Identification and Characterization of HS4-Mediated Hybrid Seed Shattering in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
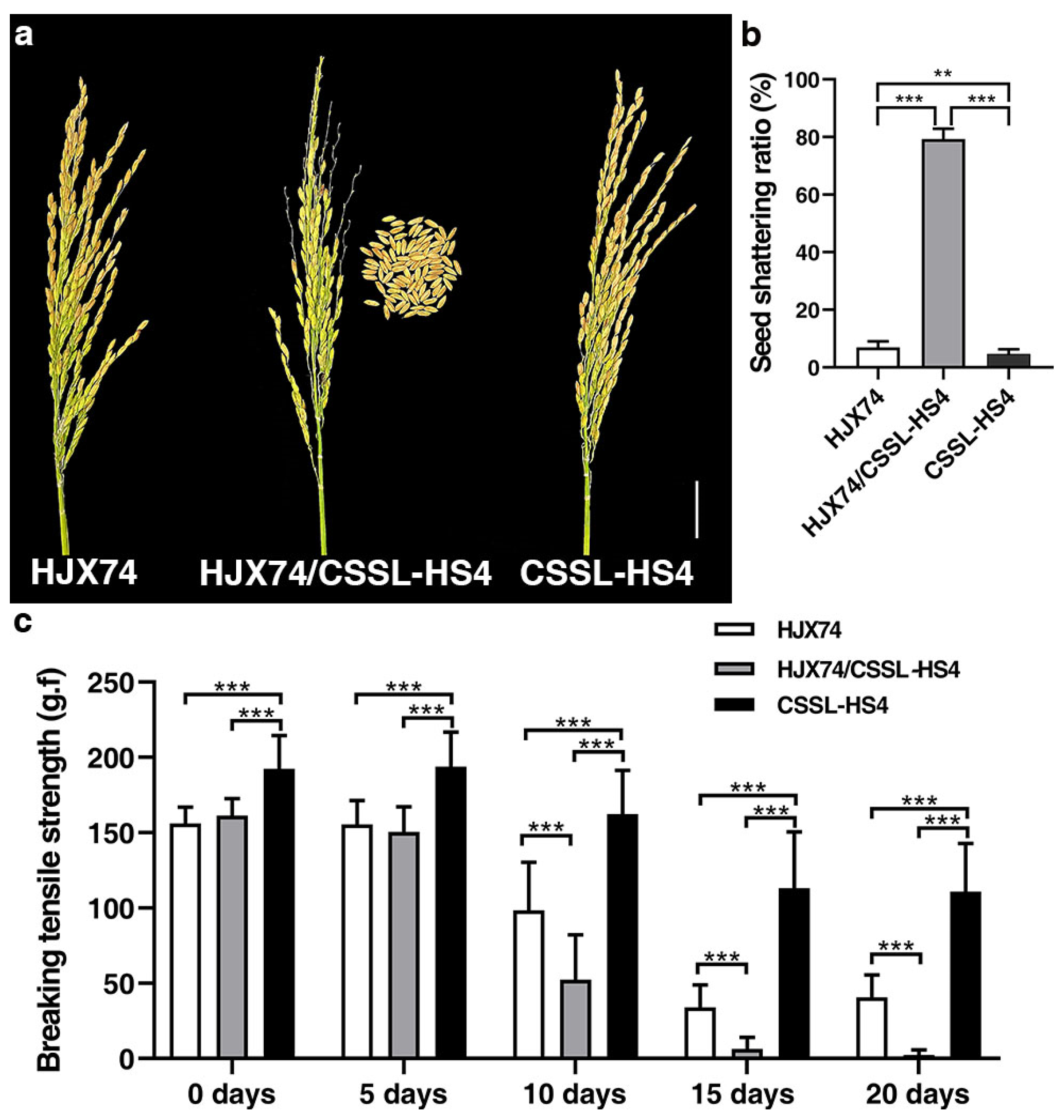
2.2. Evaluation of Shattering
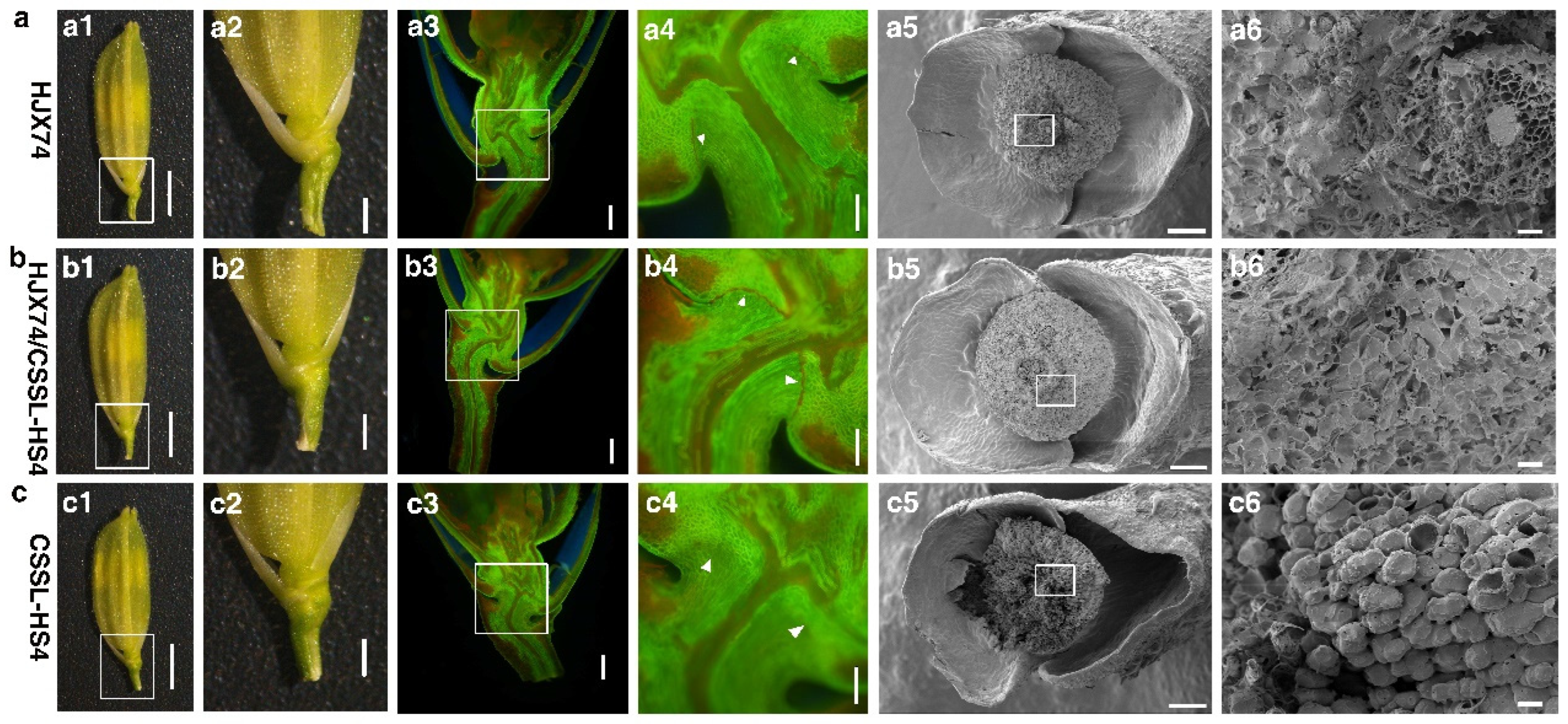
2.3. Cytological Analysis
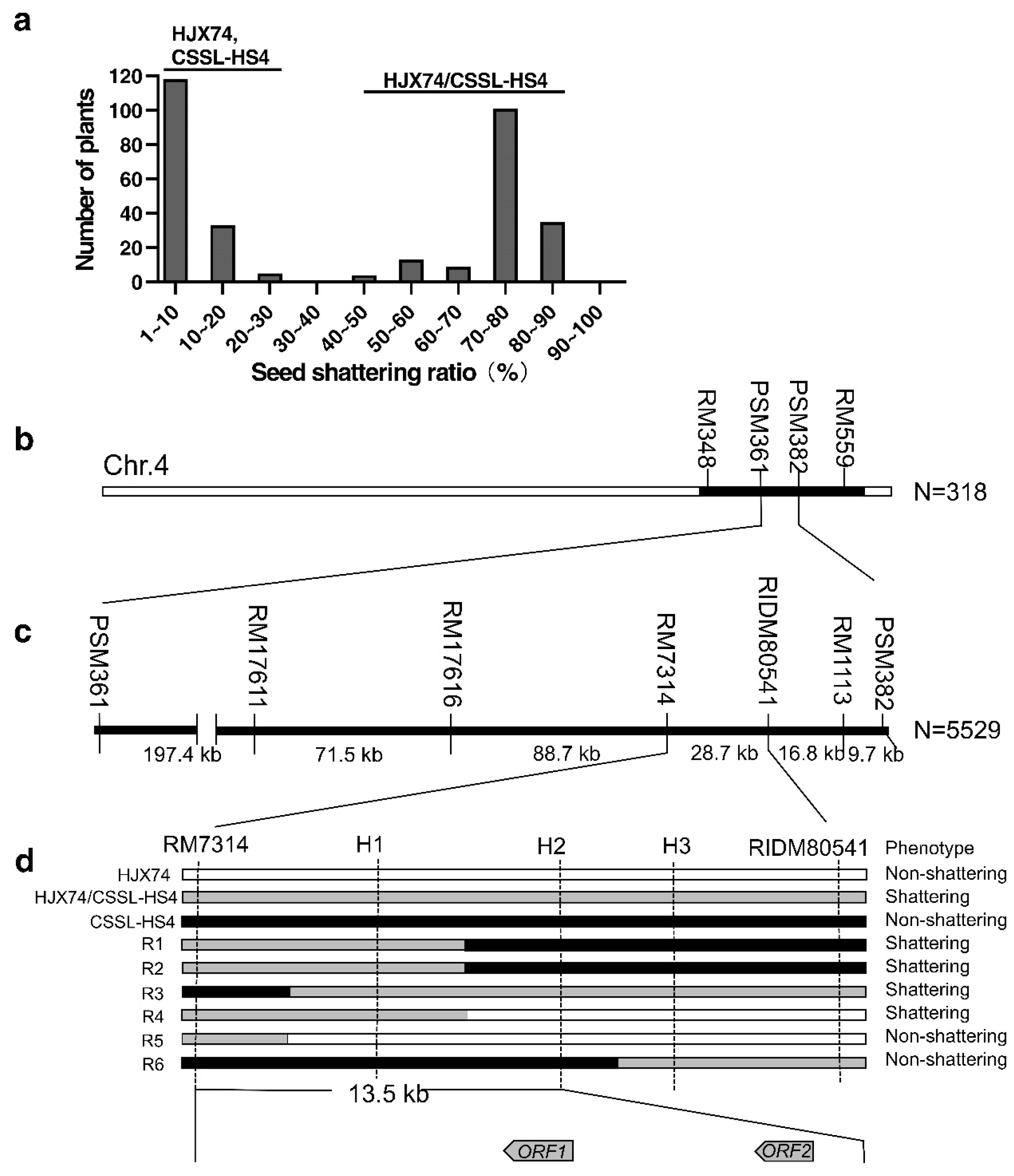
2.4. Fine Mapping and Sequencing Analysis
2.5. RNA Isolation and qRT-PCR Analysis
2.6. Subcellular Localization
2.7. Yeast Two-Hybrid Analysis
3. Results
3.1. Identification of HS4 Controlling Hybrid Seed Shattering
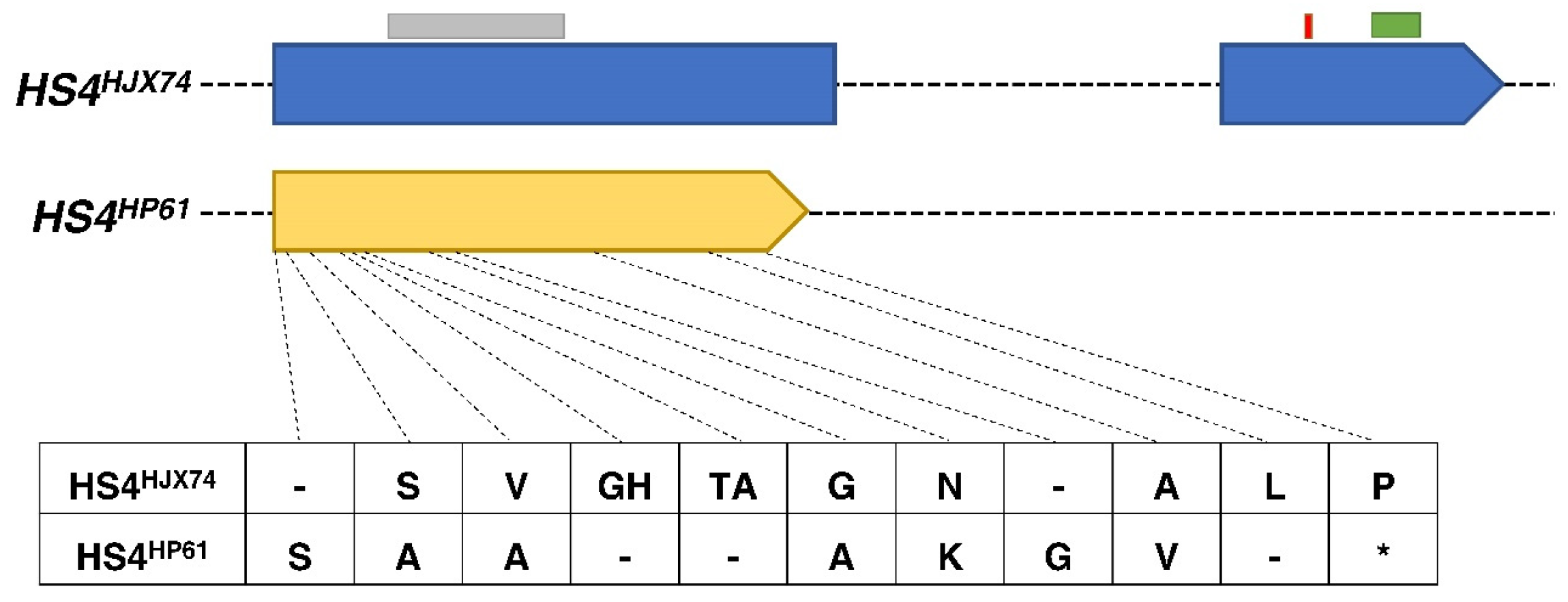
3.2. Fine Mapping and Candidate Gene Analysis of HS4
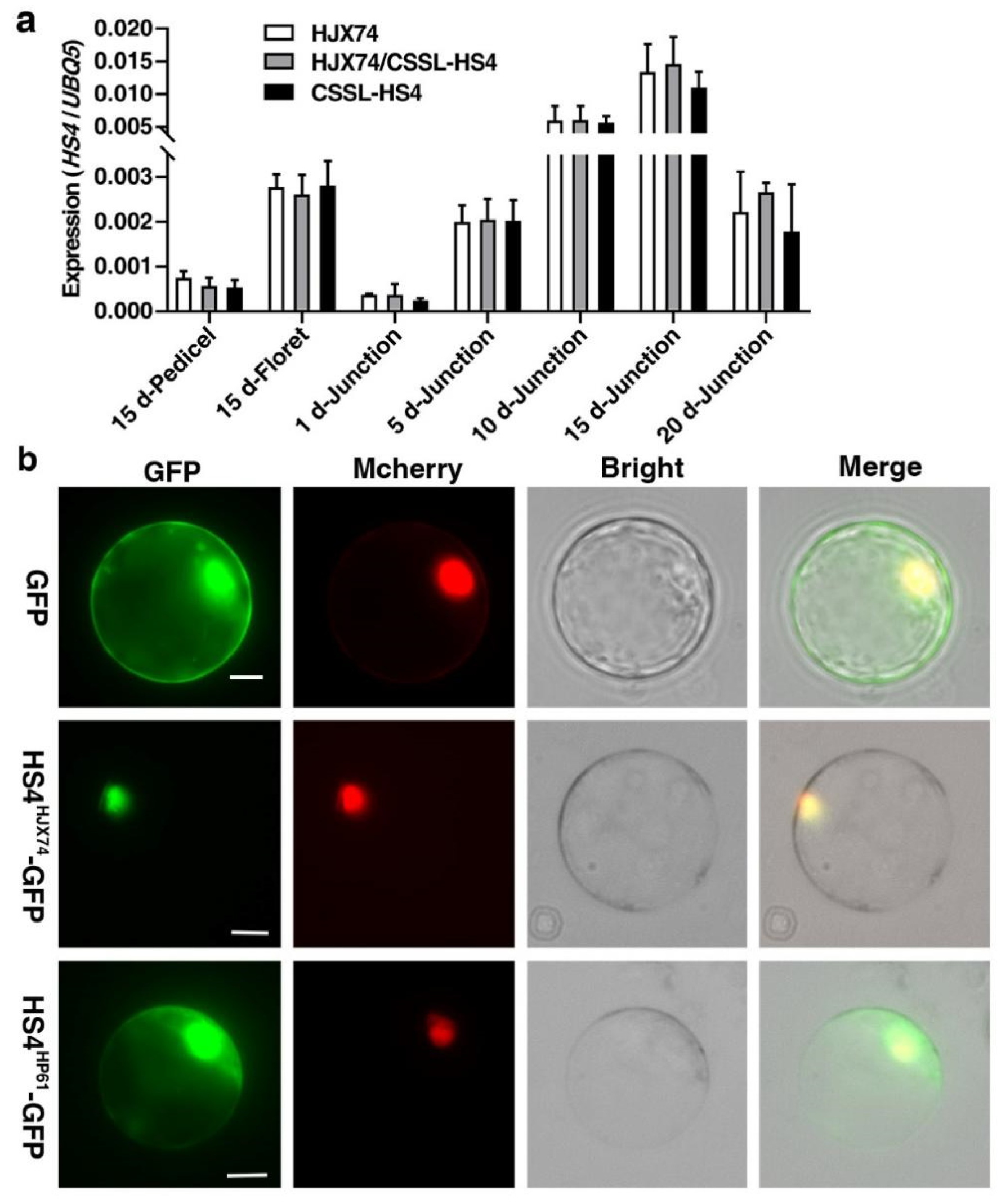
3.3. Expression Analysis and Subcellular Localization of HS4
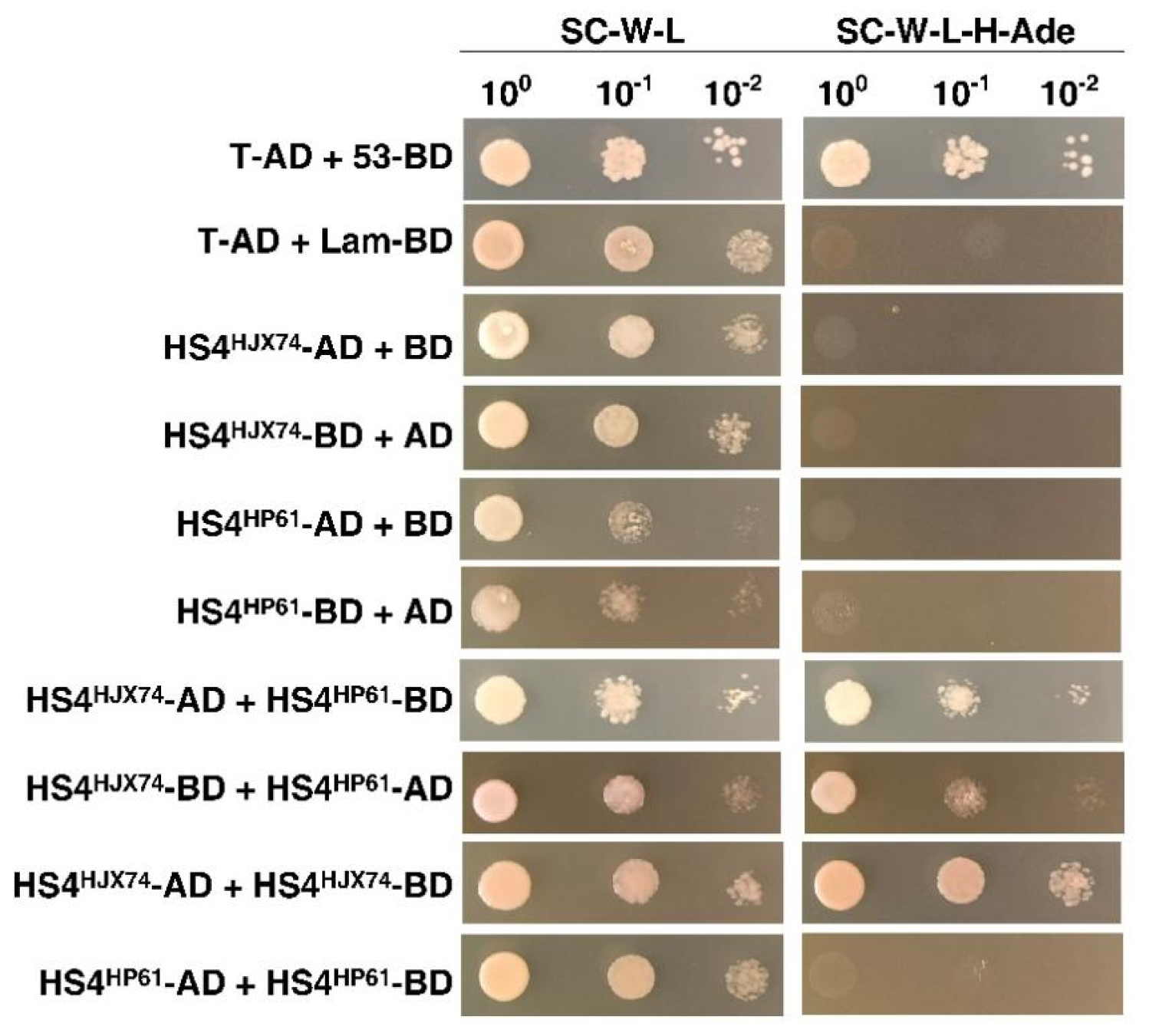
3.4. Preliminary Research on the Molecular Mechanism of HS4-Mediated Hybrid Seed Shattering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Zhou, A.; Sang, T. Rice domestication by reducing shattering. Science 2006, 311, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Estornell, L.H.; Agustí, J.; Merelo, P.; Talón, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199–200, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Lamichaney, A.; Joshi, D.C.; Bajwa, A.; Subramanian, N.; Walsh, M.; Bagavathiannan, M. Seed shattering: A trait of evolutionary importance in plants. Front. Plant Sci. 2021, 12, 657773. