Short-Term Straw Return Combined with Nitrogen Fertilizer Alters the Soil Nitrogen Supply in Rice–Rapeseed Planting Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Grain Yield and N Uptake
2.4. Soil Sampling and Measurement
2.5. qPCR and High-Throughput Sequencing
2.6. Data Analysis
3. Results
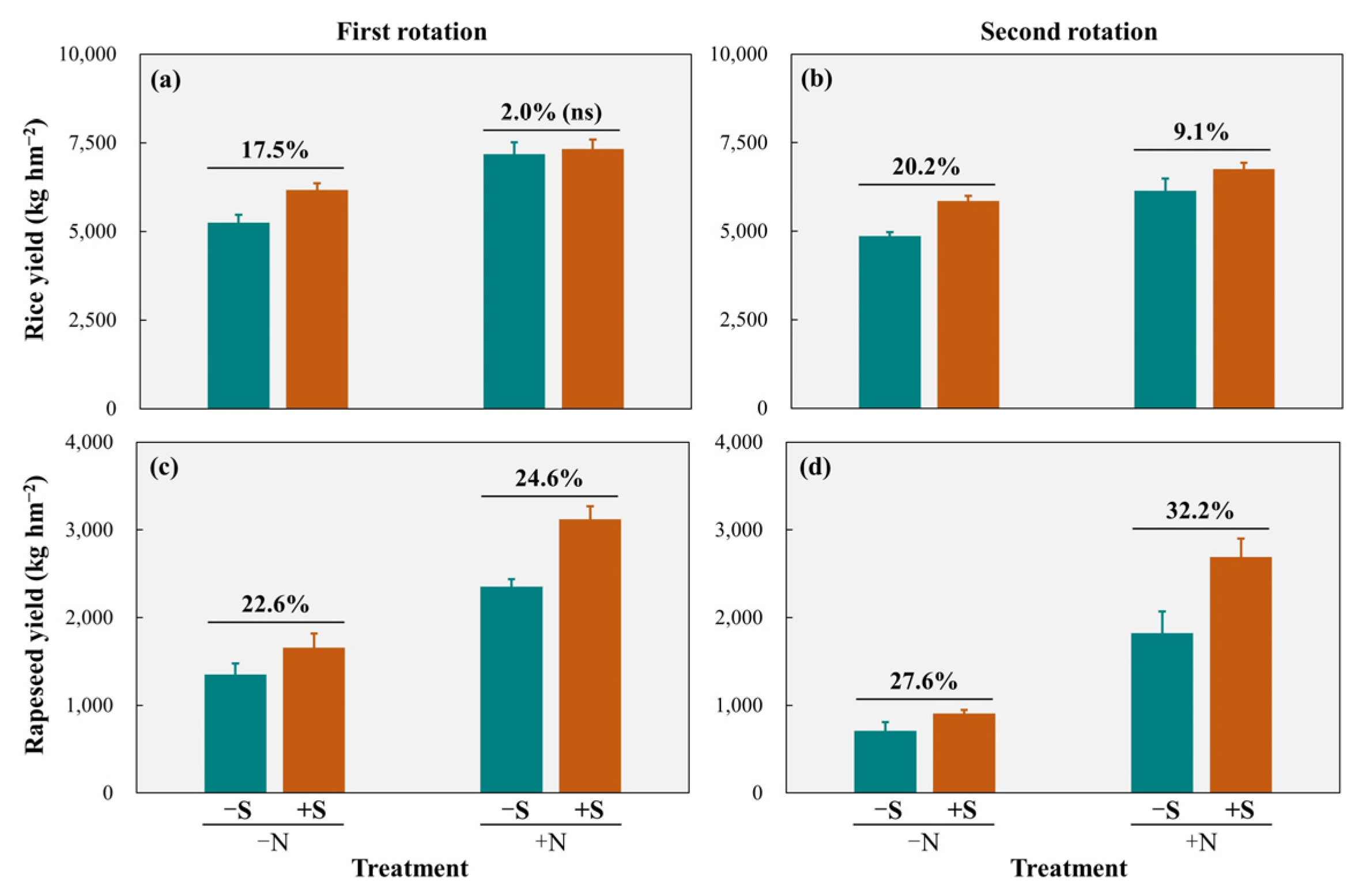
3.1. Grain Yield
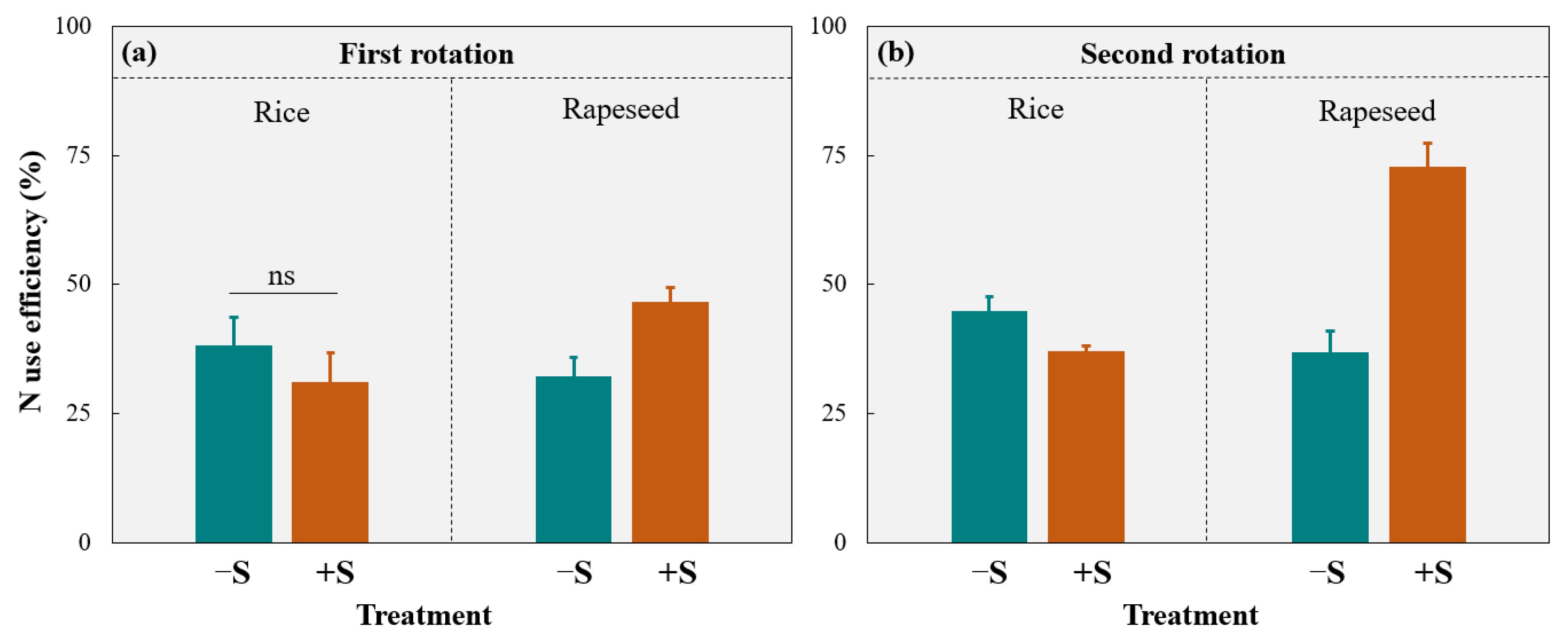
3.2. N Uptake and Use Efficiency
3.3. Soil Properties
3.4. Bacterial Community Composition
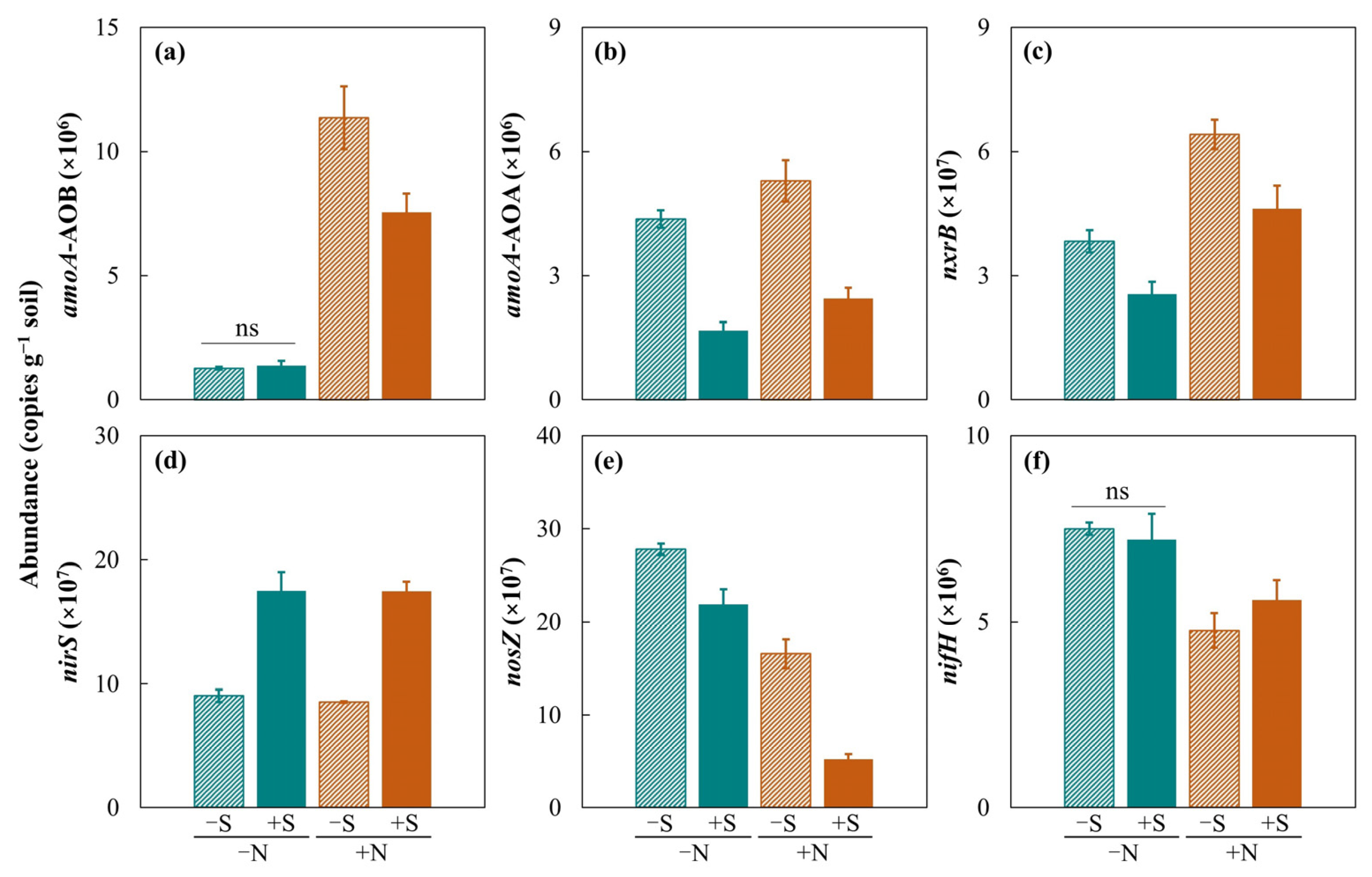
3.5. Soil Nitrogen Cycling Genes
4. Discussion
4.1. Effects of Straw Return on Crop Yield and N Uptake
4.2. Effects of Straw Return on Soil Properties
4.3. Effects of Straw Return on the Bacterial Community Composition
4.4. Effects of Straw Return on Soil Nitrogen Cycling Genes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, Y.; Sidhu, H.S. Management of cereal crop residues for sustainable rice-wheat production system in the Indo-Gangetic plains of India. Proc. Natl. Acad. Sci. USA 2014, 80, 95–114. [Google Scholar] [CrossRef]
- Raffa, D.W.; Bogdanski, A.; Tittonell, P.A. How does crop residue removal affect soil organic carbon and yield? A hierarchical analysis of management and environmental factors. Biomass Bioenergy 2015, 81, 345–355. [Google Scholar] [CrossRef]
- Li, J.; Gan, G.; Chen, X.; Zou, J. Effects of long-term straw management and potassium fertilization on crop yield, soil properties, and microbial community in a rice-oilseed rape rotation. Agriculture 2021, 11, 1233. [Google Scholar] [CrossRef]
- Lv, X.; Wang, Z.; Ma, L.; Cao, N.; Meng, Y.; Zhou, Z. Crop residue incorporation combined with potassium fertilizer increased cotton canopy apparent photosynthesis and seed cotton yield in barley-cotton rotation system. Arch. Agron. Soil Sci. 2021, 67, 300–312. [Google Scholar] [CrossRef]
- Wang, K.; Hu, W.; Xu, Z.; Xue, Y.; Zhang, Z.; Liao, S.; Zhang, Y.; Li, X.; Ren, T.; Cong, R.; et al. Seasonal temporal characteristics of in situ straw decomposition in different types and returning methods. J. Soil Sci. Plant Nutr. 2022, 22, 4228–4240. [Google Scholar] [CrossRef]
