Exploring the Impact of Tea (Camellia sinensis (L.) O. Ktze.)/Trachelospermum jasminoides (Lindl.) Lem. Intercropping on Soil Health and Microbial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Study Area
2.2. Soil Sample Collection
2.3. Determination of Soil Physicochemical Properties and Enzyme Activities
2.4. Soil Total DNA Extraction
2.5. High-Throughput Sequencing Analysis of 16S/ITS rDNA
2.6. Data Analysis
3. Results
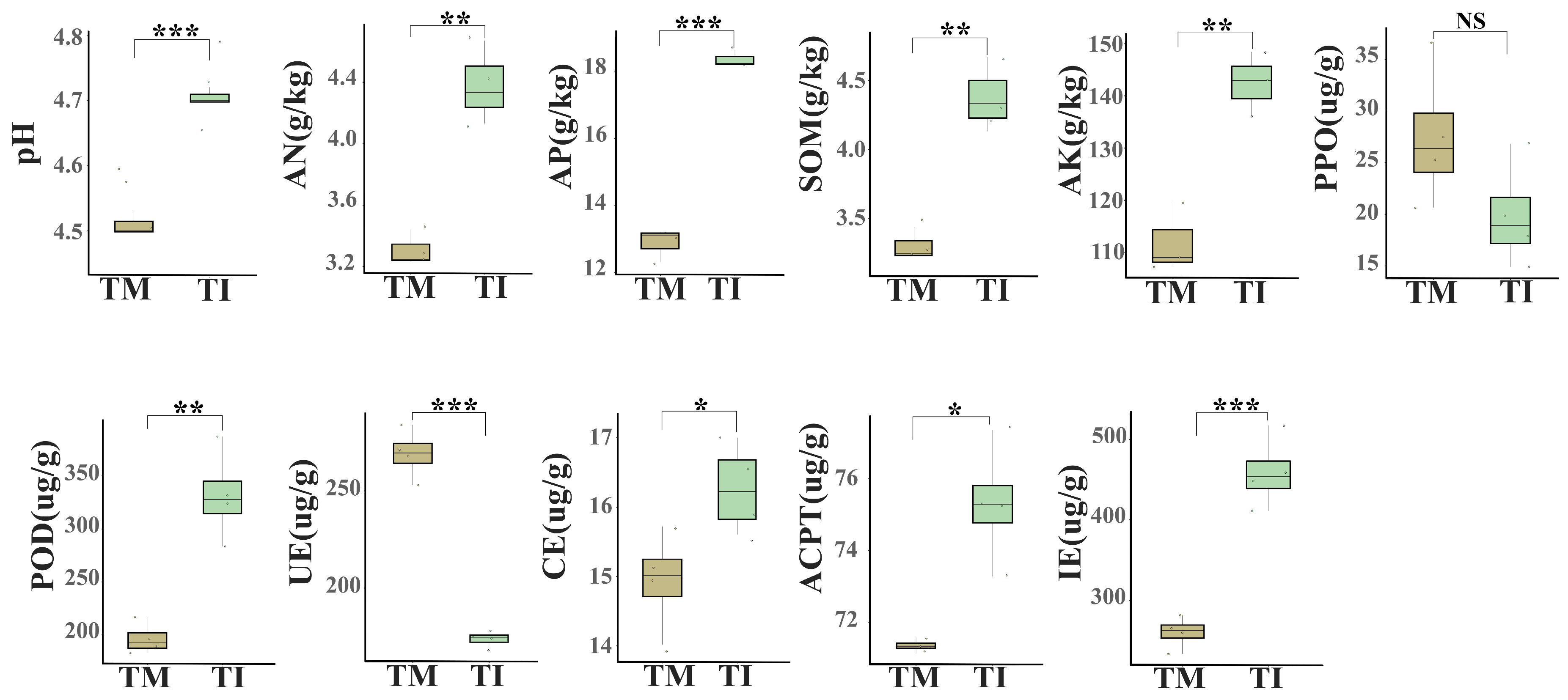
3.1. Impacts of Intercropping on Soil Physicochemical Properties and Enzyme Activities
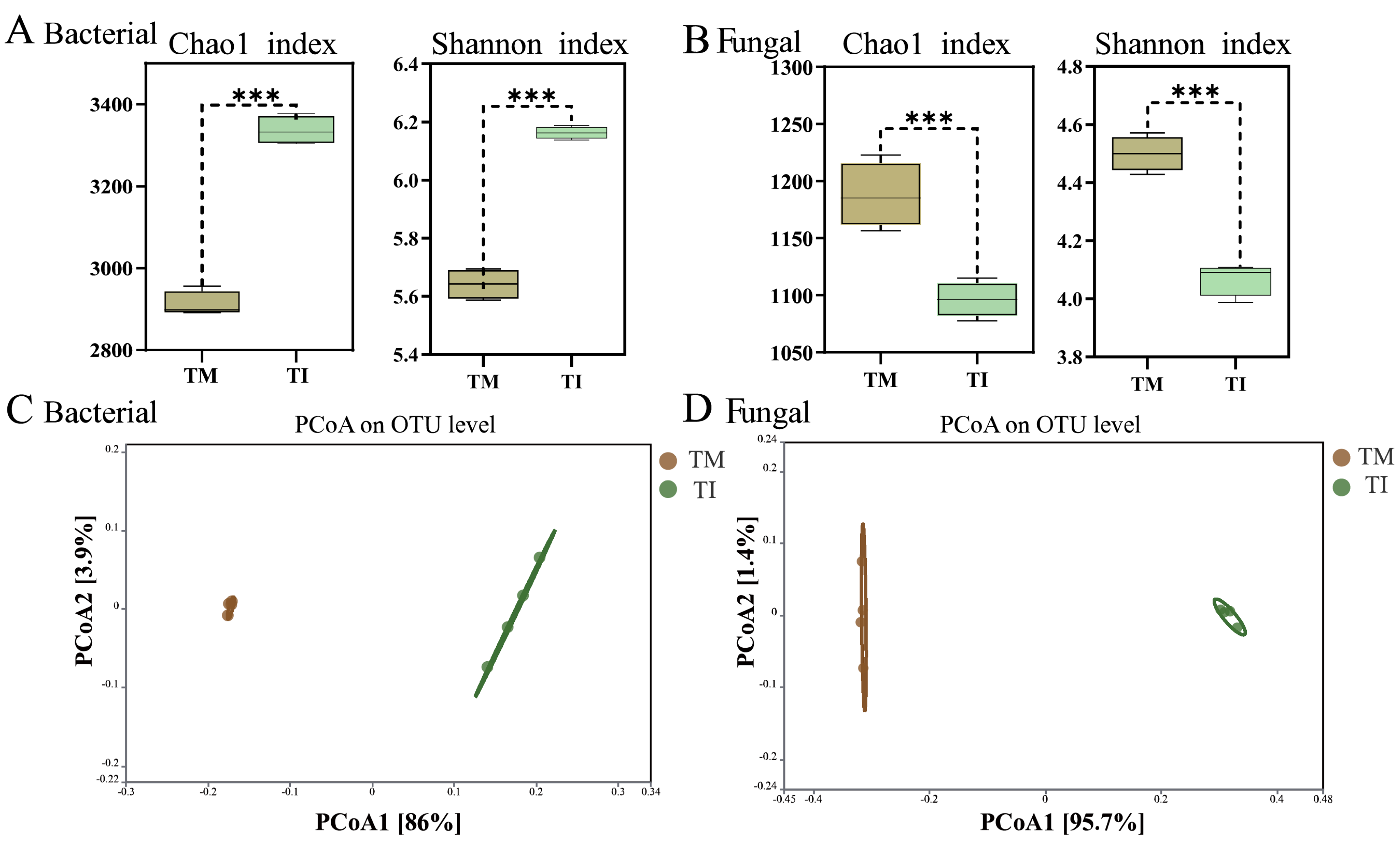
3.2. Effects of Intercropping on α-Diversity and β-Diversity of Soil Microorganisms
3.3. Impact of Intercropping on Soil Microbial Co-Occurrence Networks
3.4. Impact of Intercropping on Soil Microbial Community Structure
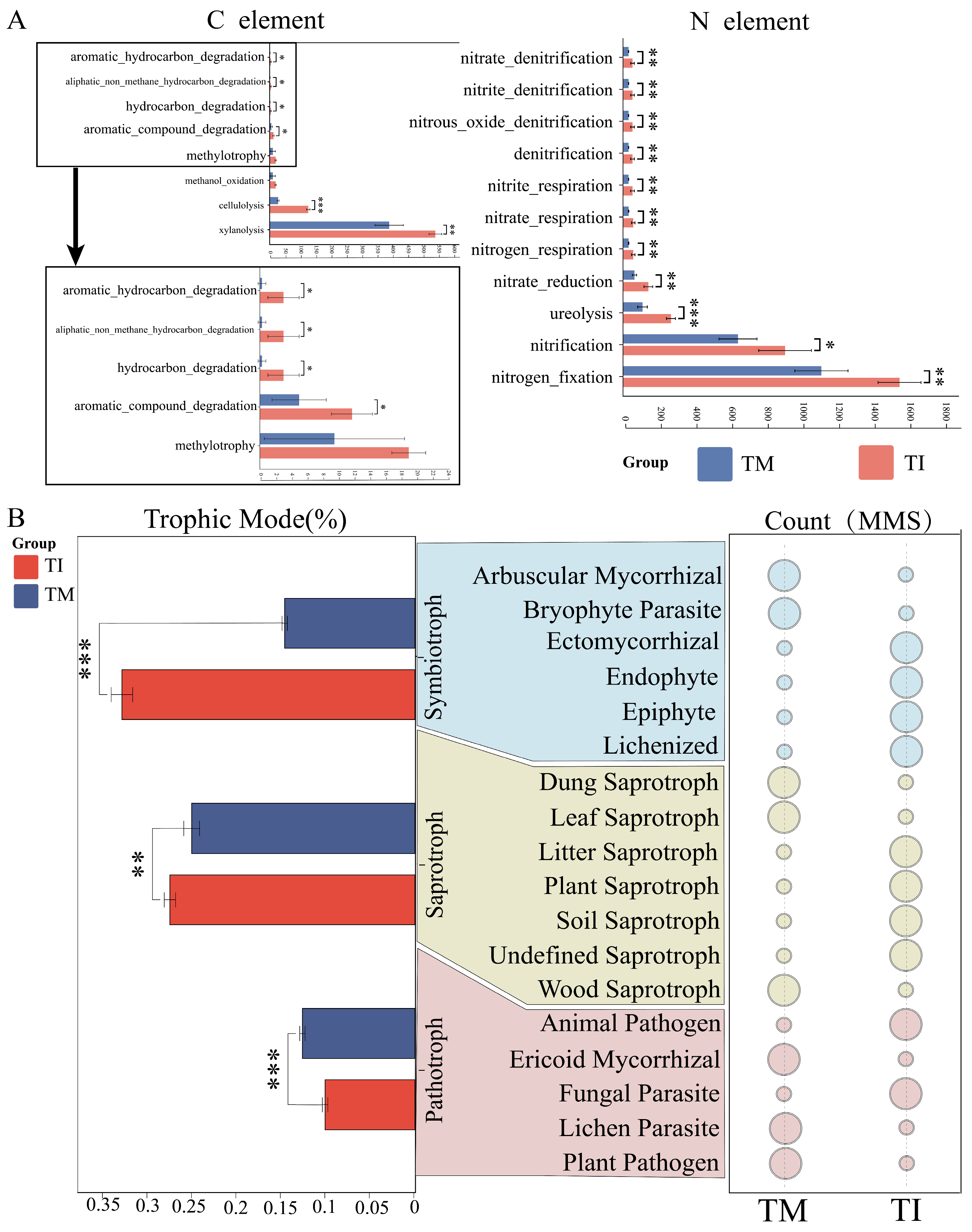
3.5. Impact of Intercropping on Soil Bacterial Functions and Fungal Nutrient Modes
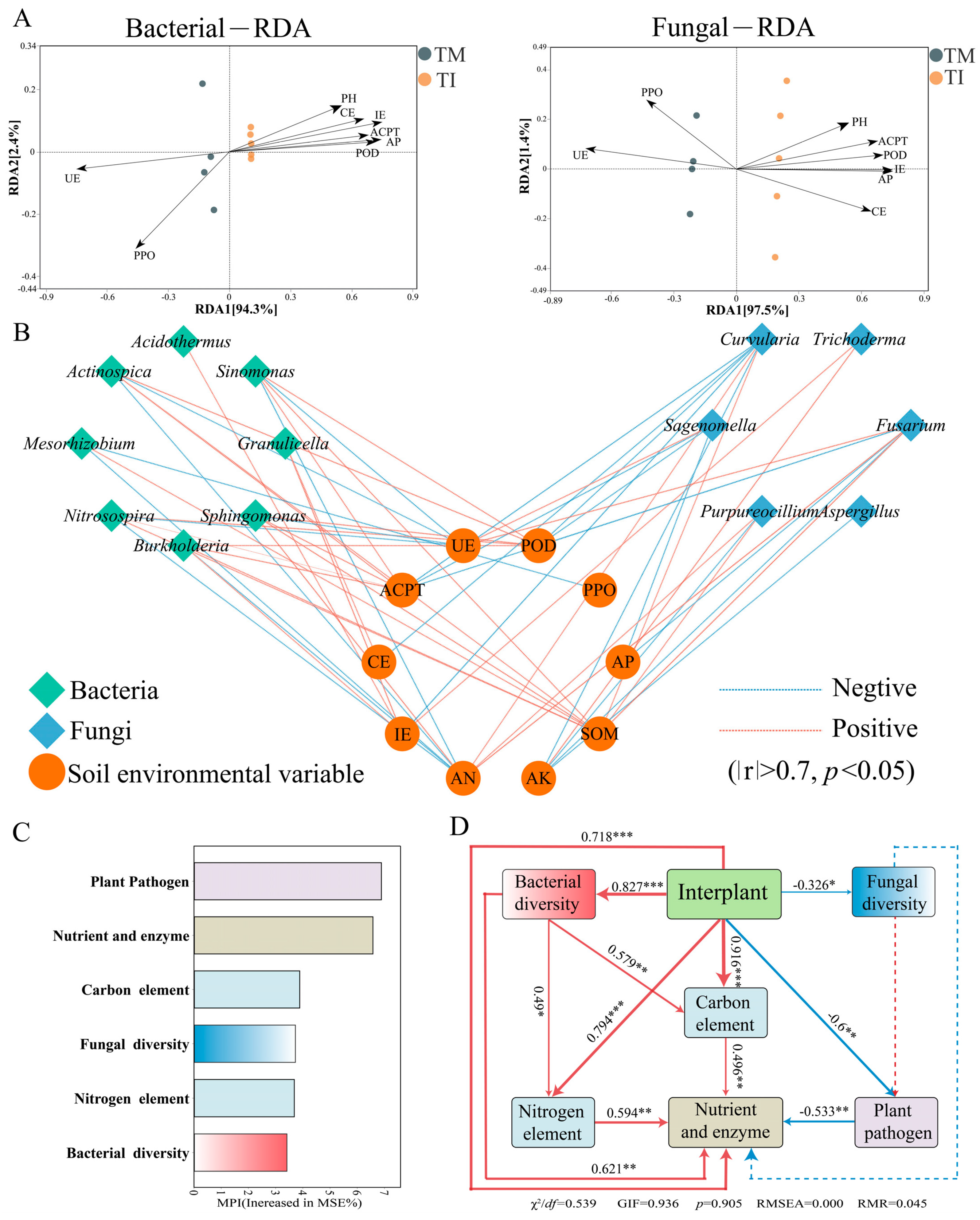
3.6. Impact of Intercropping on Soil Environmental Variables and Microbial Correlations
4. Discussion
4.1. Intercropping Significantly Influences the Microbial Community Structure in Tea Soil
4.2. Intercropping Cultivation Improves Soil Nutrient Levels by Promoting Soil C and N Cycling
4.3. Intercropping Cultivation Suppresses Soil Pathogenic Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.H.; Won, Y.S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green Tea Catechin Metabolites Exert Immunoregulatory Effects on CD4 (+) T Cell and Natural Killer Cell Activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tian, C.; Zhu, C.; Lai, Z.; Lin, Y.; Guo, Y. Hidden players in the regulation of secondary metabolism in tea plant: Focus on non-coding RNAs. Beverage Plant Res. 2022, 2, 19. [Google Scholar] [CrossRef]