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Castillo, C.C.; Fuller, D.Q. Genetic evaluation of domestication-related traits in rice: Implications for the archaeobotany of rice origins. Archaeol. Anthropol. Sci. 2020, 12, 197. [Google Scholar] [CrossRef]
- Purugganan, M.D.; Fuller, D.Q. The nature of selection during plant domestication. Nature 2009, 457, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Chakraborty, D. Shattering or not shattering: That is the question in domestication of rice (Oryza sativa L.). Genet. Resour. Crop Evol. 2017, 65, 391–395. [Google Scholar] [CrossRef]
- Oba, S.; Sumi, N.; Fujimoto, F. Association between grain shattering habit and formation of abscission layer controlled by grain shattering gene Sh-2 in rice (Oryza sativa L.). Jpn. J. Crop Sci. 1995, 64, 607–615. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Wang, M.; Meyer, R.S.; Luo, X.; Ndjiondjop, M.N.; Tan, L.; Zhang, J.; Wu, J.; Cai, H.; et al. A single-nucleotide polymorphism causes smaller grain size and loss of seed shattering during African rice domestication. Nat. Plants 2017, 3, 17064. [Google Scholar] [CrossRef]
- Lin, Z.; Griffith, M.E.; Li, X.; Zhu, Z.; Tan, L.; Fu, Y.; Zhang, W.; Wang, X.; Xie, D.; Sun, C. Origin of seed shattering in rice (Oryza sativa L.). Planta 2007, 226, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Konishi, S.; Izawa, T.; Lin, S.Y.; Ebana, K.; Fukuta, Y.; Sasaki, T.; Yano, M. An SNP caused loss of seed. Science 2006, 312, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Win, K.T.; Yamagata, Y.; Doi, K.; Uyama, K.; Nagai, Y.; Toda, Y.; Kani, T.; Ashikari, M.; Yasui, H.; Yoshimura, A. A single base change explains the independent origin of and selection for the nonshattering gene in African rice domestication. New Phytol. 2017, 213, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Kaplan-Levy, R.N.; Brewer, P.B.; Quon, T.; Smyth, D.R. The trihelix family of transcription factors—light, stress and development. Trends Plant Sci. 2012, 17, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, D.; Li, C.; Luo, J.; Zhu, B.F.; Zhu, J.; Shangguan, Y.; Wang, Z.; Sang, T.; Zhou, B.; et al. Genetic control of seed shattering in rice by the APETALA2 transcription factor SHATTERING ABORTION1. Plant Cell 2012, 24, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, L.H.; Kim, S.L.; Choi, H.; Koh, H.J.; An, G. The BEL1-type homeobox gene SH5 induces seed