- Li, X.; Zuo, Q.; Chang, H.; Bai, G.; Kuai, J.; Zhou, G. Higher density planting benefits mechanical harvesting of rapeseed in the Yangtze River Basin of China. Field Crops Res. 2018, 218, 97–105. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhuang, M. Bottom-up re-estimations of greenhouse gas and atmospheric pollutants derived from straw burning of three cereal crops production in China based on a national questionnaire. Environ. Sci. Pollut. Res. 2021, 28, 65410–65415. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Liu, T.; Zhou, M. Straw burning, PM2.5, and death: Evidence from China. J. Dev. Econ. 2020, 145, 102468. [Google Scholar] [CrossRef]
- Qiao, Y.; Miao, S.; Zhong, X.; Zhao, H.; Pan, S. The greatest potential benefit of biochar return on bacterial community structure among three maize-straw products after eight-year field experiment in Mollisols. Appl. Soil Ecol. 2020, 147, 103432. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xin, L.; Liu, J.T.; Yuan, M.Z.; Liu, S.T.; Jiang, W.; Chen, J.P. Changes in bacterial community of soil induced by long-term straw returning. Sci. Agric. 2017, 74, 349–356. [Google Scholar] [CrossRef]
- Nannipieri, P.; Eldor, P. The chemical and functional characterization of soil N and its biotic components. Soil Boil. Biochem. 2009, 41, 2357–2369. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Coffman, C.B.; Mangum, R.W. Potential long-term benefits of no-tillage and organic cropping systems for grain production and soil improvement. Agron. J. 2007, 99, 1297–1305. [Google Scholar] [CrossRef]
- Anglade, J.; Billen, G.; Garnier, J.; Makridis, T.; Puech, T.; Tittel, C. Nitrogen soil surface balance of organic vs conventional cash crop farming in the Seine watershed. Agric. Syst. 2015, 139, 82–92. [Google Scholar] [CrossRef]
- Wang, Z.; Miao, Y.; Li, S. Effect of ammonium and nitrate nitrogen fertilizers on wheat yield in relation to accumulated nitrate at different depths of soil in drylands of China. Field Crops Res. 2015, 183, 211–224. [Google Scholar] [CrossRef]
