Unlocking the Potential of Inoculation with Bradyrhizobium for Enhanced Growth and Symbiotic Responses in Soybean Varieties under Controlled Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of the Experimental Materials
2.2. Bacterial Growth and Growth Medium
2.3. Authentication Test and Seedling Management
2.4. Data Collection
2.5. Determination of Symbiotic Effectiveness Indices of Bradyrhizobia Strains
2.6. Statistical Data Analysis
3. Results
3.1. Growth, Nodulation, and Leaf Color Response of Soybean Varieties to Inoculation
3.1.1. Plant Height
3.1.2. Nodule Number and Dry Weight Plant−1
3.1.3. Shoot and Root Dry Weight
3.1.4. Leaf Color
3.2. Symbiotic Effectiveness of Bradyrhizobia Strains
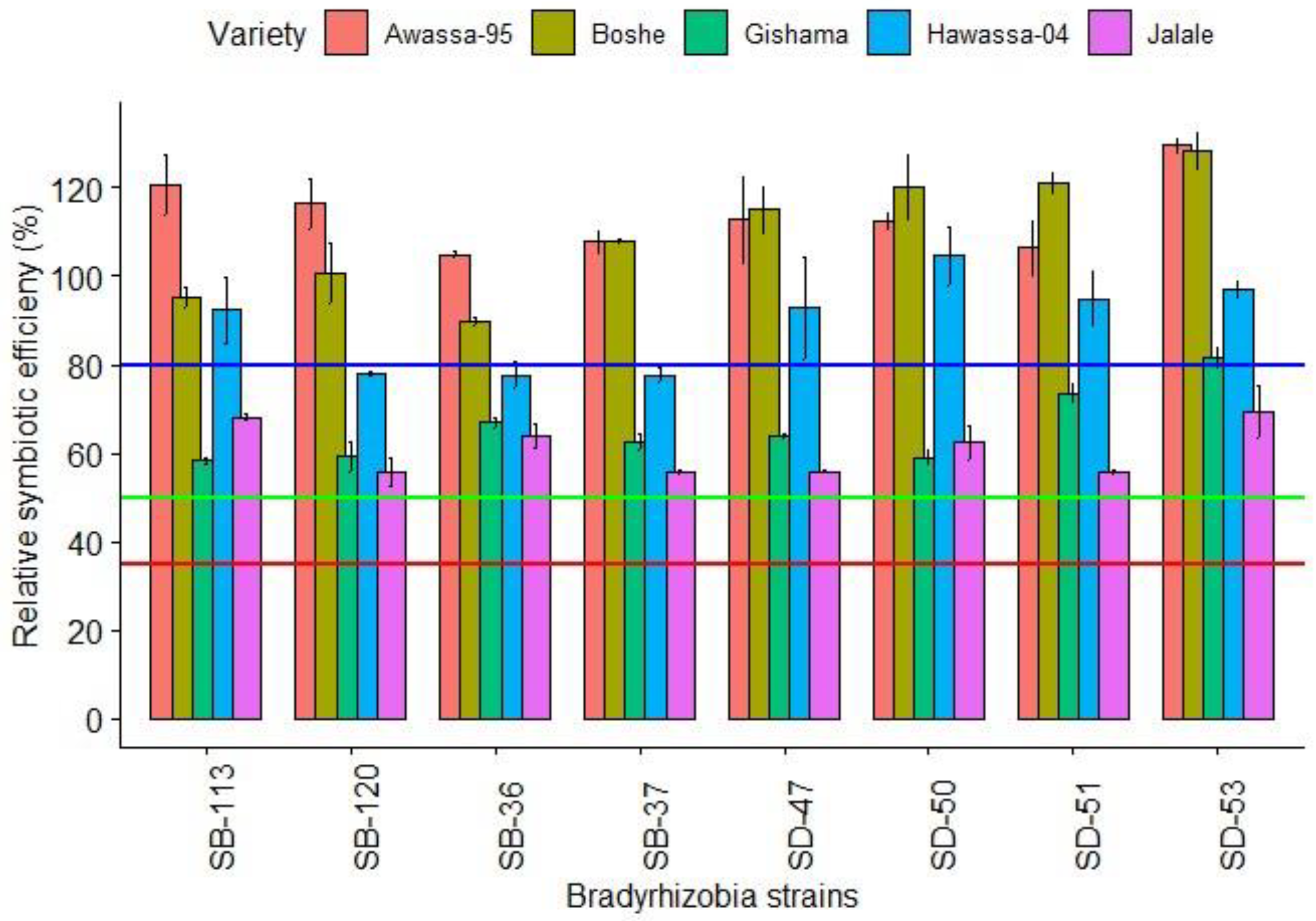
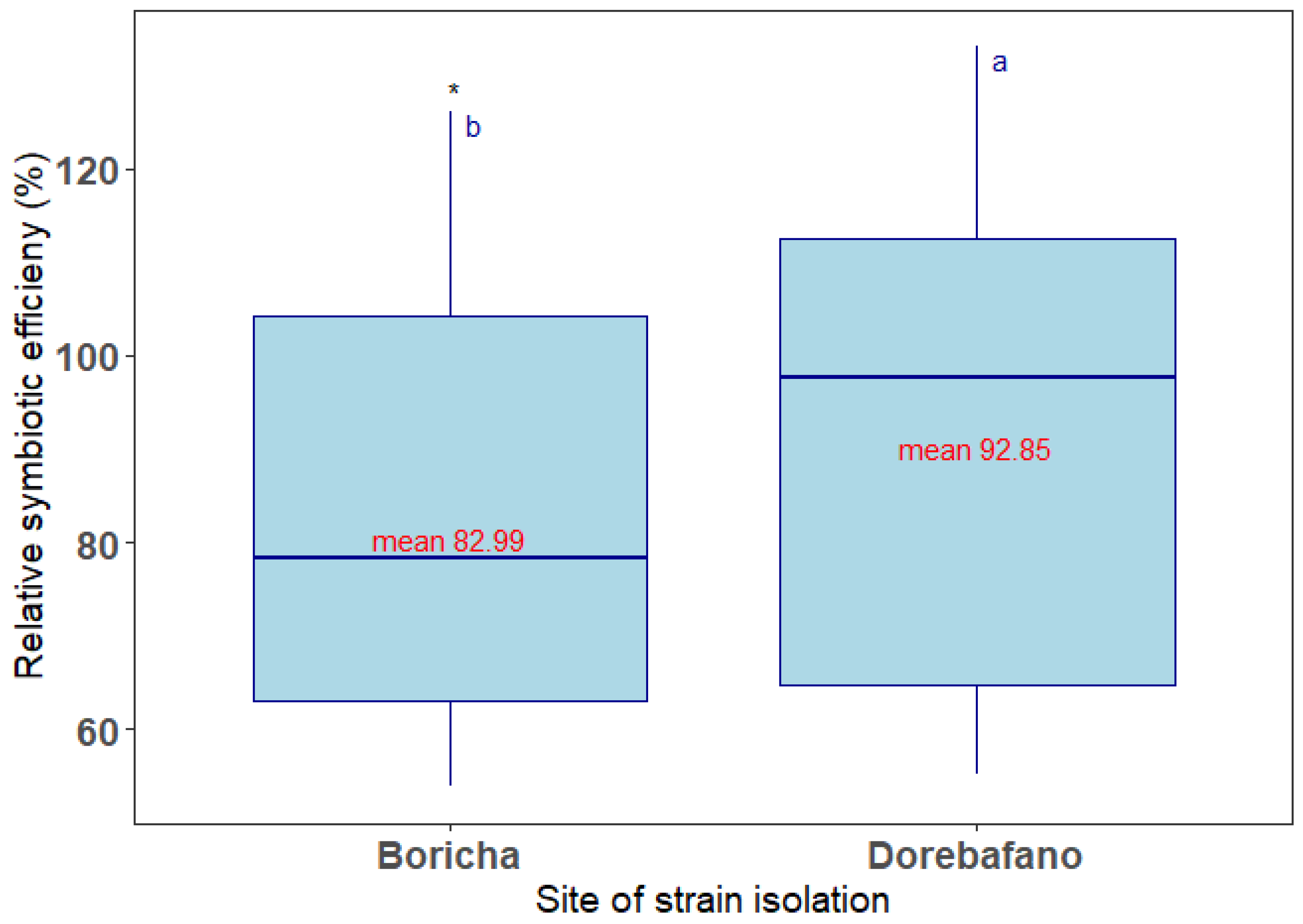
3.2.1. Relative Symbiotic Effectiveness
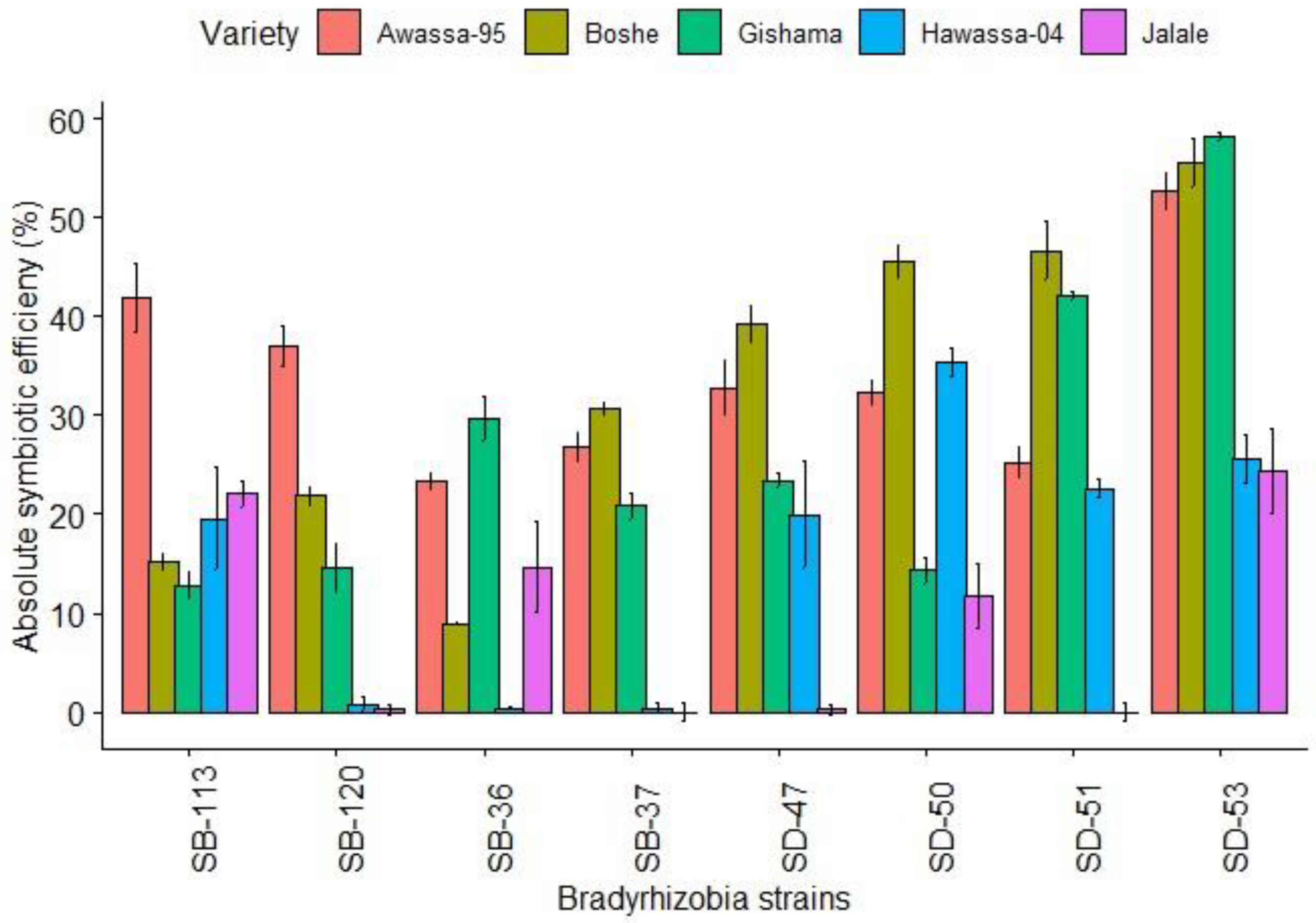
3.2.2. Absolute Symbiotic Effectiveness
4. Discussion
4.1. Growth, Nodulation, and Leaf Color Response of Soybean Varieties to Inoculation
4.1.1. Plant Height
4.1.2. Nodule Number and Dry Weight Plant−1
4.1.3. Shoot and Root Dry Weight
4.1.4. Leaf Color
4.2. Symbiotic Effectiveness of Bradyrhizobia Strains
4.2.1. Relative Symbiotic Effectiveness (RSE)
