Boosting Drought Tolerance in Tomatoes through Stimulatory Action of Salicylic Acid Imparted Antioxidant Defense Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Planting Material and Experimental Layout
2.2. Preparation of Salicylic Acid Solution for Foliar Application
2.3. Assessment of Morphological Parameters
2.4. Assessment of Physiological Parameters
2.4.1. Leaf Membrane Stability Index
2.4.2. Leaf Relative Water Content
2.5. Assessment of Biochemical Parameters
2.5.1. Lipid Peroxidation
2.5.2. Antioxidant Enzyme Activity
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Result
3.1. Morphological Parameters
3.1.1. Plant Height
3.1.2. Leaf Area
3.1.3. Shoot Fresh and Dry Weight
3.1.4. Root Fresh and Dry Weight
3.2. Physiological Parameters
3.2.1. Leaf Membrane Stability Index (LMSI%)
3.2.2. Leaf Relative Water Content (%)
3.2.3. Lipid Peroxidation (MDA nmol g−1 fw)
3.2.4. Anti-Oxidant Enzyme Activity
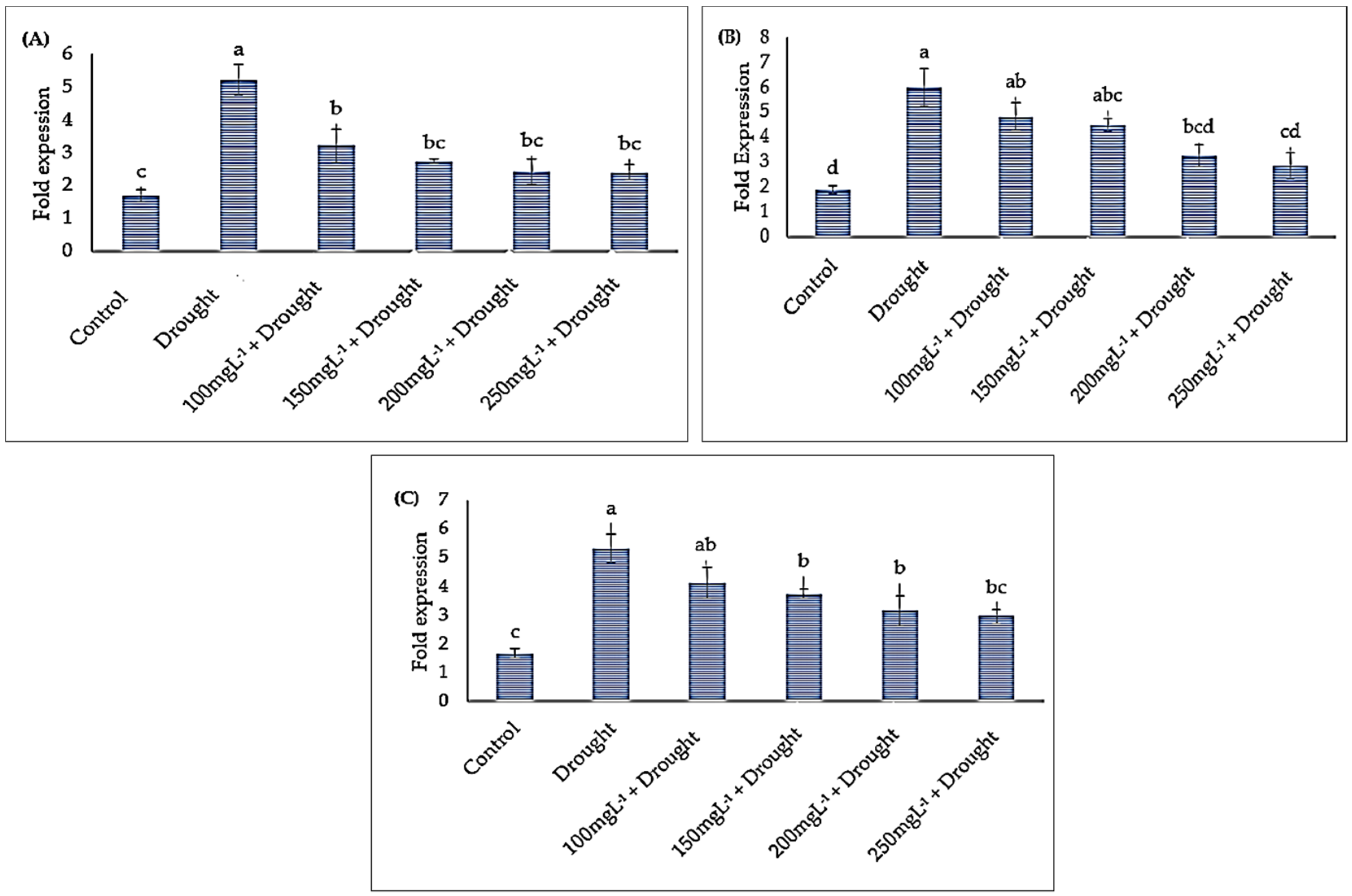
3.3. Anti-Oxidant Genes Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aires, E.S.; Ferraz, A.K.L.; Carvalho, B.L.; Teixeira, F.P.; Rodrigues, J.D.; Ono, E.O. Foliar Application of Salicylic Acid Intensifies Antioxidant System and Photosynthetic Efficiency in Tomato Plants. Bragantia 2022, 81, e1522. [Google Scholar] [CrossRef]
