Regulated Deficit Irrigation Perspectives for Water Efficiency in Apricot Cultivation: A Review
Abstract
:1. Introduction
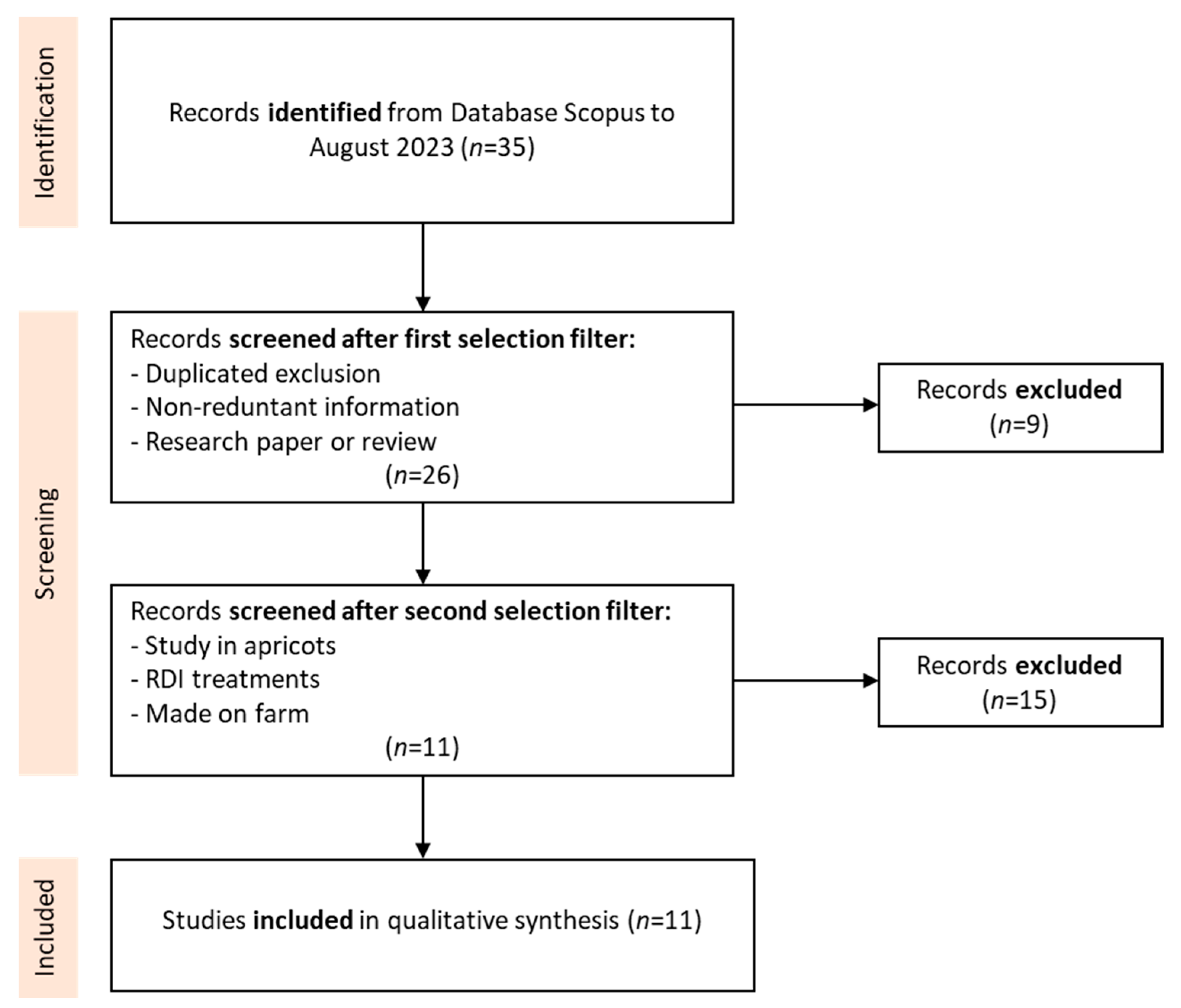
2. Methodology for the Literature Review
3. Results and Discussion
3.1. Crop Water Status
3.1.1. Volumetric Water Content
3.1.2. Stomatal Conductance and Net Photosynthesis
3.1.3. Water Potential
3.2. Crop Productivity
3.2.1. Vegetative Growth
3.2.2. Evolution of Floral Buds
3.2.3. Productive Efficiency
3.3. Fruit Quality
3.3.1. Colorimetric Parameters
3.3.2. Fruit Weight and Size
3.3.3. Fruit Shelf Life
3.3.4. Chemical Quality Indices
4. Future Prospectus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berbel, J.; Esteban, E. Droughts as a catalyst for water policy change. Analysis of Spain, Australia (MDB), and California. Glob. Environ. Chang. 2019, 58, 101969. [Google Scholar] [CrossRef]
- Torrecillas, A.; Domingo, R.; Galego, R.; Ruiz-Sánchez, M.C. Apricot tree response to withholding irrigation at different phenological periods. Sci. Hortic. 2000, 85, 201–215. [Google Scholar] [CrossRef]
- Torrecillas, A.; Galego, R.; Pérez-Pastor, A.; Ruiz-Sánchez, M.C. Gas exchange and water relations of young apricot plants under drought conditions. J. Agric. Sci. 1999, 132, 445–452. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. Data from Apricot Producing Nations. 2023. Available online: http://www.fao.org/faostat/en/#data/ (accessed on 20 March 2024).
