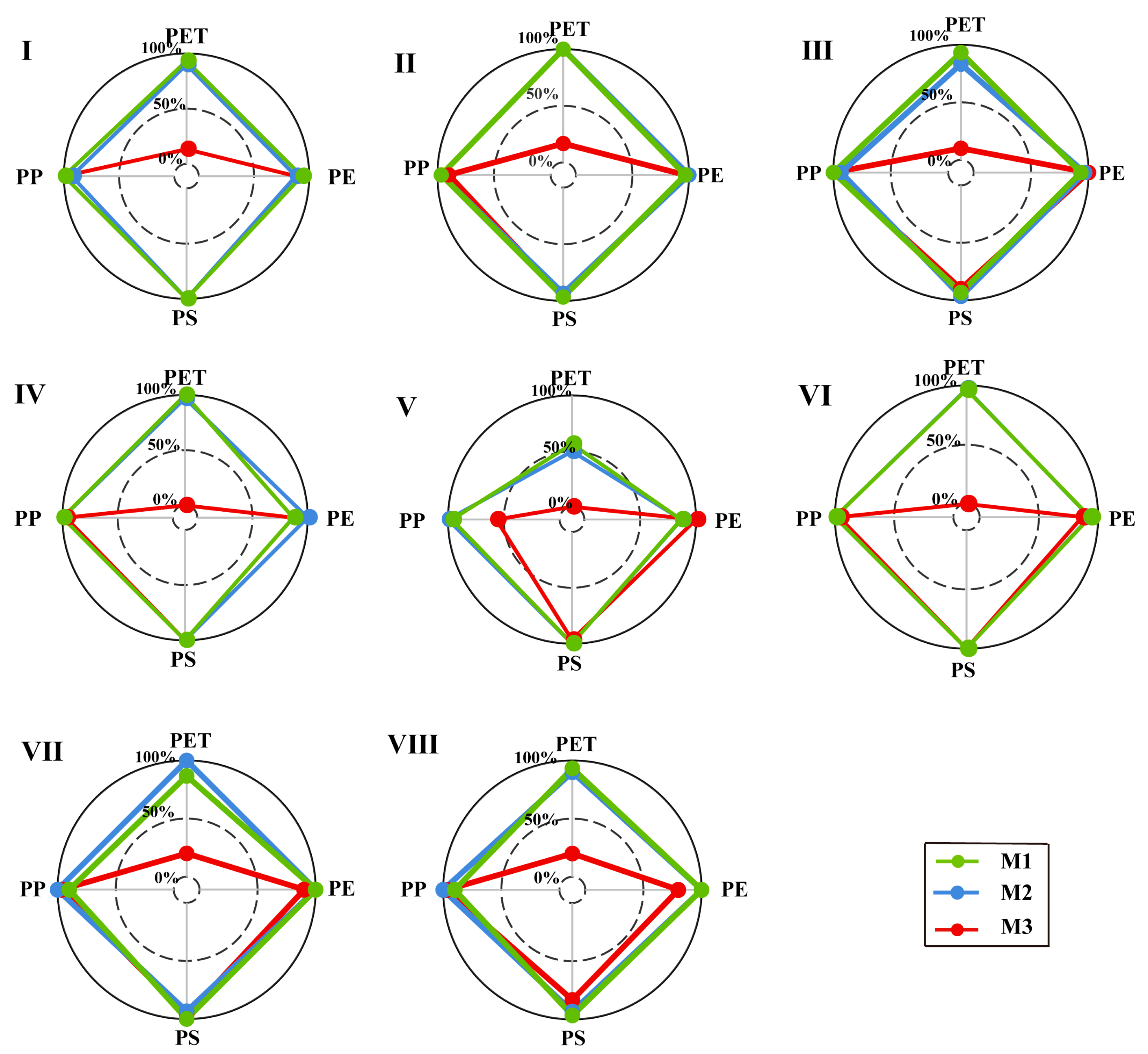
3.1. Extraction Rates of Microplastics and Comparison the Influence of Different Method
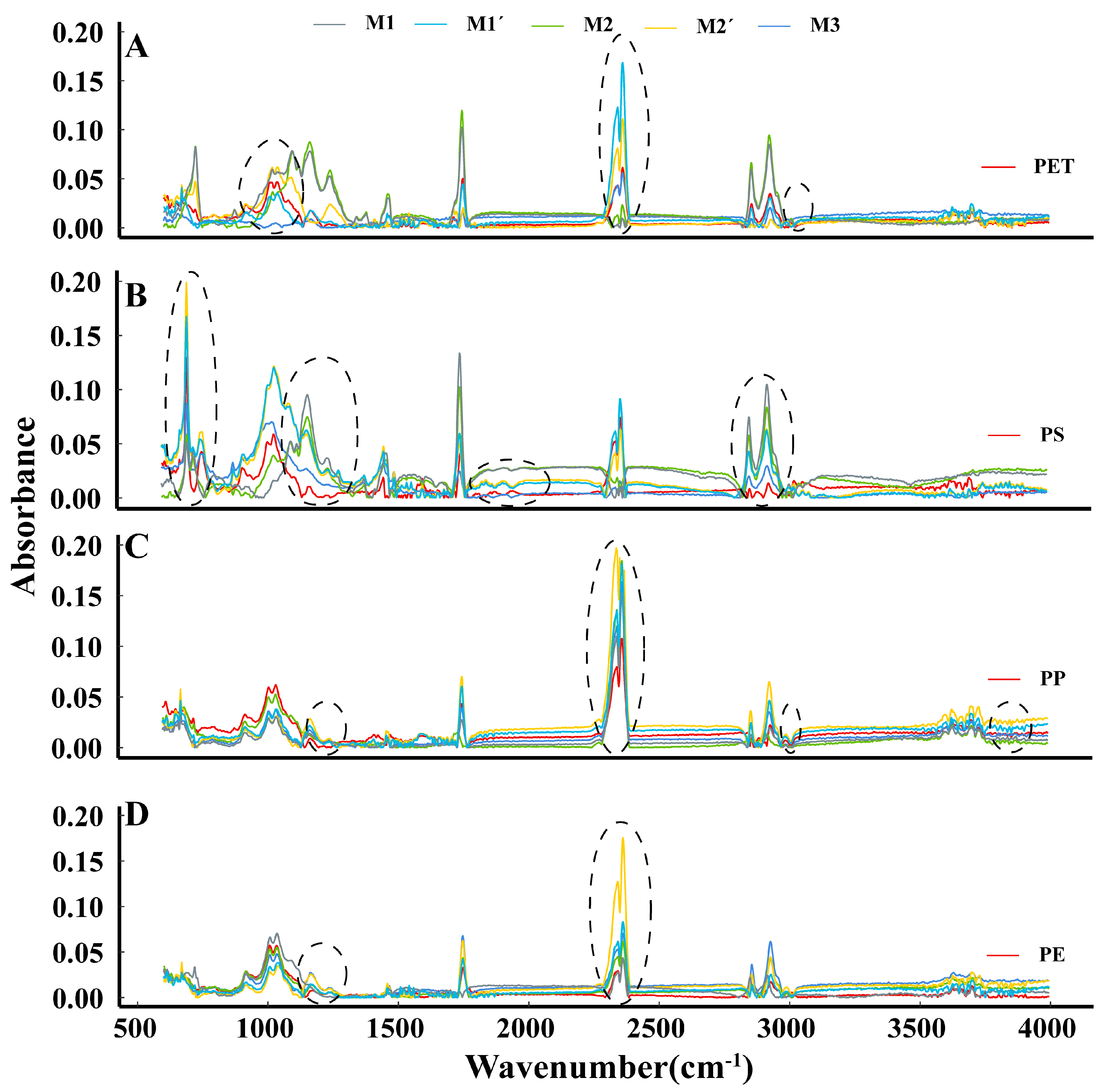
3.2. Changes in Microplastic Spectrograms under the Different Methods
3.3. Appropriate Methods for Microplastic Detection in Different Agricultural Soils
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, A.-T.; Zhou, R.; Mo, F.; Liu, S.-T.; Li, J.-Y.; Chen, Y.; Zhao, L.; Xiong, Y.-C. Soil water balance dynamics under plastic mulching in dryland rainfed agroecosystem across the Loess Plateau. Agric. Ecosyst. Environ. 2021, 312, 107354. [Google Scholar] [CrossRef]
- Lv, H.; Zhou, W.; Dong, J.; He, S.; Chen, F.; Bi, M.; Wang, Q.; Li, J.; Liang, B. Irrigation amount dominates soil mineral nitrogen leaching in plastic shed vegetable production systems. Agric. Ecosyst. Environ. 2021, 317, 107474. [Google Scholar] [CrossRef]
- Fan, Y.n.; Zhang, Y.; Wan, M.; Hu, W.; Chen, Z.; Huang, B. Plastic shed production intensified secondary soil salinization in perennial fruit production systems. Agric. Ecosyst. Environ. 2021, 316, 107469. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.Y.; Sun, Q.; Ni, H.G.; Wang, J. A minimalist approach to quantify emission factor of microplastic by mechanical abrasion. Chemosphere. 2020, 245, 125630. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ge, W.; Hei, Z.; Cong, C.; Ma, C.; Xie, M.; Liu, B.; Feng, W.; Wang, F.; Jiao, J. Agricultural land use and management weaken the soil erosion induced by extreme rainstorms. Agric. Ecosyst. Environ. 2020, 301, 107047. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Horodytska, O.; Valdes, F.J.; Fullana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jia, Q.; Zhao, X.; Li, L.; Nie, Y.; Liu, H.; Ye, J. The occurrence of microplastics in water bodies in urban agglomerations: Impacts of drainage system overflow in wet weather, catchment land-uses, and environmental management practices. Water Res. 2020, 183, 116073. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liang, C.; Li, Y.; Qiao, L.; Ai, Z.; Xue, S.; Liu, G. Interactive effects of microplastics and glyphosate on the dynamics of soil dissolved organic matter in a Chinese loess soil. Catena 2019, 182, 104177. [Google Scholar] [CrossRef]
- Berger, S.; Kim, Y.; Kettering, J.; Gebauer, G. Plastic mulching in agriculture—Friend or foe of N2O emissions? Agric. Ecosyst. Environ. 2013, 167, 43–51. [Google Scholar] [CrossRef]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Roy, P.D.; Jeyanthi Kumari, V. Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal south India. J. Hazard. Mater. 2021, 402, 123786. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, C.; Chu, Q.; Wang, F.; Lan, T.; Wang, J. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere 2020, 244, 125491. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Rahman, M.S.; Alom, J.; Hasan, M.S.; Johir, M.A.H.; Mondal, M.I.H.; Lee, D.Y.; Park, J.; Zhou, J.L.; Yoon, M.H. Microplastic particles in the aquatic environment: A systematic review. Sci. Total Environ. 2021, 775, 145793. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Lu, H.; Liu, Y. The occurrence of microplastics in farmland and grassland soils in the Qinghai-Tibet plateau: Different land use and mulching time in facility agriculture. Environ. Pollut. 2021, 279, 116939. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zeng, A.; Jiang, X.; Gu, X. Are microplastics correlated to phthalates in facility agriculture soil? J. Hazard. Mater. 2021, 412, 125164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, X.; Liu, H.; Huang, X.; Chen, Q.; Guo, X.; Zhang, J. A systematic review of microplastics in the environment: Sampling, separation, characterization and coexistence mechanisms with pollutants. Sci. Total Environ. 2023, 859, 160151. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Sethupathi, S.; Bashir, M.J.K.; Munusamy, Y.; Chan, C.W. A systematic review of microplastics occurrence, characteristics, identification techniques and removal methods in ASEAN and its future prospects. J. Environ. Chem. Eng. 2024, 12, 112305. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; An, S.; Li, H.; Deng, X.; Wang, P.; Fan, M. Land-use patterns determine the distribution of soil microplastics in typical agricultural areas on the eastern Qinghai-Tibetan Plateau. J. Hazard. Mater. 2022, 426, 127806. [Google Scholar] [CrossRef] [PubMed]
- Nuelle, M.T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; Wang, J. Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci. Total Environ. 2019, 694, 133798. [Google Scholar] [CrossRef] [PubMed]
- Crichton, E.M.; Noël, M.; Gies, E.A.; Ross, P.S. A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Anal. Methods 2017, 9, 1419–1428. [Google Scholar] [CrossRef]
- Heo, N.W.; Hong, S.H.; Han, G.M.; Hong, S.; Lee, J.; Song, Y.K.; Jang, M.; Shim, W.J. Distribution of small plastic debris in cross-section and high strandline on Heungnam beach, South Korea. Ocean. Sci. J. 2013, 48, 225–233. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.; Ma, S.; Guo, X.; Zhu, L. High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants. Water Res. 2020, 174, 115634. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, B.; Li, Q.; Liu, N.; Xia, B.; Zhu, L.; Qu, K. Toxicities of polystyrene nano- and microplastics toward marine bacterium Halomonas alkaliphila. Sci. Total Environ. 2018, 642, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Okoffo, E.D.; Rauert, C.; Thomas, K.V. Mass quantification of microplastic at wastewater treatment plants by pyrolysis-gas chromatography-mass spectrometry. Sci. Total Environ. 2023, 856, 159251. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.F.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Renner, G.; Schmidt, T.C.; Schram, J. Analytical methodologies for monitoring micro(nano)plastics: Which are fit for purpose? Curr. Opin. Environ. Sci. Health 2018, 1, 55–61. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Geng, Y.; Zhou, W.; Shao, X.; Lin, H.; Zhou, Y. Development of a multi-spectroscopy method coupling mu-FTIR and mu-Raman analysis for one-stop detection of microplastics in environmental and biological samples. Sci. Total Environ. 2024, 917, 170396. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Dai, H.; He, L. Migration testing of microplastics from selected water and food containers by Raman microscopy. J. Hazard. Mater. 2024, 462, 132798. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lin, H.; Xu, S.; He, L. Recent Advances in Spectroscopic Techniques for the Analysis of Microplastics in Food. J. Agric. Food Chem. 2022, 70, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Goydaragh, G.M.; Taghizadeh-Mehrjardi, R.; Golchin, A.; Asghar Jafarzadeh, A.; Lado, M. Predicting weathering indices in soils using FTIR spectra and random forest models. Catena 2021, 204, 105437. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. Microplastic particles increase arsenic toxicity to rice seedlings. Environ. Pollut. 2020, 259, 113892. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Rossiter, D.G.; Zhang, G.-L.; Li, D.-C. A soil colour map of China. Geoderma 2020, 379, 114556. [Google Scholar] [CrossRef]
- Zhao, G.; Yue, X.; Tian, P.; Mu, X.; Xu, W.; Wang, F.; Gao, P.; Sun, W. Comparison of the suspended sediment dynamics in Two Loess Plateau Catchments, China. Land Degrad. Dev. 2017, 28, 1398–1411. [Google Scholar] [CrossRef]
- Liu, K.; Du, J.; Zhong, Y.; Shen, Z.; Yu, X. The response of potato tuber yield, nitrogen uptake, soil nitrate nitrogen to different nitrogen rates in red soil. Sci. Rep. 2021, 11, 22506. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Tang, X.; Zhou, X. Transport of 3,5,6-trichloro-2-pyrdionl (a main pesticide degradation product) in purple soil: Experimental and modeling. Appl. Geochem. 2018, 88, 179–187. [Google Scholar] [CrossRef]
- Wang, L. Study on Soil Properties Changed after the Enclosurein the Loess Hilly-Gully Region Ordos; Inner Mongolia Agricultural University: Hohhot, China, 2017. [Google Scholar]
- Molatudi, R.L. Effect of Switchgrass and Energy Sorghum on Main Physicaland Chemical Properties of Soil in Marginal Land, InnerMongolia; China Agricultural University: Beijing, China, 2016. [Google Scholar]
- Xue, Z. Assessment of Soil Quality and Pollution Riskin Main Sewage-Irrigated Area of Hebei Province; Hebei Agricultural University: Baoding, China, 2012. [Google Scholar]
