Transcriptome and Flavonoid Compounds Metabolome Analyses Reveal the Mechanisms of Heat Stress in Rhododendron with Exogenously Applied Calcium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Measurements of Related Physiological Indices
2.3. RNA Isolation, Library Establishment, RNA-Sequencing, and Data Interpretation
2.4. Untargeted Metabolite Profiling and Data Analysis
2.5. Correlation Analysis of Metabolites and Transcript Profiles
2.6. Expression Pattern Analysis
3. Results
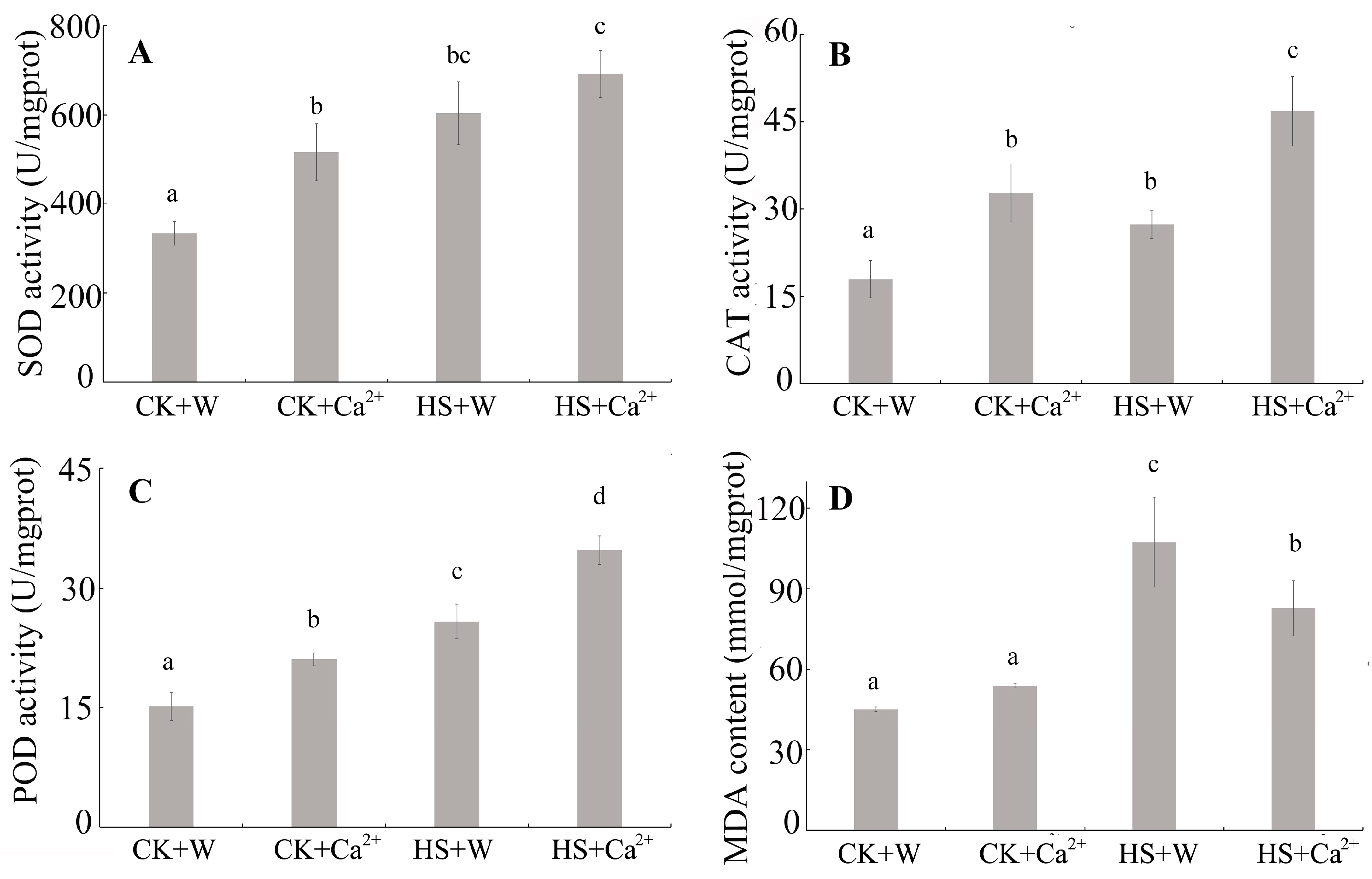
3.1. Heat Stress and External CaCl2 Influence the Antioxidant System
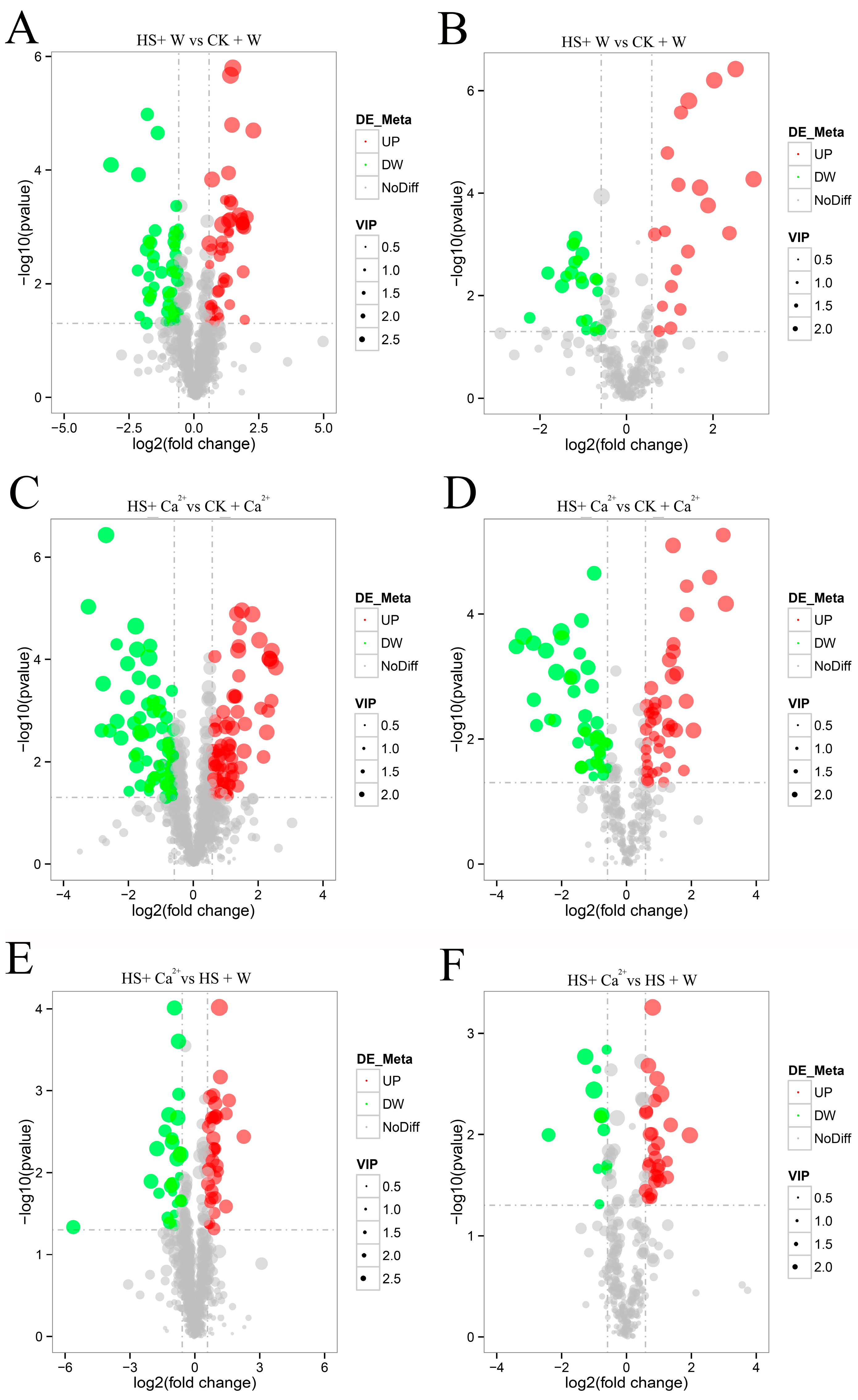
3.2. Heat Stress and External CaCl2 Influence Gene Expression
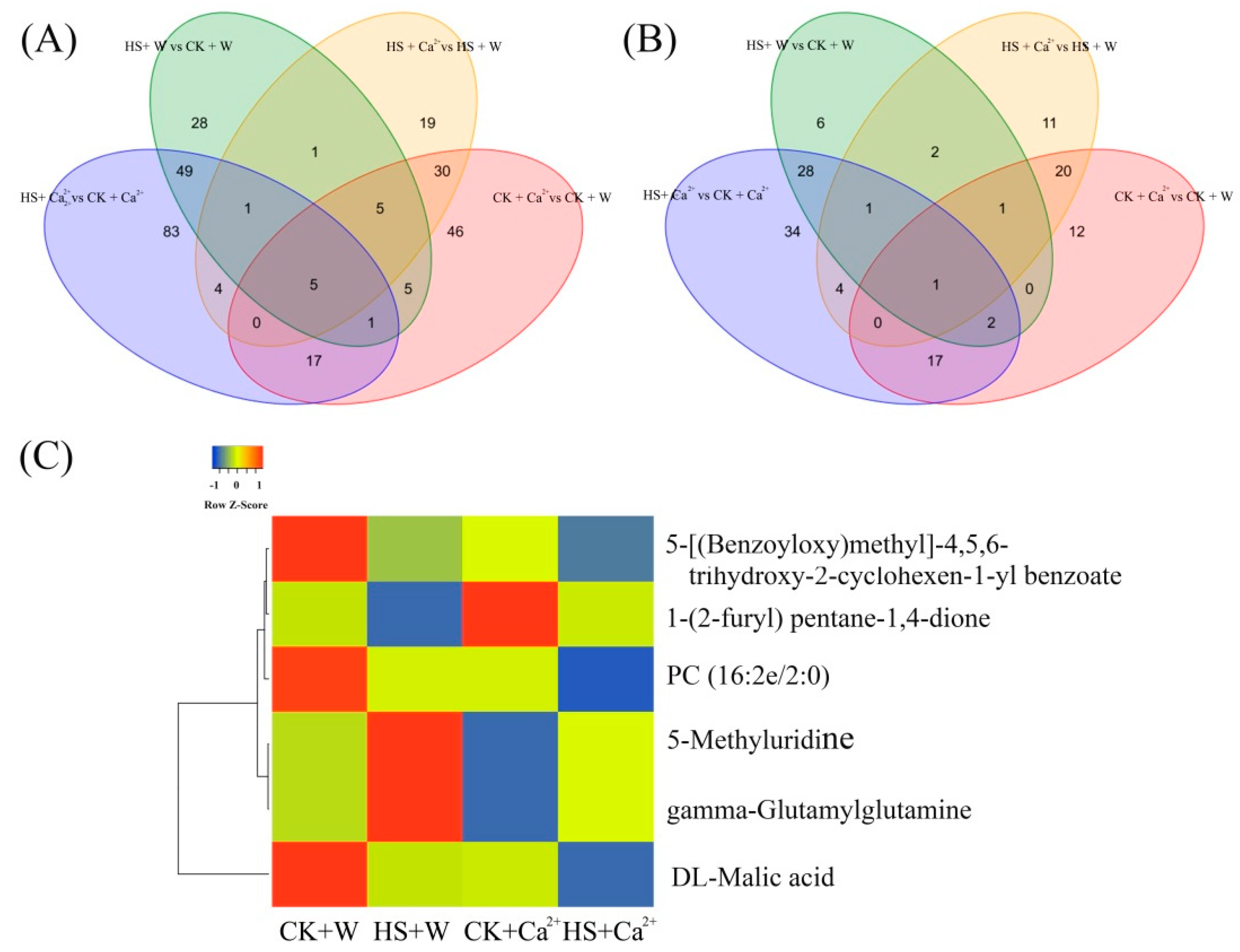
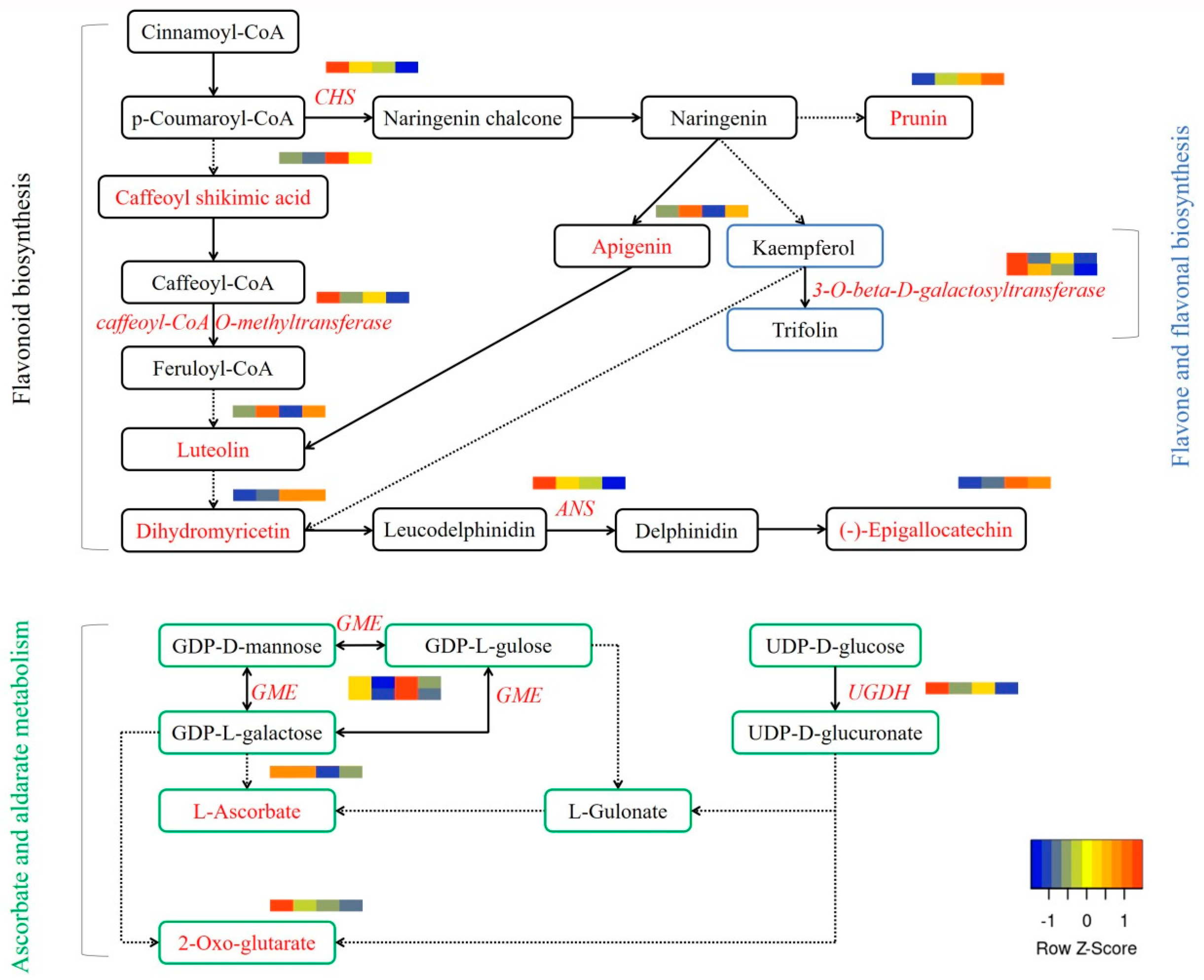
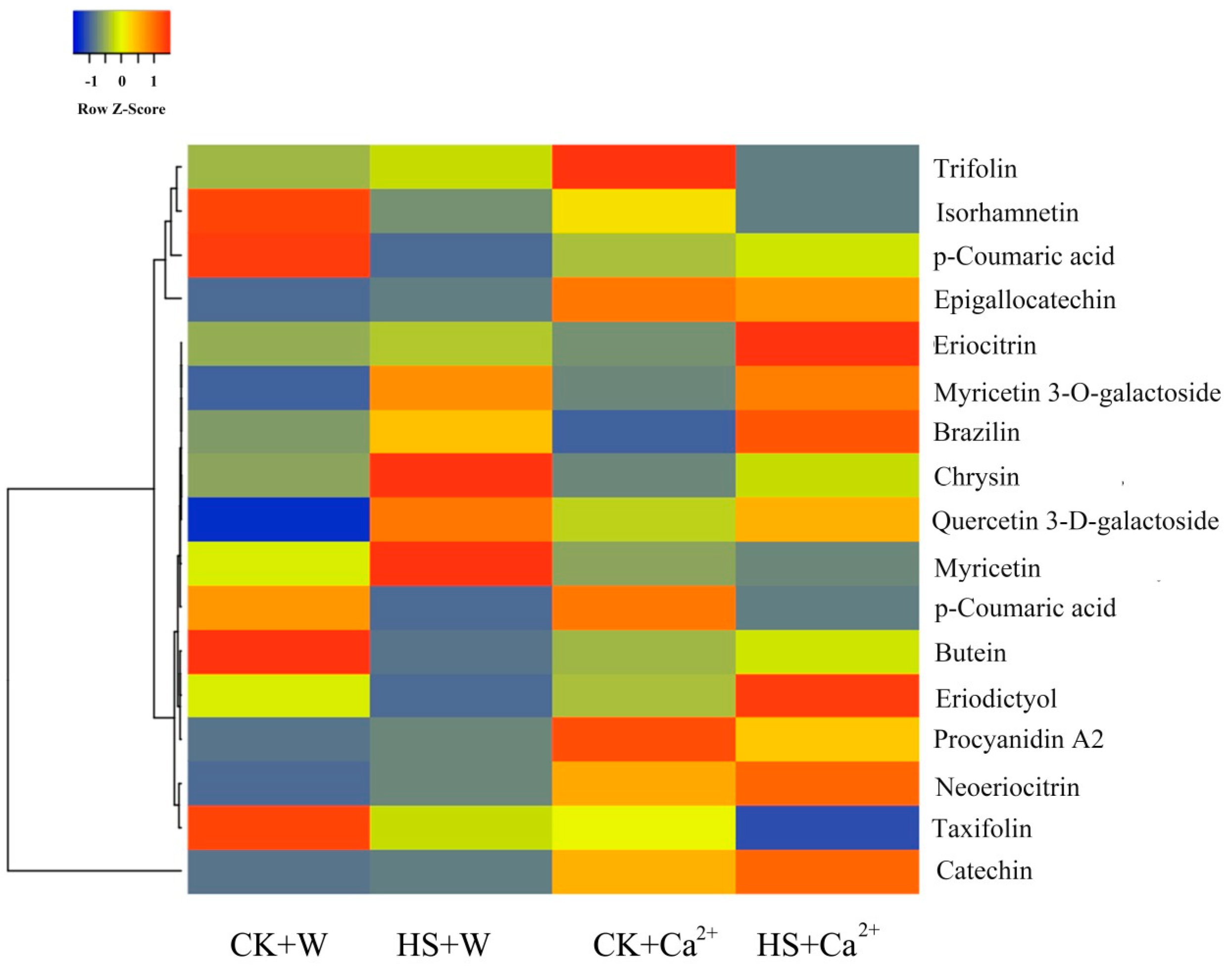
3.3. Heat Stress and External CaCl2 Influence Metabolite Changes