- Duan, Y.; Shang, X.; Liu, G.; Zou, Z.; Zhu, X.; Ma, Y.; Li, F.; Fang, W. The effects of tea plants-soybean intercropping on the secondary metabolites of tea plants by metabolomics analysis. BMC Plant Biol. 2021, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.G.; Huang, J.A.; Li, J.; Yu, P.H.; Xiong, Z.; Zhang, J.W.; Gong, Y.S.; Liu, Z.H.; Chen, J.H. Black tea increased survival of Caenorhabditis elegans under stress. J. Agric. Food Chem. 2014, 62, 11163–11169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, T.; Zheng, Z. Effect of tea plantation age on the distribution of soil organic carbon and nutrient within micro-aggregates in the hilly region of western Sichuan, China. Ecol. Eng. 2016, 90, 113–119. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Y.; Rensing, C.; Ye, J.; Qiu, J.; Li, Q.; Wu, L.; Lu, Q.; Lin, Y.; Jia, X. Improvement of phenolic acid autotoxicity in tea plantations by Pseudomonas fluorescens ZL22. J. Hazard. Mater. 2023, 458, 131957. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.; Li, Z.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef]
- Willey, R.W. Resource use in intercropping systems. Agric. Water Manag. 1990, 17, 215–231. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Zhou, B.; Tang, H.; Chen, Y.; Qiao, X.; Tang, J. Habitat management as a safe and effective approach for improving yield and quality of tea (Camellia sinensis) leaves. Sci. Rep. 2019, 9, 433. [Google Scholar] [CrossRef]
- Wang, Z.; Geng, Y.; Liang, T. Optimization of reduced chemical fertilizer use in tea gardens based on the assessment of related environmental and economic benefits. Sci. Total Environ. 2020, 713, 136439. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Y.; Jiang, J.; Meng, X.; Huang, Z.; Wu, H.; He, L.; Xiong, F.; Liu, J.; Zhong, R.; et al. Sugarcane/peanut intercropping system improves physicochemical properties by changing N and P cycling and organic matter turnover in root zone soil. PeerJ 2021, 9, e10880. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, Z.; Wang, C.; Zhong, Z. Effects of intercropping with persimmon on the rhizosphere environment of tea. Front. Biol. China 2006, 1, 407–410. [Google Scholar] [CrossRef]
- Ma, Y.H.; Fu, S.L.; Zhang, X.P.; Zhao, K.; Chen, H.Y.H. Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Appl. Soil Ecol. 2017, 119, 171–178. [Google Scholar] [CrossRef]