- MAPA, Ministerio de Agricultura, Pesca y Alimentación. Anuario de Estadística 2023. Análisis Provincial de Superficie, Árboles Diseminados, Rendimiento y Producción. 2022. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/ (accessed on 20 March 2024).
- Pérez-Pastor, A.; Ruiz-Sánchez, M.C.; Domingo, R. Effects of timing and intensity of deficit irrigation on vegetative and fruit growth of apricot trees. Agric. Water Manag. 2014, 134, 110–118. [Google Scholar] [CrossRef]
- Morote Seguido, Á.F.; Rico Amorós, A.M. Perspectivas de funcionamiento del Trasvase Tajo-Segura (España): Efectos de las nuevas reglas de explotación e impulso de la desalinización como recurso sustitutivo. Boletín Asoc. Geógrafos Españoles 2018, 2754. [Google Scholar] [CrossRef]
- Chalmers, D.J.; Mitchell, P.D.; Van Heek, L. Control of Peach Tree Growth and Productivity by Regulated Water Supply, Tree Density, and Summer Pruning. JASHS 1981, 106, 307–312. [Google Scholar] [CrossRef]
- Sepaskhah, A.R.; Ahmadi, S.H. A review on partial root-zone drying irrigation. Int. J. Plant Prod. 2012, 4, 241–258. [Google Scholar] [CrossRef]
- Baldicchi, A.; Farinelli, D.; Micheli, M.; Di Vaio, C.; Moscatello, S.; Battistelli, A.; Walker, R.P.; Famiani, F. Analysis of seed growth, fruit growth and composition and phospoenolpyruvate carboxykinase (PEPCK) occurrence in apricot (Prunus armeniaca L.). Sci. Hortic. 2015, 186, 38–46. [Google Scholar] [CrossRef]
- Pérez-Pastor, A.; Ruiz-Sánchez, M.C.; Domingo, R.; Torrecillas, A. Growth and phenological stages of Búlida apricot trees in south-east Spain. Agronomie 2004, 24, 93–100. [Google Scholar] [CrossRef]
- Westwood, M.N. Temperate-Zone Pomology; Timber Press: Portland, ME, USA, 1993. [Google Scholar]
- Moriana, A.; Girón, I.F.; Martín-Palomo, M.J.; Conejero, W.; Ortuño, M.F.; Torrecillas, A.; Moreno, F. New approach for olive trees irrigation scheduling using trunk diameter sensors. Agric. Water Manag. 2010, 97, 1822–1828. [Google Scholar] [CrossRef]
- Domingo, R.; Ruiz-Sánchez, M.C.; Sánchez-Blanco, M.J.; Torrecillas, A. Water relations, growth and yield of Fino lemon trees under regulated deficit irrigation. Irrig. Sci. 1996, 16, 115–123. [Google Scholar] [CrossRef]
- Boland, A.-M.; Mitchell, P.D.; Jerie, P.H.; Goodwin, I. The effect of regulated deficit irrigation on tree water use and growth of peach. J. Hortic. Sci. 1993, 68, 261–274. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Arzani, K.; Wood, D.; Stephen Lawes, G. Influence of first season application of paclobutrazol, root-pruning and regulated deficit irrigation on second season flowering and fruiting of mature “sundrop” apricot trees. Acta Hortic. 2000, 516, 75–82. [Google Scholar] [CrossRef]
- Ezzat, A.; Salama, A.-M.; Szabó, S.; Yaseen, A.A.; Molnár, B.; Holb, I.J. Deficit irrigation strategies on tree physiological and chemical properties: Treatment effects, prediction based model analyses and inter-correlations. Agronomy 2021, 11, 1361. [Google Scholar] [CrossRef]
- Kumar, P.; Thakur, J.; Agrawal, G. Evaluation of regulated deficit drip irrigation strategies in apricot. J. Plant Nutr. 2022, 45, 3109–3117. [Google Scholar] [CrossRef]
- Kaya, S.; Evren, S.; Dasci, E.; Adiguzel, M.C.; Yilmaz, H. Effects of different irrigation regimes on vegetative growth, fruit yield and quality of drip-irrigated apricot trees. Afr. J. Biotechnol. 2010, 9, 5902–5907. [Google Scholar]