3.4. Correlation between the Metabolome and Transcriptome Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, V.; Suri, S.; Prasad, R.; Gat, Y.; Sangma, C.; Jakhu, H.; Sharma, M. Bioactive compounds, health benefits and utilization of Rhododendron: A comprehensive review. Agric. Food Secur. 2019, 8, 6. [Google Scholar] [CrossRef]
- Cullen, J. Hardy Rhododendron Species: A Guide to Identification; Timber Press Inc.: Portland, OR, USA, 2005. [Google Scholar]
- Krebs, S.L. Rhododendron. In Ornamental Crops, Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Deepak, H.V.; Swamy, M.M.M.; Murai, Y.; Suga, Y.; Monde, K. Daurichromenic acid from the chinese traditional medicinal plant Rhododendron dauricum inhibits sphingomyelin synthase and aβ aggregation. Molecules 2020, 25, 4077. [Google Scholar] [CrossRef]
- Chai, W.M.; Wang, R.; Wei, M.K.; Zou, Z.R.; Deng, R.G.; Liu, W.S.; Peng, Y.Y. Proanthocyanidins extracted from Rhododendron pulchrum leaves as source of tyrosinase inhibitors: Structure, activity, and mechanism. PLoS ONE 2015, 10, e0145483. [Google Scholar] [CrossRef]
- Saklani, S.; Chandra, S. Evaluation of in vitro antimicrobial activity, nutritional profile and phytochemical screening of Rhododendron arboreum. World J. Pharm. Sci. 2015, 4, 962–971. Available online: https://www.wjpps.com/wjpps_controller/abstract_id/3250 (accessed on 20 March 2024).