- Lian, T.; Mu, Y.; Jin, J.; Ma, Q.; Cheng, Y.; Cai, Z.; Nian, H. Impact of intercropping on the coupling between soil microbial community structure, activity, and nutrient-use efficiencies. PeerJ 2019, 7, e6412. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhong, R.; Jiang, J.; He, L.; Huang, Z.; Shi, G.; Wu, H.; Liu, J.; Xiong, F.; Han, Z.; et al. Cassava/peanut intercropping improves soil quality via rhizospheric microbes increased available nitrogen contents. BMC Biotechnol. 2020, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.-T.; Lin, S.-H. Priming Effects of Cover Cropping on Bacterial Community in a Tea Plantation. Sustainability 2021, 13, 4345. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, C.; Cao, Y.; Dai, J.; Cheng, X.; Hua, S.; Wang, W.; Duan, Y.; Petropoulos, E.; Wang, H.; et al. Tea plant-legume intercropping simultaneously improves soil fertility and tea quality by changing bacillus species composition. Hortic. Res. 2022, 9, uhac046. [Google Scholar] [CrossRef]
- McSpadden Gardener, B.B. Ecology of Bacillus and Paenibacillus spp. in Agricultural Systems. Phytopathology 2004, 94, 1252–1258. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Ma, D.; Wang, L.; Zhang, X.; Ding, Y.; Fan, K.; Xu, Z.; Yuan, C.; Jia, H.; et al. Differential responses of the rhizosphere microbiome structure and soil metabolites in tea (Camellia sinensis) upon application of cow manure. BMC Microbiol. 2022, 22, 55. [Google Scholar] [CrossRef]
- Diana, A.F.; Widowati, W.; Tjahjana, R.H.; Triyana, E. Stability Analysis of Dynamical Model Interactions Tea Plants, Pests, and Diseases with Fungicides and Insecticides Controls. Int. J. Math. Comput. Res. 2023, 11, 3209–3219. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, Y.; Liu, P.; Xu, R.; Rensing, C.; Fu, X.; Liao, H. Genotype and rhizobium inoculation modulate the assembly of soybean rhizobacterial communities. Plant Cell Environ. 