- Geisseler, D.; Lazicki, P.A.; Scow, K.M. Mineral nitrogen input decreases microbial biomass in soils under grasslands but not annual crops. Appl. Soil Ecol. 2016, 106, 1–10. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Till. Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.; Huang, Q.; Zhang, R.; Li, R.; Shen, B.; Shen, Q. Effects of organic-inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice-wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Saha, R.; Mishra, V.K.; Majumdar, B.; Laxminarayana, K.; Ghosh, P.K. Effect of integrated nutrient management on soil physical properties and crop productivity under a maize (Zea mays)-mustard (Brassica campestris) cropping sequence in acidic soils of Northeast India. Commun. Soil Sci. Plant Anal. 2010, 41, 2187–2200. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Hati, K.M.; Swarup, A.; Mishra, B.; Manna, M.C.; Wanjari, R.H.; Mandal, K.G.; Misra, A.K. Impact of long-term application of fertilizer, manure and lime under intensive cropping on physical properties and organic carbon content of an Alfisol. Geoderma 2008, 148, 173–179. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y.; Wu, Z.; Yan, X.; Gunina, A.; Kuzyakov, Y.; Xiong, Z. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J. Clean. Prod. 2020, 242, 118435. [Google Scholar] [CrossRef]
- Brar, B.S.; Singh, J.; Singh, G.; Kaur, G. Effects of long term application of inorganic and organic fertilizers on soil organic carbon and physical properties in maize–wheat rotation. Agronomy 2015, 5, 220–238. [Google Scholar] [CrossRef]
- Gao, Y.; Song, X.; Liu, K.; Li, T.; Zheng, W.; Wang, Y.; Liu, Z.; Zhang, M.; Chen, Q.; Li, Z.; et al. Mixture of controlled-release and conventional urea fertilizer application changed soil aggregate stability, humic acid molecular composition, and maize nitrogen uptake. Sci. Total Environ. 2021, 789, 147778. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xue, S.; Wang, G.; Liu, G. Effects of nitrogen addition on soil oxidisable organic carbon fractions in the rhizospheric and bulk soils of Chinese pines in north-western China. Soil Res. 2018, 56, 192–203. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Marschner, P. Soil respiration, microbial biomass and nutrient availability in soil after addition of residues with adjusted N and P concentrations. Pedosphere 2017, 27, 76–85. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jin, S.; Xiao, H.; Zhang, Z.; Hu, C.; Qiao, Y.; Liu, D.; Guo, X.; Peng, X. Effects of combined application of animal slurry and mineral fertiliser on rice yield and soil nitrogen cycle microbes. Plant Soil Environ. 2024, 70, 220–228. [Google Scholar] [CrossRef]
