Synergistic Remediation of Cd-Contaminated Soil with Pure Natural Adsorption Material and Hyperaccumulator Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Contaminated Soil
2.2. Preparation and Characterization of Adsorbent Materials
2.3. Experimental Design of Synergistic Remediation
2.4. Analysis Method of Plant Samples
2.5. Analysis Method of Soil Samples
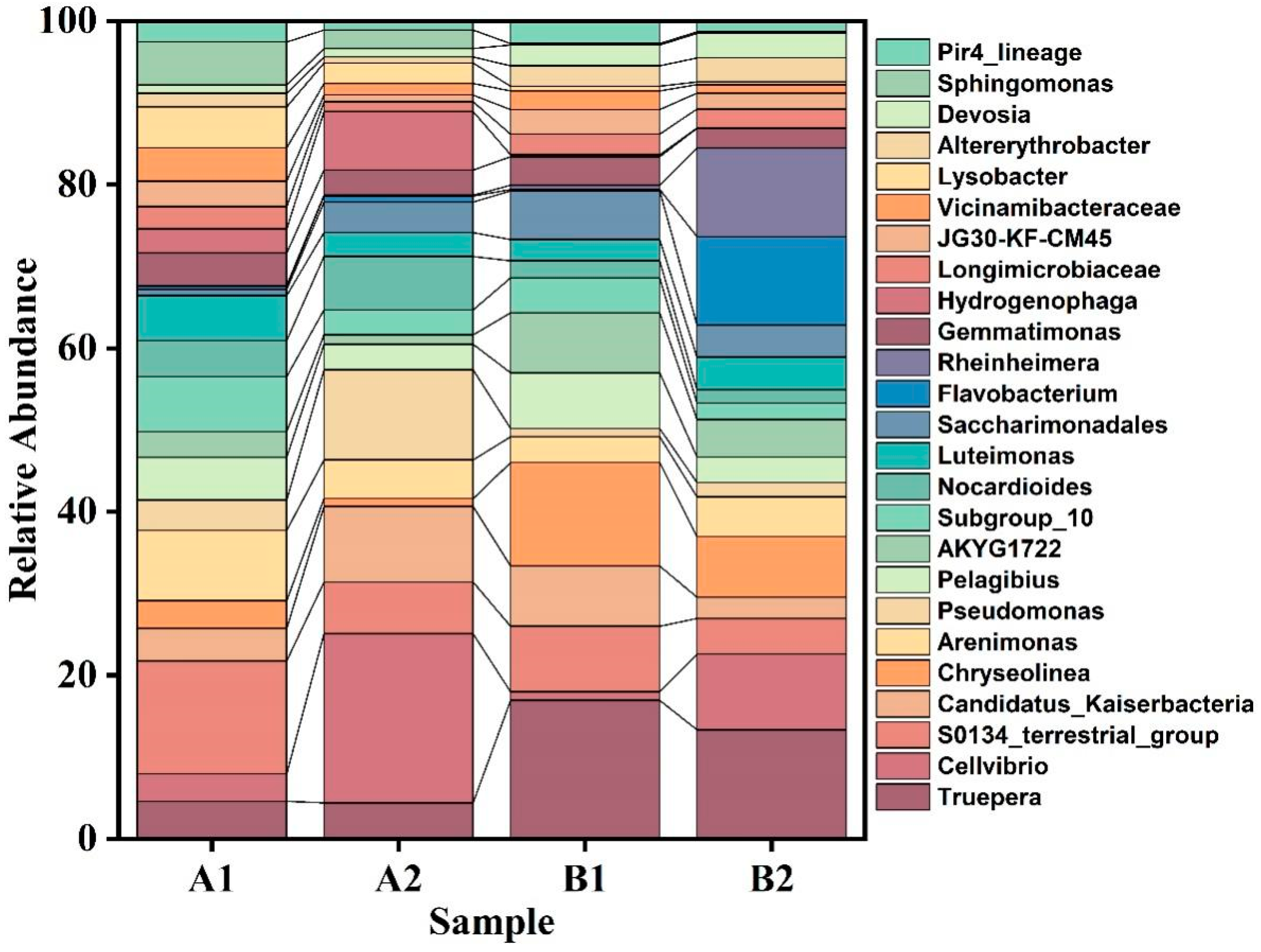