4.2.2. Absolute Symbiotic Effectiveness (ASE)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shurtleff, W.; Aoyagi, A. History of Soybeans and Soyfoods in Africa (1857–2009): Extensively Annotated Bibliography and Sourcebook; Soyinfo Center: Lafayette, CA, USA, 2009. [Google Scholar]
- Desissa, D.H. Soybean research and development in Ethiopia. Acta Sci. Agric. 2019, 3, 192–194. [Google Scholar] [CrossRef]
- Ogbemudia, F.; Denise, E.; Ogie-Odia, E.; Omonhinmin, A. Comparative germination studies of cowpea (Vigna unguiculata Linn. Walp) and soy bean (Glycine max Linn. Merr) on whole and water saturated fractions of hydrocarbon (hexane). Ann. Biol. Res. 2010, 1, 34–40. [Google Scholar]
- Meena, B.; Fagodiya, R.; Prajapat, K.; Dotaniya, M.; Kaledhonkar, M.; Sharma, P.; Meena, R.S.; Mitran, T.; Kumar, S. Legume green manuring: An option for soil sustainability. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 387–408. [Google Scholar]
- Temesgen, D.; Assefa, F. Inoculation of native symbiotic effective Sinorhizobium spp. enhanced soybean [Glycine max (L.) Merr.] grain yield in Ethiopia. Environ. Syst. Res. 2020, 9, 38. [Google Scholar] [CrossRef]
- Gitonga, N.M.; Njeru, E.M.; Cheruiyot, R.; Maingi, J.M. Bradyrhizobium inoculation has a greater effect on soybean growth, production and yield quality in organic than conventional farming systems. Cogent Food Agric. 2021, 7, 1935529. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Nakei, M.D.; Venkataramana, P.B.; Ndakidemi, P.A. Soybean-Nodulating Rhizobia: Ecology, Characterization, Diversity and, Plant Growth Promoting Functions. Front. Sustain. Food Syst. 2022, 6, 824444. [Google Scholar] [CrossRef]
- Agoyi, E.; Afutu, E.; Tumuhairwe, J.; Odong, T.; Tukamuhabwa, P. Screening soybean genotypes for promiscuous symbiotic association with Bradyrhizobium strains. Afr. Crop Sci. J. 2016, 24, 49–59. [Google Scholar] [CrossRef]
- Getachew Gebrehana, Z.; Abeble Dagnaw, L. Response of soybean to Rhizobial inoculation and starter N fertilizer on Nitisols of Assosa and Begi areas, Western Ethiopia. Environ. Syst. Res. 2020, 9, 14. [Google Scholar] [CrossRef]
- Omari, R.A.; Yuan, K.; Anh, K.T.; Reckling, M.; Halwani, M.; Egamberdieva, D.; Ohkama-Ohtsu, N. Enhanced soybean productivity by inoculation with indigenous bradyrhizobium strains in agroecological conditions of northeast Germany. Front. Plant Sci. 2022, 12, 707080. [Google Scholar] [CrossRef]
- Argaw, A. Symbiotic effectiveness of inoculation with Bradyrhizobium isolates on soybean [Glycine max (L.) Merrill] genotypes with different maturities. SpringerPlus 2014, 3, 753. [Google Scholar] [CrossRef]