- Yang, L.; Tan, L.; Zhang, F.; Sang, W. Duration of continuous cropping with straw return affects the composition and structure of soil bacterial communities in cotton fields. Can. J. Microbiol. 2018, 64, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, S.; Hua, Q.; Qiu, C.; Wu, P.; Liu, X.; Lin, X. The long-term effects of using phosphate-solubilizing bacteria and photosynthetic bacteria as biofertilizers on peanut yield and soil bacteria community. Front. Microbiol. 2021, 12, 693535. [Google Scholar] [CrossRef]
- Pan, H.; Ying, S.; Liu, H.; Zeng, L.; Zhang, Q.; Liu, Y.; Xu, J.; Li, Y.; Di, H. Microbial pathways for nitrous oxide emissions from sheep urine and dung in a typical steppe grassland. Biol. Fertil. Soils 2018, 54, 717–730. [Google Scholar] [CrossRef]
- Lin, J.; Xu, Z.; Xue, Y.; Sun, R.; Yang, R.; Cao, X.; Li, H.; Shao, Q.; Lou, Y.; Wang, H.; et al. N2O emissions from soils under short-term straw return in a wheat-corn rotation system are associated with changes in the abundance of functional microbes. Agric. Ecosyst. Environ. 2023, 341, 108217. [Google Scholar] [CrossRef]
- Fu, G.; Wu, J.; Han, J.; Zhao, L.; Leong, K. Effects of substrate type on denitrification efficiency and microbial community structure in constructed wetlands. Bioresour. Technol. 2020, 307, 123222. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, J.; Wang, Z.; Muhammad, R.K.; Lu, J.; Li, X. Soil available potassium affected by rice straw incorporation and potassium fertilizer application under a rice–oilseed rape rotation system. Soil Use Manag. 2019, 35, 503–510. [Google Scholar] [CrossRef]
- Tekliye, L.; Mekuriaw, Y.; Asmare, B.; Mehret, F. Nutrient intake, digestibility, growth performance and carcass characteristics of Farta sheep fed urea-treated rice straw supplemented with graded levels of dried Sesbania sesban leaves. Agric. Food Secur. 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Hu, C.; Xia, X.; Chen, Y.; Qiao, Y.; Liu, D.; Fan, J.; Li, S. Yield, nitrogen use efficiency and balance response to thirty-five years of fertilization in paddy rice-upland wheat cropping system. Plant Soil Environ. 2019, 65, 55–62. [Google Scholar] [CrossRef]