2019, 42, 2028–2044. [Google Scholar] [CrossRef] [PubMed]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Frankeberger, W.T.; Johanson, J.B. Method of measuring invertase activity in soils. Plant Soil 1983, 74, 301–311. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. BioRxiv 2016, 081257. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Zheng, H.; Hu, B.; Dai, X.; Meng, N.; Zhu, J.; Yan, D. Environmental changes drive soil microbial community assembly across arid alpine grasslands on the Qinghai-Tibetan Plateau, China. CATENA 2023, 228, 107175. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Merloti, L.F.; Mendes, L.W.; Pedrinho, A.; de Souza, L.F.; Ferrari, B.M.; Tsai, S.M. Forest-to-agriculture conversion in Amazon drives soil microbial communities and N-cycle. Soil Biol. Biochem. 2019, 137, 107567. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Di Martino, C.; Torino, V.; Minotti, P.; Pietrantonio, L.; Del Grosso, C.; Palmieri, D.; Palumbo, G.; Crawford, T.W.; Carfagna, S. Mycorrhized Wheat Plants and Nitrogen Assimilation in Coexistence and Antagonism with Spontaneous Colonization of Pathogenic and Saprophytic Fungi in a Soil of Low Fertility. Plants 2022, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-Y.; Li, H.; Hao, M.-M.; Ren, Y.-N.; Zhang, M.-K.; Liu, R.-Y.; Zhang, Y.; Li, G.; Chen, J.-S.; Ning, T.-Y.; et al. Nitrogen fixation and crop productivity enhancements co-driven by intercrop root exudates and key rhizosphere bacteria. J. Appl. Ecol. 2021, 58, 2243–2255. [Google Scholar] [CrossRef]
- Wang, G.; Bei, S.; Li, J.; Bao, X.; Zhang, J.; Schultz, P.A.; Li, H.; Li, L.; Zhang, F.; Bever, J.D.; et al. Soil microbial legacy drives crop diversity advantage: Linking ecological plant–soil feedback with agricultural intercropping. J. Appl. Ecol. 2021, 58, 496–506. [Google Scholar] [CrossRef]