- Egamberdiyeva, D.; Qarshieva, D.; Davranov, K. Growth and yield of soybean varieties inoculated with Bradyrhizobium spp in N-deficient calcareous soils. Biol. Fertil. Soils 2004, 40, 144–146. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Bobrecka-Jamro, D.; Pikuła, W.; Jańczak-Pieniążek, M. Effect of nitrogen fertilization and inoculation with Bradyrhizobium japonicum on nodulation and yielding of soybean. Agronomy 2023, 13, 1341. [Google Scholar] [CrossRef]
- Anwar, A.; Podder, A.; Hasem, M.; Bala, P.; Islam, M. Effect of Bradyrhizobium inoculants on the growth and yield of soybean varieties PB-1 and G-2. J. Soil Nat. 2010, 4, 39–48. [Google Scholar]
- Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Nikolić, Z.; Tintor, B.; Đukić, V. Competitiveness of Bradyrhizobium japonicum inoculation strain for soybean nodule occupancy. Plant Soil Environ. 2022, 68, 59–64. [Google Scholar] [CrossRef]
- Samudin, S.; Kuswantoro, H. Effect of Rhizobium inoculation to nodulation and growth of soybean [Glycine max (L.) Merrill] germplasm. Legume Res. Int. J. 2018, 41, 303–310. [Google Scholar] [CrossRef]
- Desta, M.; Akuma, A.; Minay, M.; Yusuf, Z.; Baye, K. Effects of Indigenous and Commercial Rhizobia on Growth and Nodulation of Soybean (L) under Greenhouse Condition. Open Biotechnol. J. 2023, 17, e187407072302070. [Google Scholar] [CrossRef]
- Jones, K.; Nti, F. Impacts and Repercussions of Price Increases on the Global Fertilizer Market; USDA Foreign Agricultural Service: Washington, DC, USA, 2022.