- Wilcox, J.K.; Catiganani, G.L.; Lazarus, S. Tomato and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2003, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.K.; Parveen, A.; Jamwal, G.; Basu, U.; Kumar, R.R.; Rai, P.K.; Sharma, J.P.; Alalawy, A.I.; Al-Duais, M.A.; Hossain, M.A.; et al. Leaf Proteome Response to Drought Stress and Antioxidant Potential in Tomato (Solanum lycopersicum L.). Atmosphere 2021, 12, 1021. [Google Scholar] [CrossRef]
- Di Cesare, L.F.; Migliori, C.; Ferrari, V.; Parisi, M.; Campanelli, G.; Candido, V.; Perrone, D. Effects of irrigation-fertilization and irrigation-mycorrhization on the alimentary and nutraceutical properties of tomatoes. In Irrigation Systems and Practices in Challenging Environments; Lee, T.S., Ed.; TECH Press: Rijeka, Croatia, 2012; pp. 207–332. [Google Scholar]
- Singh, J.; Rai, G.; Upadhyay, A.; Kumar, R.; Singh, K. Antioxidant phytochemicals in tomato (Lycopersicon esculentum). Indian J. Agric. Sci. 2004, 74, 3–5. [Google Scholar]
- Rai, G.K.; Kumar, R.; Singh, A.K.; Rai, P.K.; Rai, M.; Chaturvedi, A.K.; Rai, A.B. Changes in antioxidant and phytochemical properties of tomato (Lycopersicon esculentum mill.) under ambient condition. Pak. J. Bot. 2012, 44, 667–670. [Google Scholar]
- Kissoudis, C.; Sunarti, S.; Van deWiel, C.; Visser, R.G.F.; van der Linden, C.G.; Bai, Y. Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J. Exp. Bot. 2016, 67, 5119–5132. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. 2022. Available online: http://faostat3.fao.org/home/E (accessed on 5 April 2024).