- Kaya, S.; Evren, S.; Dasci, E.; Cemal Adiguzel, M. Fruit physical characteristics responses of young apricot trees to different irrigation regimes and yield, quality, vegetative growth, and evapotranspiration relations. Int. J. Phys. Sci. 2011, 6, 3134–3142. [Google Scholar]
- Pérez-Pastor, A.; Ruiz-Sánchez, M.C.; Martínez, J.A.; Nortes, P.A.; Artés, F.; Domingo, R. Effect of deficit irrigation on apricot fruit quality at harvest and during storage. J. Sci. Food Agric. 2007, 87, 2409–2415. [Google Scholar] [CrossRef]
- Pérez-Pastor, A.; Domingo, R.; Torrecillas, A.; Ruiz-Sánchez, M.C. Response of apricot trees to deficit irrigation strategies. Irrig. Sci. 2009, 27, 231–242. [Google Scholar] [CrossRef]
- Pérez-Sarmiento, F.; Mirás-Avalos, J.M.; Alcobendas, R.; Alarcón, J.J.; Mounzer, O.; Nicolás, E. Effects of regulated deficit irrigation on physiology, yield and fruit quality in apricot trees under Mediterranean conditions. Span. J. Agric. Res. 2016, 14, e1205. [Google Scholar] [CrossRef]
- Kaya, S.; Evren, S.; Dasci, E.; Cemal Adiguzel, M.; Yilmaz, H. Evapotranspiration, irrigation water applied, and vegetative growth relations of young apricot trees under different irrigation regimes. Sci. Res. Essays 2011, 6, 738–747. [Google Scholar]
- Pereira, L.S. Higher performance through combined improvements in irrigation methods and scheduling: A discussion. Agric. Water Manag. 1999, 40, 153–169. [Google Scholar] [CrossRef]
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Parkash, V.; Singh, S. A review on potential plant-based water stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Pask, A. (Ed.) Physiological Breeding. II, A Field Guide to Wheat Phenotyping; CIMMYT: Mexico City, Mexico, 2012. [Google Scholar]
- Bartolini, S.; Lo Piccolo, E.; Remorini, D. Different summer and autumn water deficit affect the floral differentiation and flower bud growth in apricot (Prunus armeniaca L.). Agronomy 2020, 10, 914. [Google Scholar] [CrossRef]
- Losciale, P.; Gaeta, L.; Corsi, M.; Galeone, C.; Tarricone, L.; Leogrande, R.; Stellacci, A.M. Physiological responses of apricot and peach cultivars under progressive water shortage: Different crop signals for anisohydric and isohydric behaviours. Agric. Water Manag. 2023, 286, 108384. [Google Scholar] [CrossRef]
- Torrecillas, A.; Corell, M.; Galindo, A.; Pérez-López, D.; Memmi, H.; Rodríguez, P.; Cruz, Z.N.; Centeno, A.; Intrigliolo, D.S.; Moriana, A. Agronomical effects of deficit irrigation in apricot, peach, and plum trees. In Water Scarcity and Sustainable Agriculture in Semiarid Environment: Tools, Strategies, and Challenges for Woody Crops; García, I.F., Durán, V.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 87–109. [Google Scholar] [CrossRef]
- Nicolás, E.; Torrecillas, A.; Amico, J.D.; Alarcón, J.J. Sap flow, gas exchange, and hydraulic conductance of young apricot trees growing under a shading net and different water supplies. J. Plant Physiol. 2005, 162, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sánchez, M.C.; Domingo, R.; Pérez-Pastor, A. Daily variations in water relations of apricot trees under different irrigation regimes. Biol. Plant. 2007, 51, 735–740. [Google Scholar] [CrossRef]