- Zhang, M.; Pan, D.R.; Zhou, Y.F.; Zhu, Q.Q.; Wand, S.M. Analysis of the flavonoids in the leaves of Rhododendron pulchrum sweet by HPLC-MS. Med. Plant 2012, 3, 21–24. [Google Scholar]
- Jaiswal, P.; Cheruku, J.R.; Kumar, K.; Yadav, S.; Singh, A.; Kumari, P.; Dube, S.C.; Upadhyaya, K.C.; Verma, P.K. Differential transcript accumulation in chickpea during early phases of compatible interaction with a necrotrophic fungus ascochyta rabiei. Mol. Biol. Rep. 2012, 39, 4635–4646. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Rastogi, A.; Yadav, S. Effects of heat stress and molecular mitigation approaches in orphan legume, chickpea. Mol. Biol. Rep. 2020, 47, 4659–4670. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Rather, R.A.; Bhagat, M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: Molecular mechanisms and implications in human health. Cancer Med. 2020, 9, 9181–9192. [Google Scholar] [CrossRef]
- Soares, J.C.; Rosalen, P.L.; Lazarini, J.G.; Massarioli, A.P.; da Silva, C.F.; Nani, B.D.; Franchin, M.; de Alencar, S.M. Comprehensive characterization of bioactive phenols from new Brazilian superfruits by LC-ESI-QTOF-MS, and their ROS and RNS scavenging effects and anti-inflammatory activity. Food Chem. 2019, 281, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Zuo, A.R.; Dong, H.H.; Yu, Y.Y.; Shu, Q.L.; Zheng, L.X.; Yu, X.Y.; Cao, S.W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lou, B.Y.; Zhang, Y.J.; Lin, Q.; Huang, Y.L.; Li, Y. Analysis of flavonoids in Rhododendron pulchrum flowers by HPLC-MS/MS. Int. J. Agric. Biol. 2020, 24, 1464–1468. [Google Scholar] [CrossRef]
- Braam, J. Regulated expression of the calmodulin-related TCH genes in cultured Arabidopsis cells: Induction by calcium and heat shock. Proc. Natl. Acad. Sci. USA 1992, 89, 3213–3216. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, K.R.; Alharby, H.F.; Pirzadah, T.B. Exogenously applied calcium regulates antioxidative system and reduces cadmium-uptake in Fagopyrum esculentum. Plant Physiol. Bioch. 2022, 180, 17–26. [Google Scholar] [CrossRef]
- Shen, H.; Bing, Z.; Xu, J.; Zheng, X.; Huang, W. Effects of salicylic acid and calcium chloride on heat tolerance in Rhododendron ‘fen zhen zhu’. J. Am. Soc. Hortic. Sci. 2016, 141, 363–372. [Google Scholar] [CrossRef]
- Han, Y.; Wan, H.H.; Cheng, T.R.; Wang, J.; Yang, W.R.; Pan, H.T.; Zhang, Q.X. Comparative RNA-seq analysis of transcriptome dynamics during petal development in Rosa chinensis. Sci. Rep. 2017, 7, 43382. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Lin, S.; Yang, S.; Yan, X.; Bendahmane, M.; Bao, M.; Fu, X. Mapping a double flower phenotype-associated gene DcAP2L in Dianthus chinensis. J. Exp. Bot. 2020, 71, 1915–1927. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L.P. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar]
- Xie, Z.; Wang, J.; Wang, W.; Wang, Y.; Xu, J.; Li, Z.; Zhao, X.; Fu, B. Integrated analysis of the transcriptome and metabolome revealed the molecular mechanisms underlying the enhanced salt tolerance of rice due to the application of exogenous melatonin. Front. Plant Sci. 2021, 11, 618680. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Z.; Xiang, Z.; Yang, Z. Exogenous application of citric acid ameliorates the adverse effect of heat stress in tall fescue (Lolium arundinaceum). Front. Plant Sci. 2016, 7, 179. [Google Scholar] [CrossRef]
- Gong, M.; Chen, S.N.; Song, Y.Q.; Li, Z.G. Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. Funct. Plant Biol. 1997, 24, 371–379. [Google Scholar] [CrossRef]
- Xing, C.; Li, J.; Yuan, H.; Yang, J. Physiological and transcription level responses of microalgae auxenochlorella protothecoides to cold and heat induced oxidative stress. Environ. Res. 2022, 211, 113023. [Google Scholar] [CrossRef]