- Kaur, G.; Kumar, S.; Nayyar, H.; Upadhyaya, H.D. Cold stress injury during the pod-filling phase in chickpea (Cicer arietinum L.): Effects on quantitative and qualitative components of seeds. J. Agron. Crop Sci. 2008, 194, 457–464. [Google Scholar] [CrossRef]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Pang, E. 2012. Abiotic stress responses in plants: Present and future. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2015; pp. 1–19. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought stress responses in plants, oxidative stress, and antioxidant defense. In Climate Change and Plant Abiotic Stress Tolerance; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 209–250. [Google Scholar]
- Hayat, S.; Hasan, S.A.; Fariduddin, Q.; Ahmad, A. Growth of Tomato (Lycopersicon esculentum) in Response to Salicylic Acid under Water Stress. J. Plant Interact. 2008, 3, 297–304. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Rai, G.K.; Bagati, S.; Rai, P.K. (Eds.) Reactive Oxygen Species Generation, Antioxidants and Regulating Genes in Crops under Abiotic Stress Conditions. In Abiotic Stress Tolerance Mechanisms in Plants; Narendra Publishing House: New Delhi, India, 2018. [Google Scholar]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef]
- Senaratna, T.; Merritt, D.; Dixon, K.; Bunn, E.; Touchell, D.; Sivasithamparam, K. Benzoic acid may act as the functional group in salicylic acid and derivatives in the induction of multiple stress tolerance in plants. Plant Growth Regul. 2003, 39, 77–81. [Google Scholar] [CrossRef]
- Souri, M.K.; Tohidloo, G. Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem. Biol. Technol. Agric. 2019, 6, 26. [Google Scholar] [CrossRef]
- Yusuf, M.; Hayat, S.; Alyemeni, M.N.; Fariduddin, Q.; Ahmad, A. Salicylic acid: Physiological Roles in Plants. In Salicylic Acid; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–30. [Google Scholar]
- Lakzayi, M.; Sabbagh, E.; Rigi, K.; Keshtehgar, A. Effect of salicylic acid on activities of antioxidant enzymes, flowering and fruit yield and the role on reduce of drought stress. Int. J. Farming Allied Sci. 2014, 3, 980–987. [Google Scholar]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Chakma, R.; Biswas, A.; Saekong, P.; Ullah, H.; Datta, A. Foliar application and seed priming of salicylic acid affect growth, fruit yield, and quality of grape tomato under drought stress. Sci. Hortic. 2021, 280, 109904. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. 2011. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Orabi, S.A.; Dawood, M.G.; Salman, S.R. Comparative study between the physiological role of hydrogen peroxide and salicylic acid in alleviating the harmful effect of low temperature on tomato plants grown under sandponic culture. Sci. Agric. 2015, 9, 49–59. [Google Scholar]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Vinod, S. Salicylic acid functionalized chitosan nanoparticle: A sustainable biostimulant for plant. Int. J. Biol. Macromol. 2019, 123, 59–69. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, L.; Shi, Y.; Su, D.; Lu, W.; Cheng, Y.; Li, Z. Stress-responsive tomato gene SlGRAS4 function in drought stress and abscisic acid signalling. Plant Sci. 2021, 304, 110804. [Google Scholar] [CrossRef] [PubMed]
- El-Hady, N.A.A.A.; ElSayed, A.I.; El-Saadany, S.S.; Deligios, P.A.; Ledda, L. Exogenous application of foliar salicylic acid and propolis enhances antioxidant defenses and growth parameters in tomato plants. Plants 2021, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. J. Agric. Sci. 1990, 115, 63–66. [Google Scholar] [CrossRef]