shattering by enhancing abscission-zone development and inhibiting lignin biosynthesis. Plant J. 2014, 79, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, S.R.; Kim, Y.H.; Kim, H.; Eun, M.Y.; Jin, I.D.; Cha, Y.S.; Yun, D.W.; Ahn, B.O.; Lee, M.C.; et al. Inactivation of the CTD phosphatase-like gene OsCPL1 enhances the development of the abscission layer and seed shattering in rice. Plant J. 2010, 61, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; He, W.; Wu, L.; Chang, L.; Hu, M.; Fu, Y.; Liu, F.; Sun, H.; Gu, P.; Ndjiondjop, M.N.; et al. The MYB transcription factor seed shattering 11 controls seed shattering by repressing lignin synthesis in African rice. Plant Biotechnol. J. 2023, 21, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, Q.; Wang, Q. Advances in rice seed shattering. Int. J. Mol. Sci. 2023, 24, 8889. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.I. Experimental studies on the origin of cultivated rice. Genetics 1974, 78, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T. The origin, evolution, cultivation, dissemination, and diversification of Asian and African rices. Euphytica 1976, 25, 425–441. [Google Scholar] [CrossRef]
- Shen, S.Q.; Zhuang, J.Y.; Wang, S.Z.; Bao, J.S.; Zheng, K.L.; Shu, Q.R.; Xia, Y.W. Mapping QTLs for Rice Shattering Trait and Analysis Major Effects. Mol. Plant Breed. 2004, 2, 627–632. (In Chinese) [Google Scholar]
- Chen, J.; Li, X.; Cheng, C.; Wang, Y.; Qin, M.; Zhu, H.; Zeng, R.; Fu, X.; Liu, Z.; Zhang, G. Characterization of epistatic interaction of QTLs LH8 and EH3 controlling heading date in rice. Sci. Rep. 2014, 4, 4263. [Google Scholar] [CrossRef] [PubMed]
- Bassam, B.J.; Gresshoff, P.M. Silver staining DNA in polyacrylamide gels. Nat. Protoc. 2007, 2, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Chen, S.; Ning, Y.; Wang, G.L. Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Curr. Protoc. Plant Biol. 2016, 1, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, P.; Huang, J.; Ma, S.; Xie, X.; Tao, D.; Chen, L.; Liu, Y.G. Interspecific hybrid sterility in rice is mediated by OgTPR1 at the S1 locus encoding a peptidase-like protein. Mol. Plant 2017, 10, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Ogino, A.; Yoshikawa, T.; Kitashima, Y.; Saito, N.; Kanaoka, Y.; Onishi, K.; Yoshitake, Y.; Tsukiyama, T.; Saito, H.; et al. Lineage-specific gene acquisition or loss is involved in interspecific hybrid sterility in rice. Proc. Natl. Acad. Sci. USA 2018, 115, E1955–E1962. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tang, J.; Xie, X.; Li, X.; Huang, J.; Fei, Y.; Han, J.; Chen, S.; Tang, H.; Zhao, X.; et al. An asymmetric allelic interaction drives allele transmission bias in interspecific rice hybrids. Nat. Commun. 2019, 10, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, Y.; Haberer, G.; Marri, P.R.; Fan, C.; Goicoechea, J.L.; Zuccolo, A.; Song, X.; Kudrna, D.; Ammiraju, J.S.S.; et al. The genome sequence of African rice (Oryza glaberrima) and evidence for independent domestication. Nat. Genet. 2014, 46, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kurata, N.; Wei, X.; Wang, Z.X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 2012, 490, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Pourkheirandish, M.; Hensel, G.; Kilian, B.; Senthil, N.; Chen, G.; Sameri, M.; Azhaguvel, P.; Sakuma, S.; Dhanagond, S.; Sharma, R.; et al. Evolution of the grain dispersal system in barley. Cell 2015, 162, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhou, H.; Yu, Y.; Chen, L.; Hao, P.; Li, X. The evolutionary landscape of intergenic trans-splicing events in insects. Nat. Commun. 2015, 6, 8734. [Google Scholar] [CrossRef]
- Lei, Q.; Li, C.; Zuo, Z.; Huang, C.; Cheng, H.; Zhou, R. Evolutionary insights into RNA trans-splicing in vertebrates. Genome Biol. Evol. 2016, 8, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.M.; Caspar, T.; Dehesh, K.; Quail, P.H. DNA binding factor GT-2 from Arabidopsis. Plant Mol. Biol. 1993, 23, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Yook, K. Complementation. In WormBook: The Online Review of C. elegans Biology; WormBook: Pasadena, CA, USA, 2005; pp. 1–17. [Google Scholar]
- Juers, D.H.; Matthews, B.W.; Huber, R.E. LacZ β-galactosidase: Structure and function of an enzyme of historical and molecular biological importance. Protein Sci. 2012, 21, 1792–1807. [Google Scholar] [CrossRef]
- Cheng, S.H.; Zhuang, J.Y.; Fan, Y.Y.; Du, J.H.; Cao, L.Y. Progress in research and development on hybrid rice: A super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, X.; Li, W.; Zhu, W.; Zhu, H.; Liu, Z.; Luan, X.; Dai, Z.; Liu, G.; Zhang, Z.; et al. Overcoming inter-subspecific hybrid sterility in rice by developing indica-compatible japonica lines. Sci. Rep. 2016, 6, 26878. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Deng, Y.; Ding, Y.; Guo, J.; Qiu, J.; Wang, B.; Wang, C.; Xie, Y.; Zhang, Z.; Chen, J.; et al. Rice functional genomics: Decades’ efforts and roads ahead. Sci. China Life Sci. 2022, 65, 33–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Yang, Y.; Elgamal, W.H.; Xu, P.; Li, J.; El-Refaee, Y.Z.; Hao, S.; Tao, D. Two