- Wu, C.; Ma, Y.; Wang, D.; Shan, Y.; Song, X.; Hu, H.; Ren, X.; Ma, X.; Cui, J.; Ma, Y. Integrated microbiology and metabolomics analysis reveal plastic mulch film residue affects soil microorganisms and their metabolic functions. J. Hazard. Mater. 2022, 423, 127258. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Z.; Wang, J.; Qin, X.; Chen, J.; Wu, L.; Lin, S.; Rensing, C.; Lin, W. Bio-fertilizer Amendment Alleviates the Replanting Disease under Consecutive Monoculture Regimes by Reshaping Leaf and Root Microbiome. Microb. Ecol. 2022, 84, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Senghor, Y.; Balde, A.B.; Manga, A.G.B.; Affholder, F.; Letourmy, P.; Bassene, C.; Kanfany, G.; Ndiaye, M.; Couedel, A.; Leroux, L.; et al. Intercropping millet with low-density cowpea improves millet productivity for low and medium N input in semi-arid central Senegal. Heliyon 2023, 9, e17680. [Google Scholar] [CrossRef]
- Tariq, A.; Sardans, J.; Peñuelas, J.; Zhang, Z.; Graciano, C.; Zeng, F.; Olatunji, O.A.; Ullah, A.; Pan, K. Intercropping of Leguminous and Non-Leguminous Desert Plant Species Does Not Facilitate Phosphorus Mineralization and Plant Nutrition. Cells 2022, 11, 998. [Google Scholar] [CrossRef]
- Ablimit, R.; Li, W.; Zhang, J.; Gao, H.; Zhao, Y.; Cheng, M.; Meng, X.; An, L.; Chen, Y. Altering microbial community for improving soil properties and agricultural sustainability during a 10-year maize-green manure intercropping in Northwest China. J. Environ. Manag. 2022, 321, 115859. [Google Scholar] [CrossRef]
- Yu, H.; Chen, S.; Zhang, X.; Zhou, X.; Wu, F. Rhizosphere bacterial community in watermelon-wheat intercropping was more stable than in watermelon monoculture system under Fusarium oxysporum f. sp. niveum invasion. Plant Soil 2019, 445, 369–381. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Selvaraj, G.; Bais, H.P. Functional soil microbiome: Belowground solutions to an aboveground problem. Plant Physiol. 2014, 166, 689–700. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, L.; Xu, R.; Xu, H.; Sun, L.; Liao, H. Intercropping Tea Plantations with Soybean and Rapeseed Enhances Nitrogen Fixation through Shifts in Soil Microbial Communities. Front. Agric. Sci. Eng. 2022, 9, 344–355. [Google Scholar] [CrossRef]