- Li, Y.; Song, A.; Lu, Y.; Wang, E.; Tang, Z.; Liu, x.; Fan, F. Volatile organic compound compositions and microbial communityproperties in maize rhizosphere among different soils. J. Plant Nutr. Fertil. 2019, 25, 1633–1645. [Google Scholar] [CrossRef]
- Meng, Y.; Xue, J.; Sha, L.; Tang, J. Variations of soil NH4-N,NO-N and N mineralization under differntforests in xishuangbanna, southwest China. Acta Phytoecol. Sin. 2001, 25, 99–104. [Google Scholar]
- Zheng, X. Effect of Plowing Depth and Straw Returned on SoilProperties and Crop Growth; Yunnan Agricultural University: Kunming, China, 2017. [Google Scholar]
- Liu, L.; Zhao, G.; Mu, X.; Gao, P.; Sun, W.; Han, J. Soil Quality Evaluation Along the Qinghai-Tibet HighwayBased on the Minimum Data Set. Res. Soil Water Conserv. 2022, 29, 125–131. [Google Scholar] [CrossRef]
- Dong, M.; Luo, Z.; Xing, X.; Zhang, Q.; Sun, Y. Separation of Microplastics in Soils and Sediments with Oil Extraction Protocol. Res. Environ. Sci. 2020, 33, 1522–1529. [Google Scholar]
- Lekše, N.; Žgajnar Gotvajn, A.; Zupančič, M.; Griessler Bulc, T. Oil-based extraction as an efficient method for the quantification of microplastics in environmental samples. Environ. Sci. Eur. 2024, 36, 68. [Google Scholar] [CrossRef]
- Gu, W.; Yang, G.; Liu, Y.; Mao, Y.; Li, H.; Ai, H.; He, Q. Treatment and detection methods of microplastics from environment media: A review. J. Civ. Environ. Eng. 2020, 42, 135–143. [Google Scholar]
- Wei, X.; Ji, H.; Wang, S.; Chu, H.; Song, C. The formation of representative lateritic weathering covers in south-central Guangxi (southern China). Catena 2014, 118, 55–72. [Google Scholar] [CrossRef]
- Cai, L.; Hu, L.; Shi, H.; Ye, J.; Zhang, Y.; Kim, H. Effects of inorganic ions and natural organic matter on the aggregation of nanoplastics. Chemosphere 2018, 197, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lyu, X.; Li, Z.; Gao, B.; Zeng, X.; Wu, J.; Sun, Y. Transport of polystyrene nanoplastics in natural soils: Effect of soil properties, ionic strength and cation type. Sci. Total Environ. 2020, 707, 136065. [Google Scholar] [CrossRef] [PubMed]
- Xu, l.; Li, H.; Han, L.; Zou, G.; Chen, Y.; Liu, D.; Xue, Y.; Lu, A. Research progress on the adsorption and desorption between microplastics and typical pollutants. Chin. J. Eco-Agric. 2021, 29, 1–9. [Google Scholar]
- Turner, A.; Holmes, L.A. Adsorption of trace metals by microplastic pellets in fresh water. Environ. Chem. 2015, 12, 600–610. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, Y.; Su, J.; Li, H.; Wang, B.; Zhong, Y.; Hu, B.; Yang, L. Research progress on the harm and detection technologies of nanoplastics in aquatic environment. Environ. Eng. 2019, 37, 64–70. [Google Scholar]