- Hu, C.; Xia, X.; Han, X.; Chen, Y.; Qiao, Y.; Liu, D.; Li, S. Soil organic carbon sequestration as influenced by long-term manuring and fertilization in the rice-wheat cropping system. Carbon. Manag. 2018, 9, 619–629. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, W.; Li, T.; Cheng, X.; Liu, Q. Balancing straw returning and chemical fertilizers in China: Role of straw nutrient resources. Renew. Sustain. Energy. Rev. 2018, 81, 2695–2702. [Google Scholar] [CrossRef]
- Geng, J.; Sun, Y.; Zhang, M.; Li, C.; Yang, Y.; Liu, Z.; Li, S. Long-term effects of controlled release urea application on crop yields and soil fertility under rice-oilseed rape rotation system. Field Crops Res. 2015, 184, 65–73. [Google Scholar] [CrossRef]
- Yousaf, M.; Li, X.; Zhang, Z.; Ren, T.; Cong, R.; Ata-Ul-Karim, S.T.; Fahad, S.; Shah, A.N.; Lu, J. Nitrogen fertilizer management for enhancing crop productivity and nitrogen use efficiency in a rice-oilseed rape rotation system in China. Front. Plant Sci. 2016, 7, 1496. [Google Scholar] [CrossRef]
- Sanchis, E.; Ferrer, M.; Torres, A.G.; Cambra-López, M.; Calvet, S. Effect of water and straw management practices on methane emissions from rice fields: A review through a meta-analysis. Environ. Eng. Sci. 2012, 29, 1053–1062. [Google Scholar] [CrossRef]
- Shan, A.; Pan, J.; Kang, K.J.; Pan, M.; Wang, G.; Wang, M.; He, Z.; Yang, X. Effects of straw return with N fertilizer reduction on crop yield, plant diseases and pests and potential heavy metal risk in a Chinese rice paddy: A field study of 2 consecutive wheat-rice cycles. Environ. Pollut. 2021, 288, 117741. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Z.; Barberán, A.; Yang, Y.; Hu, S.; Guo, H. Nitrogen-induced acidification plays a vital role driving ecosystem functions: Insights from a 6-year nitrogen enrichment experiment in a Tibetan alpine meadow. Soil Biol. Biochem. 2021, 153, 108107. [Google Scholar] [CrossRef]
- Qiu, S.J.; Ju, X.T.; Ingwersen, J.; Guo, Z.D.; Stange, C.F.; Bisharat, R.; Streck, T.; Christie, P.; Zhang, F.S. Role of carbon substrates added in the transformation of surplus nitrate to organic nitrogen in a calcareous soil. Pedosphere 2013, 23, 205–212. [Google Scholar] [CrossRef]