- Wang, C.; Qu, L.; Yang, L.; Liu, D.; Morrissey, E.; Miao, R.; Liu, Z.; Wang, Q.; Fang, Y.; Bai, E. Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon. Glob. Change Biol. 2021, 27, 2039–2048. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Shi, S.; Lin, M. Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresour. Technol. 2017, 241, 415–423. [Google Scholar] [CrossRef]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef]
- Fang, J.; Yang, R.; Cao, Q.; Dong, J.; Li, C.; Quan, Q.; Huang, M.; Liu, J. Differences of the microbial community structures and predicted metabolic potentials in the lake, river, and wetland sediments in Dongping Lake Basin. Environ. Sci. Pollut. Res. Int. 2020, 27, 19661–19677. [Google Scholar] [CrossRef]
- Lai, C.; Sun, Y.; Guo, Y.; Cai, Q.; Yang, P. A novel integrated bio-reactor of moving bed and constructed wetland (MBCW) for domestic wastewater treatment and its microbial community diversity. Environ. Technol. 2021, 42, 2653–2668. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Baldauf, S.L.; Leyval, C.; Straczek, J.; Young, J.P.W. Extensive Fungal Diversity in Plant Roots. Science 2002, 295, 2051. [Google Scholar] [CrossRef]
- Bastian, F.; Bouziri, L.; Nicolardot, B.; Ranjard, L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Osono, T.; Takeda, H. Fungal decomposition of Abies needle and Betula leaf litter. Mycologia 2006, 98, 172–179. [Google Scholar] [CrossRef][Green Version]
- Yelle, D.J.; Ralph, J.; Lu, F.; Hammel, K.E. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ. Microbiol. 2008, 10, 1844–1849. [Google Scholar] [CrossRef]
- Kusuma, A.B.; Putra, K.E.; Vanggy, L.R.; Loh, J.; Nouioui, I.; Goodfellow, M. Actinospica acidithermotolerans sp. nov., a novel actinomycete isolated from sediment from an Indonesian hot spring. Arch. Microbiol. 2022, 204, 518. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Hu, C.; Zhang, X.; Chang, S.; Yang, L.; Li, Y.; An, Q. Plant growth-promoting nitrogen-fixing enterobacteria are in association with sugarcane plants growing in Guangxi, China. Microbes Environ. 2012, 27, 391–398. [Google Scholar] [CrossRef]