- Bozkurt, S.; Ödemiş, B.; Durgaç, C. Effects of deficit irrigation treatments on yield and plant growth of young apricot trees. N. Z. J. Crop Hortic. Sci. 2015, 43, 73–84. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.C.; Domingo, R.; Torrecillas, A.; Pérez-Pastor, A. Water stress preconditioning to improve drought resistance in young apricot plants. Plant Sci. 2000, 156, 245–251. [Google Scholar] [CrossRef]
- Joody, A.T. Reducing water stress on apricot saplings cv. Zanjelly. Plant Arch. 2020, 20, 105–108. [Google Scholar]
- Barradas, V.L.; Nicolás, E.; Torrecillas, A.; Alarcón, J.J. Transpiration and canopy conductance in young apricot (Prunus armenica L.) trees subjected to different PAR levels and water stress. Agric. Water Manag. 2005, 77, 323–333. [Google Scholar] [CrossRef]
- Robbins, N.E.; Dinneny, J.R. The divining root: Moisture-driven responses of roots at the micro- and macro-scale. J. Exp. Bot. 2015, 66, 2145–2154. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Jones, H.G. Physiological Aspects of the Control of Water Status in Horticultural Crops. HortScience 1990, 25, 19–25. [Google Scholar] [CrossRef]
- De Swaef, T.; Steppe, K.; Lemeur, R. Determining reference values for stem water potential and maximum daily trunk shrinkage in young apple trees based on plant responses to water deficit. Agric. Water Manag. 2009, 96, 541–550. [Google Scholar] [CrossRef]
- Choné, X.; Van Leeuwen, C.; Dubourdieu, D.; Gaudillère, J.P. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Temnani, A.; Berríos, P.; Zapata-García, S.; Espinosa, P.J.; Pérez-Pastor, A. Threshold values of plant water status for scheduling deficit irrigation in early apricot trees. Agronomy 2023, 13, 2344. [Google Scholar] [CrossRef]
- Mitchell, P.D.; Chalmers, D.J. The Effect of Reduced Water Supply on Peach Tree Growth and Yields. JASHS 1982, 107, 853–856. [Google Scholar] [CrossRef]
- Bussi, C.; Plenet, D. Effect of three different irrigation regimes on young apricot (Prunus armeniaca L. Batsch) trees. J. Hortic. Sci. Biotechnol. 2013, 88, 519–524. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, A.; Carbonell-Barrachina, A.A.; Martínez-Nicolás, J.J.; Legua, P.; Martínez, R.; Hernández, F. Antioxidant activity, volatile composition and sensory profile of four new very-early apricots (Prunus armeniaca L.). J. Sci. Food Agric. 2014, 94, 85–94. [Google Scholar] [CrossRef]
- Shih, H.-H.; Wu, C.-F.; Wang, S.-B.; Lin, K.-C. The influences of lighting design on fruit images. In Proceedings of the 2018 IEEE International Conference on Applied System Invention (ICASI), Chiba, Japan, 13–17 April 2018; pp. 506–509. [Google Scholar] [CrossRef]
- Gómez, R.; Costa, J.; Amo, M.; Alvarruiz, A.; Picazo, M.; Pardo, J.E. Physicochemical and colorimetric evaluation of local varieties of tomato grown in SE Spain: Quality of tomato varieties. J. Sci. Food Agric. 2001, 81, 1101–1105. [Google Scholar] [CrossRef]
- Ben Mimoun, M.; Marchand, M. Combined effect of restricted irrigation and potassium on yield and quality of apricot (Prunus armeniaca L.). Acta Hortic. 2016, 1130, 519–523. [Google Scholar] [CrossRef]
- Jackman, R.L.; Marangoni, A.G.; Stanley, D.W. Measurement of Tomato Fruit Firmness. HortScience 1990, 25, 781–783. [Google Scholar] [CrossRef]
- Ayour, J.; Benichou, M.; Alahyane, A.; Harrak, H. Relationships between Biochemical Criteria, Volatile Compounds, and Sensory Profiles of Ten Apricot Clones at Commercial and Consumption Ripening Stages. J. Food Qual. 2020, 2020, 8873835. [Google Scholar] [CrossRef]