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainability, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Sharma, L.; Sharma, A.K.; Ogram, A.; Singh, H. Enhancing Biological Nitrogen Fixation to Improve Soil Nutrient Status. EDIS 2023, 2023. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.-W.E. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
- Beyan, S.; Welde-meskel, E.; Dakora, F. Soybean genotypic variations for nodulation and N2 fixation in response to Bradyrhizobium inoculation under glasshouse conditions. S. Afr. J. Bot. 2015, 98, 171. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Beyan, S.M.; Dakora, F.D. Distribution, diversity and population composition of soybean-nodulating bradyrhizobia from different agro-climatic regions in Ethiopia. Biol. Fertil. Soils 2016, 52, 725–738. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H. Handbook for Rhzobia: Methods in Legume-Rhizobium Technology; Springer: New York, NY, USA, 1994; Volume 450. [Google Scholar]
- Purcino, H.; Festin, P.; Elkan, G. Identification of effective strains of Bradyrhizobium for Arachis pintoi. Trop. Agric. 2000, 77, 226–231. [Google Scholar]
- dos Santos, J.G.; Aguiar, A.D.; Junior, E.M.; Dadalto, D.L.; Sousa, M.R.; Xavier, G.R.; de Moura, E.G. Soil management and efficiency of rhizobia strains of cowpea Vigna unguiculata (L.) Walp. in the tropics. Chil. J. Agric. Res. 2011, 71, 594. [Google Scholar] [CrossRef]
- Box, G.E.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. Ser. B Stat. Methodol. 1964, 26, 211–243. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Horácio, E.H.; Gavilanes, F.E.Z.; Feliciano, M.V.; de Moraes, J.G.; Zucareli, C.; Andrade, D.S.; Maddela, N.R.; Prasad, R. Exploring the interaction effects between common bean cultivars and rhizobia inoculation on plant growth and yield. J. Agric. Food Res. 2024, 15, 100926. [Google Scholar] [CrossRef]
- Roriz, M.; Pereira, S.I.; Castro, P.M.; Carvalho, S.M.; Vasconcelos, M.W. Impact of soybean-associated plant growth-promoting bacteria on plant growth modulation under alkaline soil conditions. Heliyon 2023, 9, e14620. [Google Scholar] [CrossRef]
- Księżak, J.; Bojarszczuk, J. The effect of mineral n fertilization and bradyrhizobium japonicum seed inoculation on productivity of soybean (Glycine max (L.) Merrill). Agriculture 2022, 12, 110. [Google Scholar] [CrossRef]
- Samago, T.Y.; Anniye, E.W.; Dakora, F.D. Grain yield of common bean (Phaseolus vulgaris L.) varieties is markedly increased by rhizobial inoculation and phosphorus application in Ethiopia. Symbiosis 2018, 75, 245–255. [Google Scholar] [CrossRef]
- Shifa, M.; Yoseph, T.; Abate, B. Agronomic and symbiotic performances of common bean varieties inoculated with Rhizobium species combined with nitrogen fertilizer. J. Sci. Dev. (JSD) 2022, 10, 30–41. [Google Scholar]
- Ayalew, T.; Yoseph, T. Symbiotic effectiveness of inoculation with Bradyrhizobium isolates on Cowpea (Vigna unguiculata (L.) Walp) varieties. Cogent Food Agric. 2020, 6, 1845495. [Google Scholar] [CrossRef]