- Durani, A.; Brar, B.S.; Dheri, G.S. Soil nitrogen fractions in relation to rice-wheat productivity: Effects of long-term application of mineral fertilizers and organic manures. J. Crop Improv. 2016, 30, 399–420. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Yu, H.; Ling, N.; Wang, T.; Zhu, C.; Wang, Y.; Wang, S.; Gao, Q. Responses of soil biological traits and bacterial communities to nitrogen fertilization mediate maize yields across three soil types. Soil Till. Res. 2019, 185, 61–69. [Google Scholar] [CrossRef]
- Bay, S.K.; McGeoch, M.A.; Gillor, O.; Wieler, N.; Palmer, D.J.; Baker, D.J.; Chown, S.L.; Greening, C.; Gilbert, J.A. Soil bacterial communities exhibit strong biogeographic patterns at fine taxonomic resolution. mSystems 2020, 5, e00540-20. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Xu, J. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Boil. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, J.; Zhu, Q.; Zhang, Z.; Lin, X. pH is the primary determinant of the bacterial community structure in agricultural soils impacted by polycyclic aromatic hydrocarbon pollution. Sci. Rep. 2017, 7, 40093. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Rhodes, G.; He, J.; Ge, Y. Adaptive responses of comammox Nitrospira and canonical ammonia oxidizers to long-term fertilizations: Implications for the relative contributions of different ammonia oxidizers to soil nitrogen cycling. Sci. Total Environ. 2019, 668, 224–233. [Google Scholar] [CrossRef]
- Bissett, A.; Brown, M.V.; Siciliano, S.D.; Thrall, P.H. Microbial community responses to anthropogenically induced environmental change: Towards a systems approach. Ecol. Lett. 2013, 16, 128–139. [Google Scholar] [CrossRef]
- Lawson, C.E.; Lücker, S. Complete ammonia oxidation: An important control on nitrification in engineered ecosystems? Curr. Opin. Biotechnol. 2018, 50, 158–165. [Google Scholar] [CrossRef]
- Ouyang, Y.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. Effect of nitrogen fertilization on the abundance of nitrogen cycling genes in agricultural soils: A meta-analysis of field studies. Soil Boil. Biochem. 2018, 127, 71–78. [Google Scholar] [CrossRef]