| Parameter | Variety | RDI Timeframe | % ETc | Results | Reference |
|---|---|---|---|---|---|
| Trunk cross-sectional area (TCSA) | Salak | Postharvest | 0% | ↓ | [20] |
| Búlida | Fruit set + Fruit growth phases I, II + Late postharvest | 25–60% | ↓ | [24] | |
| Shaded area | Búlida | Fruit growth phases I, II + Late postharvest | 0% | = | [2] |
| Fruit growth phases I, II + Late postharvest | 40–60% | = | [6] | ||
| Búlida | Fruit growth phases I, II + Late postharvest | 0% | = | [2] | |
| Trunk size | Fruit growth phases I, II + Late postharvest | 40–60% | = | [6] | |
| Salak | Postharvest | 0% | = | [25] | |
| Búlida | Fruit growth phases I, II + Late postharvest | 40–60% | ↓ | [6] | |
| Stem size | Ninfa | Fruit growth phase I + Early postharvest | 25–50% | = | [18] |
| Canino | Fruit growth phase I + Early postharvest | 25–50% | ↓ | [18] |
| Parameter | Variety | RDI Timeframe | % ETc | Results | References |
|---|---|---|---|---|---|
| Percentage of fruit set | Sundrop | Fruit growth phases I, II | 0% | ↑ | [17] |
| Búlida | Fruit growth phases I, II + Late postharvest | 0% | = | [2] | |
| Fruit set phase + Fruit growth phases I, II + Late postharvest | 25–60% | = | [24] | ||
| Ninfa | Fruit growth phase I + Early postharvest | 25–50% | ↑ | [18] | |
| Canino | Fruit growth phase I + Early postharvest | 50% | = | ||
| Fruit growth phase I + Early postharvest | 25% | ↓ | |||
| Productive load | Sundrop Búlida Not available | Fruit growth phases I, II Fruit growth phases I, II + Late postharvest Fruit growth phases I, II + Late postharvest Fruit growth phases I, II + Late postharvest Fruit growth phases I, II + Late postharvest Fruit growth phases I, II + Late postharvest | 0% | ↑ | [17] |
| 25% | ↓ | [23] | |||
| 40% | = | ||||
| 25% | ↓ | [6] | |||
| 40–60% 60% | = = | [6] | |||
| [19] | |||||
| Total production | Sundrop | Fruit growth phases I, II | 0% | ↑ | [17] |
| Búlida | Fruit growth phases I, II + Late postharvest | 0% | = | [2] | |
| Fruit growth phases I, II + Late postharvest | 25% | ↓ | [23] | ||
| Fruit growth phases I, II + Late postharvest | 40% | = | |||
| Fruit growth phases I, II + Late postharvest | 25% | ↓ | [6] | ||
| Fruit growth phases I, II + Late postharvest | 40–60% | = | |||
| Fruit set phase + Fruit growth phases I, II + Late postharvest | 25–60% | = | [24] | ||
| Salak | Postharvest | 0% | = | [20] | |
| Ninfa | Fruit growth phase I + Early postharvest | 50% | ↑ | [18] | |
| Fruit growth phase I + Early postharvest | 25% | = | |||
| Canino | Fruit growth phase I + Early postharvest | 25–50% | ↓ | ||
| Water use efficiency (WUE) | Búlida | Fruit growth phases I, II + Late postharvest | 25% | = | [23] |
| Fruit growth phases I, II + Late postharvest | 40% | ↑ | |||
| Fruit set phase + Fruit growth phases I, II + Late postharvest | 25–60% | ↑ | [24] |
| Parameter | Variety | RDI Timeframe | % ETc | Results | References |
|---|---|---|---|---|---|
| Fruit size | Búlida | Fruit growth phases I y II + Late postharvest | 0% | = | [2] |
| Fruit growth phases I y II + Late postharvest | 25% | = | [22] | ||
| Fruit growth phases I y II + Late postharvest | 25% | = | [23] | ||
| Fruit growth phases I y II + Late postharvest | 25% | ↓ | [6] | ||