- Dong, M.; Yang, Z.; Cheng, G.; Peng, L.; Xu, Q.; Xu, J. Diversity of the Bacterial Microbiome in the Roots of Four Saccharum Species: S. spontaneum, S. robustum, S. barberi, and S. officinarum. Front. Microbiol. 2018, 9, 328131. [Google Scholar] [CrossRef]
- Soe, K.M.; Htwe, A.Z.; Moe, K.; Tomomi, A.; Yamakawa, T. Diversity and Effectivity of Indigenous Mesorhizobium Strains for Chickpea (Cicer arietinum L.) in Myanmar. Agronomy 2020, 10, 287. [Google Scholar] [CrossRef]
- Zeng, Y.; Nupur; Wu, N.; Madsen, A.M.; Chen, X.; Gardiner, A.T.; Koblížek, M. Gemmatimonas groenlandica sp. nov. Is an Aerobic Anoxygenic Phototroph in the Phylum Gemmatimonadetes. Front. Microbiol. 2020, 11, 606612. [Google Scholar] [CrossRef]
- Shivaramu, S.; Tomasch, J.; Kopejtka, K.; Nupur; Saini, M.K.; Bokhari, S.N.H.; Küpper, H.; Koblížek, M. The Influence of Calcium on the Growth, Morphology and Gene Regulation in Gemmatimonas phototrophica. Microorganisms 2022, 11, 27. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Luo, J.; Di, H.J.; Liu, D.; Fan, J.; Ding, W. Nitrosospira Cluster 8a Plays a Predominant Role in the Nitrification Process of a Subtropical Ultisol under Long-Term Inorganic and Organic Fertilization. Appl. Environ. Microbiol. 2018, 84, e01031-18. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Riaz, M.; Xia, H.; Jiang, C. Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J. Clean. Prod. 2021, 292, 126062. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Líter, J.A.; de Eugenio, L.I.; Nieto-Domínguez, M.; Prieto, A.; Martínez, M.J. Hemicellulases from Penicillium and Talaromyces for lignocellulosic biomass valorization: A review. Bioresour. Technol. 2021, 324, 124623. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; de Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Q.; Kong, C.H.; Wang, P.; Meiners, S.J. Root exudate signals in plant-plant interactions. Plant Cell Environ. 