- Abera, Y.; Masso, C.; Assefa, F. Inoculation with indigenous rhizobial isolates enhanced nodulation, growth, yield and protein content of soybean (Glycine max L.) at different agro-climatic regions in Ethiopia. J. Plant Nutr. 2019, 42, 1900–1912. [Google Scholar] [CrossRef]
- Temesgen, D. Genetic Diversity of Rhizobia and Rhizobacteria from Soybean (Glycine max (L) Merr.): Implication for the Commercial Production and Application to Enhance Soybean Production under Low Input Agriculture in Ethiopia. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2017. [Google Scholar]
- Ahmad, S.; Wang, G.-Y.; Muhammad, I.; Zeeshan, M.; Zhou, X.-B. Melatonin and KNO3 application improves growth, physiological and biochemical characteristics of maize seedlings under waterlogging stress conditions. Biology 2022, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, L.; Zhang, H.; Huang, G. Symbiotic effectiveness of pea-rhizobia associations and the implications for farming systems in the western Loess Plateau, China. Afr. J. Biotechnol. 2011, 10, 3540–3548. [Google Scholar]
- Gwata, E.; Wofford, D.S.; Boote, K.; Mushoriwa, H. Determination of effective nodulation in early juvenile soybean plants for genetic and biotechnology studies. Afr. J. Biotechnol. 2003, 2, 417–420. [Google Scholar]
- Hakan, Ş.; Nur, B.; Gülnur, A. Change of Chlorophyll Amount in Some Landscape Plants. J. Biotechnol. Sci. 2014, 2, 10–16. [Google Scholar]
- Swędrzyńska, D.; Sawicka, A. Effect of inoculation with Azospirillum brasilense on development and yielding of maize (Zea mays ssp. saccharata L.) under different cultivation conditions. Pol. J. Environ. Stud. 2000, 9, 505–509. [Google Scholar]
- Mfilinge, A.; Mtei, K.; Ndakidemi, P. Effect of Rhizobium inoculation and supplementation with phosphorus and potassium on growth and total leaf chlorophyll (Chl) content of bush bean Phaseolus vulgaris, L. Agric. Sci. 2014, 5, 1413–1426. [Google Scholar]
- Kandil, A.E.; Özdamar Ünlü, H. Effect of rhizobium inoculation on yield and some quality properties of fresh cowpea. Cogent Food Agric. 2023, 9, 2275410. [Google Scholar] [CrossRef]
- Osei, O.; Abaidoo, R.C.; Ahiabor, B.D.; Boddey, R.M.; Rouws, L.F. Bacteria related to Bradyrhizobium yuanmingense from Ghana are effective groundnut micro-symbionts. Appl. Soil Ecol. 2018, 127, 41–50. [Google Scholar] [CrossRef]
- Yates, R.; Howieson, J.; Hungria, M.; Bala, A.; O’Hara, G.; Terpolilli, J. Authentication of Rhizobia and Assessment of the Legume Symbiosis in Controlled Plant Growth Systems, in Working with Rhizobia; Australian Centre for International Agricultural Research: Canberra, Australia, 2016; pp. 73–108. [Google Scholar]
- Ebisa, N.; Kibret, K.; Tsige, A.; Assefa, F. Symbiotic effectiveness of indigenous rhizobia nodulating field pea (Pisum sativum L.) on soils of Horro Guduru and East Wollega highlands in western Ethiopia. Ethiop. J. Biol. Sci. 2017, 16, 83–107. [Google Scholar]