SNP mutations turned off seed shattering in rice. Plants 2019, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shen, R.; Chen, L.; Liu, Y.G. Molecular mechanisms of hybrid sterility in rice. Sci. China Life Sci. 2019, 62, 737–743. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Xie, W.; Chen, H.; Yang, T.; Liu, Z.; Ruan, Y.; Liu, C. Identification and Characterization of HS4-Mediated Hybrid Seed Shattering in Rice. Agronomy 2024, 14, 1218. https://doi.org/10.3390/agronomy14061218
Wang D, Xie W, Chen H, Yang T, Liu Z, Ruan Y, Liu C. Identification and Characterization of HS4-Mediated Hybrid Seed Shattering in Rice. Agronomy. 2024; 14(6):1218. https://doi.org/10.3390/agronomy14061218
Chicago/Turabian StyleWang, Daiqi, Wantong Xie, Hong Chen, Tifeng Yang, Ziqiang Liu, Ying Ruan, and Chunlin Liu. 2024. "Identification and Characterization of HS4-Mediated Hybrid Seed Shattering in Rice" Agronomy 14, no. 6: 1218. https://doi.org/10.3390/agronomy14061218
APA StyleWang, D., Xie, W., Chen, H., Yang, T., Liu, Z., Ruan, Y., & Liu, C. (2024). Identification and Characterization of HS4-Mediated Hybrid Seed Shattering in Rice. Agronomy, 14(6), 1218. https://doi.org/10.3390/agronomy14061218