- Liu, X.Z.; Huang, B.R. Carbohydrate accumulation in relation to heat stress tolerance in two creeping bentgrass cultivars. J. Am. Soc. Hortic. Sci. 2000, 125, 442–447. Available online: https://docslib.org/doc/9712751/carbohydrate-accumulation-in-relation-to-heat-stress-tolerance-in-two-creeping-bentgrass-cultivars (accessed on 20 March 2024). [CrossRef]
- Smirnoff, N. Antioxidant systems and plant response to the environment. In Environment and Plant Metabolism: Flexibility and Acclimation; Smirnoff, N., Ed.; Bios Scientific Publishers: Oxford, UK, 1995; pp. 217–243. [Google Scholar]
- Shen, J.; Cheng, H.; Li, X.; Pan, X.; Hu, Y.; Jin, S. Beneficial effect of exogenously applied calcium chloride on the anatomy and fast chlorophyll fluorescence in Rhododendron × pulchrum leaves following short-term heat stress treatment. Agronomy 2022, 12, 3226. [Google Scholar] [CrossRef]
- Hou, Y.; Zeng, W.; Ao, C.; Huang, J. Integrative analysis of the transcriptome and metabolome reveals Bacillus atrophaeus WZYH01-mediated salt stress mechanism in maize (Zea mays L.). J. Biotechnol. 2024, 383, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Wan, Z.Y.; Xu, Y.X.; Shen, J.S.; Li, X.Q.; Jin, S.H. Transcriptome and photosynthetic analyses provide new insight into the molecular mechanisms underlying heat stress tolerance in Rhododendron × pulchrum Sweet. Tree Physiol. 2023, 44, tpad133. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ban, Z.J.; Li, X.H.; Wu, M.Y.; Wang, A.L.; Jiang, Y.Q.; Jiang, Y.H. Differential expression of anthocyanin biosynthetic genes and transcription factor PcMYB10 in pears (Pyrus communis L.). PLoS ONE 2012, 7, e46070. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jia, H.; Li, J.; Yu, M.; Yang, Y.; Tian, D.; Zhang, H.; Zou, Z. Cecal gut microbiota and metabolites might contribute to the severity of acute myocardial ischemia by impacting the intestinal permeability, oxidative stress, and energy metabolism. Front. Microbiol. 2019, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Tong, J.; Dong, Y.; Xu, D.; Mao, J.; Zhou, Y. De novo RNA sequencing transcriptome of Rhododendron obtusum identified the early heat response genes involved in the transcriptional regulation of photosynthesis. PLoS ONE 2017, 12, e0186376. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.D.; Geng, X.M.; Mao, L.F.; Yi, Y.; Gong, J.Y.; Xu, X.R. Transcriptome analysis and identification of the genes associated with the heat stress response in four rhododendron species. Sci. Hortic. 2022, 303, 111176. [Google Scholar] [CrossRef]
- Wang, J.; Lv, J.; Liu, Z.; Liu, Y.; Zou, X. Integration of transcriptomics and metabolomics for pepper (Capsicum annuum L.) in response to heat stress. Int. J. Mol. Sci. 2019, 20, 5042. [Google Scholar] [CrossRef]
- Xia, X.; Gong, R.; Zhang, C. Integrative analysis of transcriptome and metabolome reveals flavonoid biosynthesis regulation in Rhododendron pulchrum petals. BMC Plant Biol. 2022, 22, 401. [Google Scholar] [CrossRef] [PubMed]
- Pahari, P.; Rohr, J. Total synthesis of psoralidin, an anticancer natural product. J. Org. Chem. 2009, 74, 2750–2754. [Google Scholar] [CrossRef]
- Khan, A.L.; Kang, S.M.; Dhakal, K.H.; Hussain, J.; Adnan, M.; Kim, J.G.; Lee, I.J. Flavonoids and amino acid regulation in Capsicum annuum L. by endophytic fungi under different heat stress regimes. Sci. Hortic. 2013, 155, 1–7. [Google Scholar] [CrossRef]
- Commisso, M.; Toffali, K.; Strazzer, P.; Stocchero, M.; Ceoldo, S.; Baldan, B.; Levi, M.; Guzzo, F. Impact of phenylpropanoid compounds on heat stress tolerance in Carrot cell cultures. Front. Plant Sci. 2016, 7, 1439. [Google Scholar] [CrossRef] [PubMed]