- Gunununu, R.P.; Mohammed, M.; Jaiswal, S.K.; Dakora, F.D. Phylogeny and symbiotic effectiveness of indigenous rhizobial microsymbionts of common bean (Phaseolus vulgaris L.) in Malkerns, Eswatini. Sci. Rep. 2023, 13, 17029. [Google Scholar] [CrossRef] [PubMed]


| Variety | Source | Breeder/Maintainer | Altitude (m.a.s.l.) | Year—Released/Registered | Maturity Group |
|---|---|---|---|---|---|
| Awassa-95 (G 2261) | PARC | AwARc/SARI | 520–1800 | 2005 | Early |
| Boshe (IAC-13-1) | PARC | BARI/OARI | 1200–1900 | 2008 | Medium |
| Gishama (PR-143-(26)) | PARC | PARC | 520–1800 | 2010 | Medium |
| Hawassa-04 (AGS-7-1) | PARC | AwARc/SARI | NA | 2012 | Medium |
| Jallale | PARC | BARC/OARI | 1300–1850 | 2003 | Medium |
| Origin of Strains (Locations) | Strains | Varieties Used for Isolation |
|---|---|---|
| Boricha | SB-36 | Wegayen |
| SB-37 | Wegayen | |
| Dore Bafano | SD-47 | TGX-3326-44 |
| SD-50 | TGX-3326-44 | |
| SD-51 | Gishama | |
| SD-53 | Gishama | |
| Boricha | SB-113 | Awassa-95 |
| SB-120 | Awassa-04 |
| Mean Square | Traits | ||||
|---|---|---|---|---|---|
| Plant Height (cm) | Number of Nodules | Shoot Dry Weight (gm plant−1) | Root Dry Weight (gm plant−1) | Nodule Dry Weight (gm plant−1) | |
| Variety (V) | 493.77 *** | 3.56 *** | 0.09 *** | 0.93 *** | 0.02 *** |
| Rhizobial strains (R) | 356.83 *** | 40.42 *** | 0.01 *** | 0.15 *** | 0.10 *** |
| V × R | 316.97 *** | 3.75 *** | 0.004 *** | 0.05 *** | 0.01 *** |
| Error | 29.14 | 7.95 | 0.0003 | 0.003 | 0.002 |
| CV | 9.65 | 16.87 | 1.73 | 4.84 | 38.08 |
| LSD | 8.74 | 4.57 | 0.13 | 0.09 | 0.03 |
| Varieties | Strain | Plant Height (cm) | Nodule Number Plant−1 | Nodule Dry Weight (g) | Root Dry Weight (g) | Shoot Dry Weight (g) |
|---|---|---|---|---|---|---|
| Gishama | −ve control | 40.67 st | 0.00 m | 0.000 j | 0.11 r–x | 0.91 xy |
| +ve control | 74.67 a–c | 0.00 m | 0.000 j | 0.29 c–h | 1.76 cd | |
| SB-36 | 64.00 d–h | 1.33 lm | 0.006 ij | 0.09 t–x | 1.18 n–r | |
| SB-37 | 49.33 l–s | 1.00 lm | 0.003 ij | 0.14 t–x | 1.10 r–v | |
| SD-47 | 44.67 o–t | 4.00 j–m | 0.011 g–j | 0.13 p–x | 1.12 q–t | |
| SD-50 | 49.67 m–t | 1.67 lm | 0.005 ij | 0.08 v–x | 1.04 t–w | |
| SD-51 | 52.33 k–q | 11.67 gh | 0.081 ab | 0.18 i–s | 1.29 l–o | |
| SD-53 | 43.67 q–t | 9.67 g–i | 0.010 g–j | 0.05 x | 1.44 g–j | |
| SB-113 | 43.67 q–t | 11.00 gh | 0.012 f–j | 0.07 wx | 1.03 t–w | |
| SB-120 | 47.67 m–s | 6.33 i–k | 0.006 ij | 0.09 u–x | 1.04 s–w | |
| Awassa–95 | −ve control | 44.33 p–t | 0.00 m | 0.000 j | 0.33 a–d | 1.24 m–q |
| +ve control | 44.67 o–t | 0.00 m | 0.000 j | 0.23 f–n | 1.46 f–i | |
| SB-36 | 46.33 m–t | 21.33 cd | 0.041 c–g | 0.23 g–o | 1.53 e–h | |
| SB-37 | 49.33 l–s | 2.33 k–m | 0.002 ij | 0.41 a | 1.57 ef | |
| SD-47 | 54.00 k–n | 4.67 j–l | 0.015 f–j | 0.26 d–k | 1.65 c–e | |
| SD-50 | 53.00 k–p | 4.00 j–m | 0.004 ij | 0.30 b–g | 1.64 de | |
| SD-51 | 57.00 h–l | 2.33 k–m | 0.004 ij | 0.19 i–r | 1.55 e–g | |
| SD-53 | 63.33 d–j | 24.67 c | 0.089 a | 0.18 k–u | 1.89 b | |
| SB-113 | 58.67 g–k | 11.00 gh | 0.056 b–e | 0.15 n–w | 1.76 c | |