- Wei, X.; Li, M.; Wang, Y.; Jin, L.; Ma, G.; Yu, H. Developing Predictive Models for Carrying Ability of Micro-Plastics towards Organic Pollutants. Molecules 2019, 24, 1784. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Loder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhao, S.; Zhu, L.; Li, D. Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci. Total Environ. 2018, 624, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, X.; Ouyang, Z.; Chen, Y.; Wang, X.; Liu, D.; Liu, S.; Yang, X.; Jia, H.; Guo, X. The occurrence of microplastic in Mu Us Sand Land soils in northwest China: Different soil types, vegetation cover and restoration years. J. Hazard. Mater. 2021, 403, 123982. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef] [PubMed]
- Felsing, S.; Kochleus, C.; Buchinger, S.; Brennholt, N.; Stock, F.; Reifferscheid, G. A new approach in separating microplastics from environmental samples based on their electrostatic behavior. Environ. Pollut. 2018, 234, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Stile, N.; Raguso, C.; Pedruzzi, A.; Cetojevic, E.; Lasagni, M.; Sanchez-Vidal, A.; Saliu, F. Extraction of microplastic from marine sediments: A comparison between pressurized solvent extraction and density separation. Mar. Pollut. Bull. 2021, 168, 112436. [Google Scholar] [CrossRef]

| Agricultural Soil Types | Treatment | ||
|---|---|---|---|
| M1 | M2 | M3 | |
| Northwest black soil area (I) | Preferred | Second | Third |
| Northern windy and sandy area (II) | Preferred | Preferred | Second |
| Northern mountain and hilly area (III) | Preferred | Second | Third |
| Northwest loess plateau (IV) | Second | Preferred | Third |
| Southwest laterite area (V) | --- | --- | --- |
| Southwest purple soil area (VI) | Preferred | Preferred | Second |
| Southwest karst area (VII) | Second | Preferred | Third |
| Qinghai–Tibet plateau (VIII) | Preferred | Second | Third |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, Z.; Zuo, J.; Cai, L.; Liu, Y.; Han, J.; Zhang, M. Comparison of Oil Extraction and Density Extraction Method to Extract Microplastics for Typical Agricultural Soils in China. Agronomy 2024, 14, 1193. https://doi.org/10.3390/agronomy14061193
Zhao X, Liu Z, Zuo J, Cai L, Liu Y, Han J, Zhang M. Comparison of Oil Extraction and Density Extraction Method to Extract Microplastics for Typical Agricultural Soils in China. Agronomy. 2024; 14(6):1193. https://doi.org/10.3390/agronomy14061193
Chicago/Turabian StyleZhao, Xiaoli, Zihan Liu, Jichao Zuo, Lu Cai, Yihang Liu, Jianqiao Han, and Man Zhang. 2024. "Comparison of Oil Extraction and Density Extraction Method to Extract Microplastics for Typical Agricultural Soils in China" Agronomy 14, no. 6: 1193. https://doi.org/10.3390/agronomy14061193
APA StyleZhao, X., Liu, Z., Zuo, J., Cai, L., Liu, Y., Han, J., & Zhang, M. (2024). Comparison of Oil Extraction and Density Extraction Method to Extract Microplastics for Typical Agricultural Soils in China. Agronomy, 14(6), 1193. https://doi.org/10.3390/agronomy14061193