2021, 44, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Madrid, A.M.; Allen, T.W.; Vargas, A.; Connor, A.; Wilkerson, T.H. First Report of Curvularia Leaf Spot of Field Corn, Caused by Curvularia lunata, in Mississippi. Plant Dis. 2022, 106, 1984. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.J.; Barrs, V.R. One-health pathogens in the Aspergillus viridinutans complex. Med. Mycol. 2018, 56, 1–12. [Google Scholar] [CrossRef]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3416–3438. [Google Scholar] [CrossRef]
- Wang, F.; Han, R.; Chen, S. An Overlooked and Underrated Endemic Mycosis-Talaromycosis and the Pathogenic Fungus Talaromyces marneffei. Clin. Microbiol. Rev. 2023, 36, e0005122. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Shao, S.; Li, D.; Liu, H.; Xie, W.; Huang, W.; Li, J.; Nie, C.; Zhang, J.; Hong, Y.; et al. Exploring the Impact of Tea (Camellia sinensis (L.) O. Ktze.)/Trachelospermum jasminoides (Lindl.) Lem. Intercropping on Soil Health and Microbial Communities. Agronomy 2024, 14, 1261. https://doi.org/10.3390/agronomy14061261
Xiong Y, Shao S, Li D, Liu H, Xie W, Huang W, Li J, Nie C, Zhang J, Hong Y, et al. Exploring the Impact of Tea (Camellia sinensis (L.) O. Ktze.)/Trachelospermum jasminoides (Lindl.) Lem. Intercropping on Soil Health and Microbial Communities. Agronomy. 2024; 14(6):1261. https://doi.org/10.3390/agronomy14061261
Chicago/Turabian StyleXiong, Yulin, Shuaibo Shao, Dongliang Li, He Liu, Wei Xie, Wei Huang, Jing Li, Chuanpeng Nie, Jianming Zhang, Yongcong Hong, and et al. 2024. "Exploring the Impact of Tea (Camellia sinensis (L.) O. Ktze.)/Trachelospermum jasminoides (Lindl.) Lem. Intercropping on Soil Health and Microbial Communities" Agronomy 14, no. 6: 1261. https://doi.org/10.3390/agronomy14061261
APA StyleXiong, Y., Shao, S., Li, D., Liu, H., Xie, W., Huang, W., Li, J., Nie, C., Zhang, J., Hong, Y., Wang, Q., Cai, P., & Li, Y. (2024). Exploring the Impact of Tea (Camellia sinensis (L.) O. Ktze.)/Trachelospermum jasminoides (Lindl.) Lem. Intercropping on Soil Health and Microbial Communities. Agronomy, 14(6), 1261. https://doi.org/10.3390/agronomy14061261