| SB-120 | 55.00 i–m | 13.67 fg | 0.056 b–e | 0.33 a–e | 1.70 cd | |
| Boshe | −ve control | 53.33 k–o | 0.00 m | 0.000 j | 0.17 k–u | 0.90 vw |
| +ve control | 45.00 o–t | 0.00 m | 0.000 j | 0.14 p–x | 1.09 r–w | |
| SB-36 | 37.67 t | 1.67 lm | 0.002 ij | 0.14 o–x | 0.98 wx | |
| SB-37 | 65.33 d–h | 1.67 lm | 0.005 ij | 0.23 g–o | 1.18 o–r | |
| SD-47 | 59.33 e–k | 23.00 c | 0.060 a–d | 0.20 h–p | 1.25 m–p | |
| SD-50 | 68.00 b–e | 7.33 h–j | 0.042 c–f | 0.33 a–d | 1.31 k–m | |
| SD-51 | 71.33 a–d | 4.33 j–m | 0.005 ij | 0.24 e–m | 1.32 k–m | |
| SD-53 | 66.33 c–g | 24.00 c | 0.064 a–c | 0.18 j–t | 1.40 i–l | |
| SB-113 | 54.00 k–n | 17.00 d–f | 0.054 b–e | 0.16 l–v | 1.04 t–w | |
| SB-120 | 43.33 r–t | 4.67 j–l | 0.037 c–h | 0.19 i–r | 1.10 r–w | |
| Hawassa–04 | −ve control | 63.33 d–j | 0.00 m | 0.000 j | 0.20 h–q | 0.82 y |
| +ve control | 45.50 n–t | 0.00 m | 0.000 j | 0.37 a–c | 1.06 r–w | |
| SB-36 | 76.00 ab | 1.33 lm | 0.006 ij | 0.17 l–v | 0.82 y | |
| SB-37 | 49.50 l–r | 1.00 lm | 0.003 ij | 0.11 r–x | 0.82 y | |
| SD-47 | 54.00 k–n | 3.33 j–m | 0.028 e–j | 0.27 d–i | 0.98 v–x | |
| SD-50 | 74.50 a–c | 2.67 k–m | 0.003 ij | 0.18 i–s | 1.11 r–u | |
| SD-51 | 75.00 a–c | 2.67 k–m | 0.002 ij | 0.24 e–l | 1.01 u–x | |
| SD-53 | 63.67 d–i | 20.33 c–e | 0.038 c–h | 0.09 s–x | 1.03 t–w | |
| SB-113 | 58.67 g–k | 24.67 c | 0.058 b–e | 0.14 n–w | 0.98 wx | |
| SB-120 | 45.33 n–t | 16.67 ef | 0.031 d–i | 0.11 q–x | 0.83 y | |
| Jalale | −ve control | 47.67 m–s | 0.00 m | 0.000 j | 0.21 h–p | 1.16 p–s |
| +ve control | 79 a | 0.00 m | 0.000 j | 0.32 a–f | 2.08 a | |
| SB-36 | 54.00 k–n | 2.00 k–m | 0.012 f–j | 0.39 ab | 1.33 j–m | |
| SB-37 | 59.00 f–k | 4.00 j–m | 0.010 h–j | 0.19 i–r | 1.16 p–s | |
| SD-47 | 51.67 k–r | 1.00 lm | 0.004 ij | 0.38 ab | 1.16 p–r | |
| SD-50 | 59.33 e–k | 1.00 lm | 0.008 h–j | 0.27 d–j | 1.30 l–n | |
| SD-51 | 54.67 j–m | 2.00 k–m | 0.006 ij | 0.30 b–g | 1.16 p–s | |
| SD-53 | 67.67 b–f | 33.00 b | 0.079 ab | 0.16 l–v | 1.44 g–j | |
| SB-113 | 70.67 a–d | 40.00 a | 0.090 a | 0.17 k–u | 1.42 h–k | |
| SB-120 | 45.67 n–t | 23.33 c | 0.055 b–e | 0.15 m–w | 1.16 q–r |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beruk, H.; Yoseph, T.; Ayalew, T. Unlocking the Potential of Inoculation with Bradyrhizobium for Enhanced Growth and Symbiotic Responses in Soybean Varieties under Controlled Conditions. Agronomy 2024, 14, 1280. https://doi.org/10.3390/agronomy14061280
Beruk H, Yoseph T, Ayalew T. Unlocking the Potential of Inoculation with Bradyrhizobium for Enhanced Growth and Symbiotic Responses in Soybean Varieties under Controlled Conditions. Agronomy. 2024; 14(6):1280. https://doi.org/10.3390/agronomy14061280
Chicago/Turabian StyleBeruk, Haimanot, Tarekegn Yoseph, and Tewodros Ayalew. 2024. "Unlocking the Potential of Inoculation with Bradyrhizobium for Enhanced Growth and Symbiotic Responses in Soybean Varieties under Controlled Conditions" Agronomy 14, no. 6: 1280. https://doi.org/10.3390/agronomy14061280