- Escandon, M.; Meijon, M.; Valledor, L.; Pascual, J.; Pinto, G.; Canal, M.J. Metabolome integrated analysis of high-temperature response in Pinus radiata. Front. Plant Sci. 2018, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Coberly, L.C.; Rausher, M.D. Analysis of a chalcone synthase mutant in Ipomoea purpurea reveals a novel function for flavonoids: Amelioration of heat stress. Mol. Ecol. 2003, 12, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, L.; Walzem, R.L.; Miller, E.G.; Pike, L.M.; Patil, B.S. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J. Agric. Food Chem. 2005, 53, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Hayder, N.; Bouhlel, I.; Skandrani, I.; Kadri, M.; Steiman, R.; Guiraud, P.; Mariotte, A.M.; Ghedira, K.; Dijoux-Franca, M.G.; Chekir-Ghedira, L. In vitro antioxidant and antigenotoxic potentials of myricetin-3-o-galactoside and myricetin-3-o-rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicol. Vitr. 2008, 22, 567–581. [Google Scholar] [CrossRef]
- Zhuang, W.B.; Li, Y.H.; Shu, X.C.; Pu, Y.T.; Wang, X.J.; Wang, T.; Wang, Z. The classification, molecular structure and biological biosynthesis of flavonoids, and their roles in biotic and abiotic stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Wang, Y.; Ding, Y.; Qian, W.; Qiu, C.; Xie, H.; Sun, L.; Jiang, Z.; Ma, Q.; Wang, L.; et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plants under drought stress. Sci. Rep. 2020, 10, 12275. [Google Scholar] [CrossRef]

| Conditions | Upregulated | Downregulated | Sub-Total |
|---|---|---|---|
| HS + W vs. CK + W | 9347 | 9603 | 18,950 |
| CK + Ca2+ vs. CK + W | 1719 | 1629 | 3348 |
| CK + Ca2+ vs. HS + W | 9568 | 9302 | 18,870 |
| HS + Ca2+ vs. CK + W | 10,146 | 10,147 | 20,293 |
| HS + Ca2+ vs. HS + W | 3318 | 3495 | 6813 |
| HS + Ca2+ vs. CK + Ca2+ | 10,278 | 9862 | 20,140 |
| Compounds | CAS Number | Formula | Molecular Weight |
|---|---|---|---|
| Brazilin | 474-07-7 | C16H14O5 | 286.08 |
| Butein | 487-52-5 | C15H12O5 | 272.07 |
| Catechin | 154-23-4 | C15H14O6 | 290.08 |
| Chrysin | 480-40-0 | C15H10O4 | 254.06 |
| Epigallocatechin | 970-74-1 | C15H14O7 | 288.06 |
| Eriocitrin | 13463-28-0 | C27H32O15 | 596.17 |
| Eriodictyol | 552-58-9 | C15H12O6 | 288.06 |
| Isorhamnetin | 480-19-3 | C16H12 O7 | 316.06 |
| Myricetin | 529-44-2 | C15H10 O8 | 318.04 |
| Myricetin 3-O-galactoside | 15648-86-9 | C21H20 O13 | 480.09 |
| Neoeriocitrin | 13241-32-2 | C27H32 O15 | 596.17 |
| p-Coumaric acid | 501-98-4 | C9H8O3 | 164.05 |
| Procyanidin A2 | 41743-41-3 | C30H24O12 | 576.13 |
| Quercetin 3-D-galactoside | 482-36-0 | C21H20O12 | 464.1 |
| Taxifolin | 480-18-2 | C15H12O7 | 304.06 |
| Trifolin | 23627-87-4 | C21H20O11 | 470.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Rong, X.; Li, X.; Ma, Y.; Cheng, H.; Sheng, J.; Huang, L.; Jin, S. Transcriptome and Flavonoid Compounds Metabolome Analyses Reveal the Mechanisms of Heat Stress in Rhododendron with Exogenously Applied Calcium. Agronomy 2024, 14, 1282. https://doi.org/10.3390/agronomy14061282
Shen J, Rong X, Li X, Ma Y, Cheng H, Sheng J, Huang L, Jin S. Transcriptome and Flavonoid Compounds Metabolome Analyses Reveal the Mechanisms of Heat Stress in Rhododendron with Exogenously Applied Calcium. Agronomy. 2024; 14(6):1282. https://doi.org/10.3390/agronomy14061282
Chicago/Turabian StyleShen, Jianshuang, Xianlin Rong, Xueqin Li, Yulei Ma, Hefeng Cheng, Jiaran Sheng, Lu Huang, and Songheng Jin. 2024. "Transcriptome and Flavonoid Compounds Metabolome Analyses Reveal the Mechanisms of Heat Stress in Rhododendron with Exogenously Applied Calcium" Agronomy 14, no. 6: 1282. https://doi.org/10.3390/agronomy14061282