| Fruit growth phases I y II + Late postharvest | 40–60% | = | |||
| Fruit set phase + Fruit growth phases I y II + Late postharvest | 25–60% | = | [24] | ||
| Salak | Postharvest | 0% | = | [21] | |
| Not available | Fruit growth phases I y II + Late postharvest | 60% | = | [19] | |
| Fresh weight | Sundrop | Fruit growth phases I y II | 0% | = | [18] |
| Búlida | Fruit growth phases I y II + Late postharvest | 0% | = | [2] | |
| Fruit growth phases I y II + Late postharvest | 25% | = | [22] | ||
| Fruit growth phases I y II + Late postharvest | 25% | = | [23] | ||
| Fruit growth phases I y II + Late postharvest | 40% | = | [6] | ||
| Fruit growth phases I y II + Late postharvest | 60% | = | |||
| Ninfa | Fruit growth phases I y II + Early postharvest | 25–50% | = | [18] | |
| Canino | Fruit growth phases I y II + Early postharvest | 25–50% | = | ||
| Salak | Postharvest | 0% | = | [21] | |
| Not available | Fruit growth phases I y II + Late postharvest | 60% | = | [19] | |
| Firmness | Búlida | Fruit growth phases I y II + Late postharvest | 0% | = | [2] |
| Fruit growth phases I y II + Late postharvest | 25% | = | [22] | ||
| Fruit growth phases I y II + Late postharvest | 25% | = | [23] | ||
| Fruit set phase + Fruit growth phases I y II + Late postharvest | 25–60% | = | [24] | ||
| Salak | Postharvest | 0% | = | [20] | |
| Titratable acidity (TA) | Búlida | Fruit growth phases I y II + Late postharvest | 25% | = | [22] |
| Fruit growth phases I y II + Late postharvest | 25% | ↑ | [23] | ||
| Fruit set phase + Fruit growth phases I y II + Late postharvest | 25–60% | = | [24] | ||
| Salak | Postharvest | 0% | = | [20] | |
| Not available | Fruit growth phases I y II + Late postharvest | 60% | = | [19] | |
| Total soluble solids (TSSs) | Búlida | Fruit growth phases I y II + Late postharvest | 0% | = | [2] |
| Fruit growth phases I y II + Late postharvest | 25% | ↑ | [22] | ||
| Fruit growth phases I y II + Late postharvest | 25% | ↑ | [22] | ||
| Fruit set phase + Fruit growth phases I y II + Late postharvest | 25–60% | ↑ | [24] | ||
| Salak | Postharvest | 0% | = | [20] | |
| Not available | Fruit growth phases I y II + Late postharvest | 60% | = | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreu-Coll, L.; Carbonell-Barrachina, Á.A.; Burló, F.; Galindo, A.; García-Brunton, J.; López-Lluch, D.B.; Martínez-Font, R.; Noguera-Artiaga, L.; Sendra, E.; Hernández-Ariola, P.; et al. Regulated Deficit Irrigation Perspectives for Water Efficiency in Apricot Cultivation: A Review. Agronomy 2024, 14, 1219. https://doi.org/10.3390/agronomy14061219
Andreu-Coll L, Carbonell-Barrachina ÁA, Burló F, Galindo A, García-Brunton J, López-Lluch DB, Martínez-Font R, Noguera-Artiaga L, Sendra E, Hernández-Ariola P, et al. Regulated Deficit Irrigation Perspectives for Water Efficiency in Apricot Cultivation: A Review. Agronomy. 2024; 14(6):1219. https://doi.org/10.3390/agronomy14061219
Chicago/Turabian StyleAndreu-Coll, Lucía, Ángel A. Carbonell-Barrachina, Francisco Burló, Alejandro Galindo, Jesús García-Brunton, David B. López-Lluch, Rafael Martínez-Font, Luis Noguera-Artiaga, Esther Sendra, Pedro Hernández-Ariola, and et al. 2024. "Regulated Deficit Irrigation Perspectives for Water Efficiency in Apricot Cultivation: A Review" Agronomy 14, no. 6: 1219. https://doi.org/10.3390/agronomy14061219