- Huang, L.; Riggins, C.W.; Rodríguez-Zas, S.; Zabaloy, M.C.; Villamil, M.B. Long-term N fertilization imbalances potential N acquisition and transformations by soil microbes. Sci. Total Environ. 2019, 691, 562–571. [Google Scholar] [CrossRef]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Henry, S.; Bru, D.; Stres, B.; Hallet, S.; Philippot, L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 2006, 72, 5181–5189. [Google Scholar] [CrossRef]
- Ouyang, Y.; Reeve, J.R.; Norton, J.M. Soil enzyme activities and abundance of microbial functional genes involved in nitrogen transformations in an organic farming system. Biol. Fertil. Soils 2018, 54, 437–450. [Google Scholar] [CrossRef]


| Treatment | Rice Season | Rapeseed Season | ||
|---|---|---|---|---|
| N | Rapeseed Straw | N | Rice Straw | |
| −N−S | 0 | 0 | 0 | 0 |
| −N+S | 0 | 6000 | 0 | 7500 |
| +N−S | 180 | 0 | 180 | 0 |
| +N+S | 180 | 6000 | 180 | 7500 |
| Year-Stage | Treatment | SOM (g kg−1) | TN (g kg−1) | NH4+ (mg kg−1) | NO3− (mg kg−1) | pH |
|---|---|---|---|---|---|---|
| 2020- Rice harvest | −N−S | 28.4 c | 1.64 a | 3.70 b | 1.08 a | 6.33 a |
| −N+S | 29.7 bc | 1.63 a | 3.70 b | 1.06 a | 6.24 a | |
| +N−S | 30.8 b | 1.66 a | 4.90 a | 1.16 a | 6.18 a | |
| +N+S | 32.3 a | 1.66 a | 4.84 a | 1.15 a | 6.21 a | |
| 2021- Rapeseed harvest | −N−S | 27.5 c | 1.51 c | 2.84 a | 1.27 b | 6.27 a |
| −N+S | 32.5 a | 1.91 a | 2.98 a | 1.44 b | 6.23 a | |
| +N−S | 30.7 b | 1.68 b | 2.90 a | 1.28 b | 6.19 a | |
| +N+S | 32.8 a | 1.82 a | 3.25 a | 1.87 a | 6.16 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhang, Z.; Hu, C.; Liu, D.; Qiao, Y.; Xiao, Z.; Wu, Y. Short-Term Straw Return Combined with Nitrogen Fertilizer Alters the Soil Nitrogen Supply in Rice–Rapeseed Planting Systems. Agronomy 2024, 14, 1226. https://doi.org/10.3390/agronomy14061226
Wu H, Zhang Z, Hu C, Liu D, Qiao Y, Xiao Z, Wu Y. Short-Term Straw Return Combined with Nitrogen Fertilizer Alters the Soil Nitrogen Supply in Rice–Rapeseed Planting Systems. Agronomy. 2024; 14(6):1226. https://doi.org/10.3390/agronomy14061226
Chicago/Turabian StyleWu, Haicheng, Zhi Zhang, Cheng Hu, Donghai Liu, Yan Qiao, Zhuoxi Xiao, and Yupeng Wu. 2024. "Short-Term Straw Return Combined with Nitrogen Fertilizer Alters the Soil Nitrogen Supply in Rice–Rapeseed Planting Systems" Agronomy 14, no. 6: 1226. https://doi.org/10.3390/agronomy14061226