- Galmes, J.; Flexas, J.; Robert, S. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: Responses to water stress and recovery. Plant Soil 2007, 290, 139–155. [Google Scholar] [CrossRef]
- Hodges, M.D.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Jogeswar, G.; Pallela, R.; Jakka, N.M.; Reddy, P.S.; Venkateswara Rao, J.; Sreenivasulu, N.; Kavi Kishor, P.B. Antioxidative response in different sorghum species under short-term salinity stress. Acta Physiol. Plant. 2006, 28, 465–475. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of Catalase and Peroxidase. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Li, B.; Chen, C.; Xu, Y.; Ji, D.; Xie, C. Validation of housekeeping genes as internal controls for studying the gene expression in Pyropia haitanensis (Bangiales, Rhodophyta) by quantitative real-time PCR. Acta Oceanol. Sin. 2014, 33, 152–159. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Song, W.; Shao, H.; Zheng, A.; Zhao, L.; Xu, Y. Advances in roles of salicylic acid in plant tolerance responses to biotic and abiotic stresses. Plants 2023, 12, 3475. [Google Scholar] [CrossRef]
- Ghanbari, F.; Saidi, M.; Akbari, S.; Gravand, S. The effects of salicylic acid and kaolin on growth, yield and some physiological responses of tomato under different irrigation intervals. J. Plant Process Funct. 2021, 44, 219–234. [Google Scholar]
- Iosob, G.A.; Cristea, T.O.; Avasiloaiei, D.I.; Bute, A.; Muscalu, S.P. Drought stress and the role of Salicylic acid in relieving the oxidative damage at tomato plants. Horticulture 2023, 67, 608–613. [Google Scholar]
- Alam, P.; Balawi, T.A.; Faizan, M. Salicylic Acid’s Impact on Growth, Photosynthesis, and Antioxidant Enzyme Activity of Triticum aestivum When Exposed to Salt. Molecules 2023, 28, 100. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.S.; MAbu-Elsaoud, A.; Hafez, Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Qadir, A.; Anjum, M.A.; Nawaz, A.; Ejaz, S.; Altaf, M.A.; Shahid, R.; Hassan, A. Growth of Cherry Tomato in Response to Salicylic Acid and Glycinebetaine under Water Stress Condition. Middle East J. 2019, 8, 762–775. [Google Scholar]
- Javanmardi, J.; Akbari, N. Salicylic acid at different plant growth stages affects secondary metabolites and phisico-chemical parameters of greenhouse tomato. Adv. Hortic. Sci. 2016, 33, 151–158. [Google Scholar]
- Hassanein, R.A.; Amin, A.A.E.; Rashad, E.S.M.; Ali, H. Effect of thiourea and salicylic acid on antioxidant defense of wheat plants under drought stress. Int. J. ChemTech Res. 2015, 7, 346–354. [Google Scholar]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Chhaya Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol. Plant. 2021, 172, 1106–1132. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Hussain, S.; Jalal, F.; Khan, M.A.; Imtiaz, M.; Said, F.; Ismail, M.; Khan, S.; Ali, H.M.; Hatamleh, A.A.; et al. Salicylic acid-mitigates abiotic stress tolerance via altering defense mechanisms in Brassica napus (L.). Front. Plant Sci. 2023, 14, 1187260. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yue, J.; Nie, J.; Luo, D.; Cao, S.; Wang, C.; Pan, J.; Chen, C.; Zhang, H.; Wu, Q.; et al. Salicylic acid alleviates the salt toxicity in kenaf by activating antioxidant system and regulating crucial pathways and genes. Ind. Crops Prod. 2023, 199, 116691. [Google Scholar] [CrossRef]
| Primer Name | Sequence | Annealing Temp. (°C) |
|---|---|---|
| β-actin (F) | TTGACTGAGGCACCACTTAACCCT | 68.7 |
| β-actin (R) | GCTTTCAGGTGGTGCAACGACTTT | 71.0 |
| SOD (F) | CACGTCTTCAAAGCAAGTGG | 63.5 |
| SOD (R) | CTAAGAAGAAGGGCATTCTTTGGCAT | 68.7 |
| CAT (F) | GATGAGCACACTTTGGAGCA | 64.1 |
| CAT (R) | TGCC CTTCTATTGTGGTTCC | 63.8 |
| APX (F) | GAAACTCAGAGGACTCATTGCTGAGAAGAATTG | 72.9 |
| APX (R) | GAAACTGCTCCCTAATGGGCTCCAAGAG | 73.9 |
| Treatments | Plant Height (cm) | Leaf Area (cm2) | Stem Fresh Weight (g) | Stem Dry Weight (g) | Root Fresh Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|
| T0-Control | 79.72 ± 1.03 a | 1651.93 ± 0.17 a | 26.70 ± 0.473 a | 13.1 ± 0.14 a | 17.42 ± 0.09 e | 1.96 ± 0.01 f |
| T1-Drought | 52.40 ± 0.47 e | 1340.92 ± 0.35 f | 18.52 ± 0.012 d | 8.37 ± 0.98 ae | 19.99 ± 0.06 | 2.10 ± 0.08 e |
| T2-100 mg L−1 + Drought | 53.90 ± 0.47 d | 1356.05 ± 0.42 e | 19.66 ± 0.018 c | 9.63 ± 0.01 d | 24.42 ± 0.09 c | 2.63 ± 0.00 d |
| T3-150 mg L−1 + Drought | 64.9 ± 0.38 c | 1562.97 ± 0.69 d | 24.54 ± 0.012 b | 12.55 ± 0.0 c | 26.46 ± 0.00 | 3.11 ± 0.00 c |
| T4-200 mg L−1 + Drought | 72.0 ± 0.45 b | 1640.02 ± 0.47 c | 26.85 ± 0.012 a | 12.84 ± 0.0 b | 27.35 ± 0.12 a | 3.22 ± 0.01 b |
| T5-250 mg L−1 + Drought | 72.11 ± 0.46 b | 1649.97 ± 0.63 b | 26.77 ± 0.018 a | 13.11 ± 0.0 a | 27.57 ± 0.38 a | 3.36 ± 0.01 a |
| Treatments | LMSI (%) | LRWC (%) | Lipid Peroxidation (nmol g−1 fw) |
|---|---|---|---|
| T0-Control | 46.92 ± 0.09 d | 73.92 ± 0.09 a | 0.67 ± 0.0 f |
| T1-Drought | 40.05 ± 0.10 f | 58.10 ± 0.08 f | 2.36 ± 0.0 a |
| T2-100 mg L−1 + Drought | 43.52 ± 0.01 e | 62.10 ± 0.08 e | 1.97 ± 0.0 b |
| T3-150 mg L−1 + Drought | 48.14 ± 0.01 b | 66.92 ± 0.09 d | 1.12 ± 0.0 c |
| T4-200 mg L−1 + Drought | 48.25 ± 0.01 a | 70.14 ± 0.00 c | 0.98 ± 0.0 e |
| T5-250 mg L−1 + Drought | 47.13 ± 0.01 c | 71.58 ± 0.00 b | 1.10 ± 0.0 d |
| Treatments | Superoxide Dismutase (Unit min−1 g−1 FW) | Ascorbate Peroxidase (μmol min−1 mg−1 FW) | Catalase (μmol min−1 mg−1 FW) |
|---|---|---|---|
| Control | 24.10 ± 0.08 e | 117.94 ± 0.87 f | 31.92 ± 0.43 f |
| Drought | 27.13 ± 0.12 d | 120.59 ± 0.91 e | 34.57 ± 0.54 e |
| Drought + 100 mg L−1 | 28.29 ± 0.12 c | 134.90 ± 0.95 d | 49.52 ± 0.71 d |
| Drought + 150 mg L−1 | 30.08 ± 0.11 b | 148.7 ± 0.68 c | 62.69 ± 0.50 c |
| Drought + 200 mg L−1 | 30.95 ± 0.50 a | 152.86 ± 0.22 b | 75.39 ± 0.47 b |
| Drought + 250 mg L−1 | 31.23 ± 0.01 a | 172.28 ± 0.53 a | 89.16 ± 0.79 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, G.K.; Magotra, I.; Khanday, D.M.; Choudhary, S.M.; Bhatt, A.; Gupta, V.; Rai, P.K.; Kumar, P. Boosting Drought Tolerance in Tomatoes through Stimulatory Action of Salicylic Acid Imparted Antioxidant Defense Mechanisms. Agronomy 2024, 14, 1227. https://doi.org/10.3390/agronomy14061227
Rai GK, Magotra I, Khanday DM, Choudhary SM, Bhatt A, Gupta V, Rai PK, Kumar P. Boosting Drought Tolerance in Tomatoes through Stimulatory Action of Salicylic Acid Imparted Antioxidant Defense Mechanisms. Agronomy. 2024; 14(6):1227. https://doi.org/10.3390/agronomy14061227
Chicago/Turabian StyleRai, Gyanendra Kumar, Isha Magotra, Danish Mushtaq Khanday, Sadiya M. Choudhary, Anil Bhatt, Vinod Gupta, Pradeep Kumar Rai, and Pradeep Kumar. 2024. "Boosting Drought Tolerance in Tomatoes through Stimulatory Action of Salicylic Acid Imparted Antioxidant Defense Mechanisms" Agronomy 14, no. 6: 1227. https://doi.org/10.3390/agronomy14061227