2.6. Total DNA Extraction, 16S rRNA Amplification and High-Throughput Sequencing
2.7. Statistical Analysis Methods
3. Results
3.1. Characterization of Materials
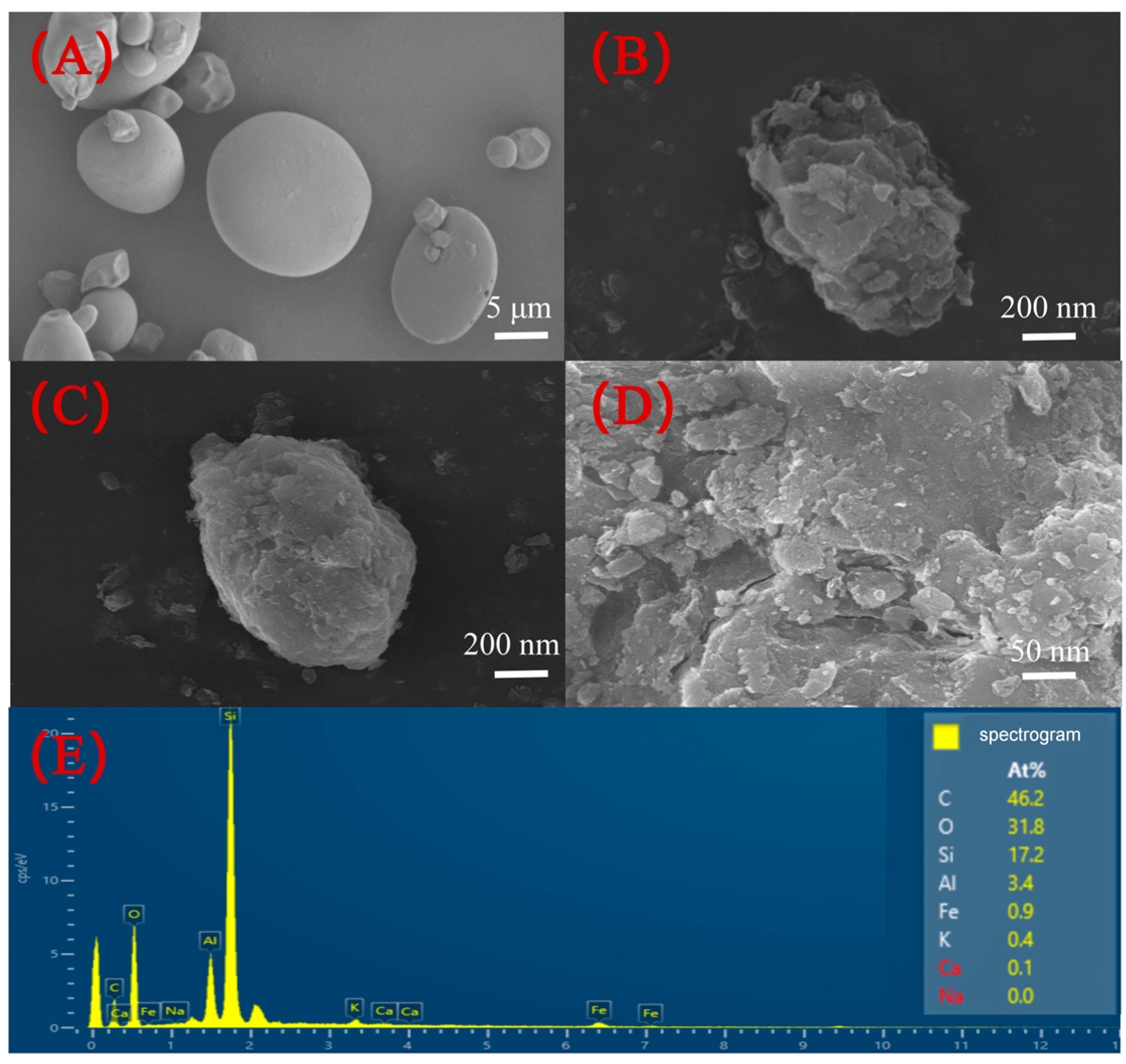
3.1.1. Surface Morphology Characterization of SMC
3.1.2. Adsorption Efficiency of SMC towards Heavy Metals in Solution
3.2. Screening of Indigenous Hyperaccumulating Plants
3.3. Remediation Effect of SMC in Cd Contaminated Soil
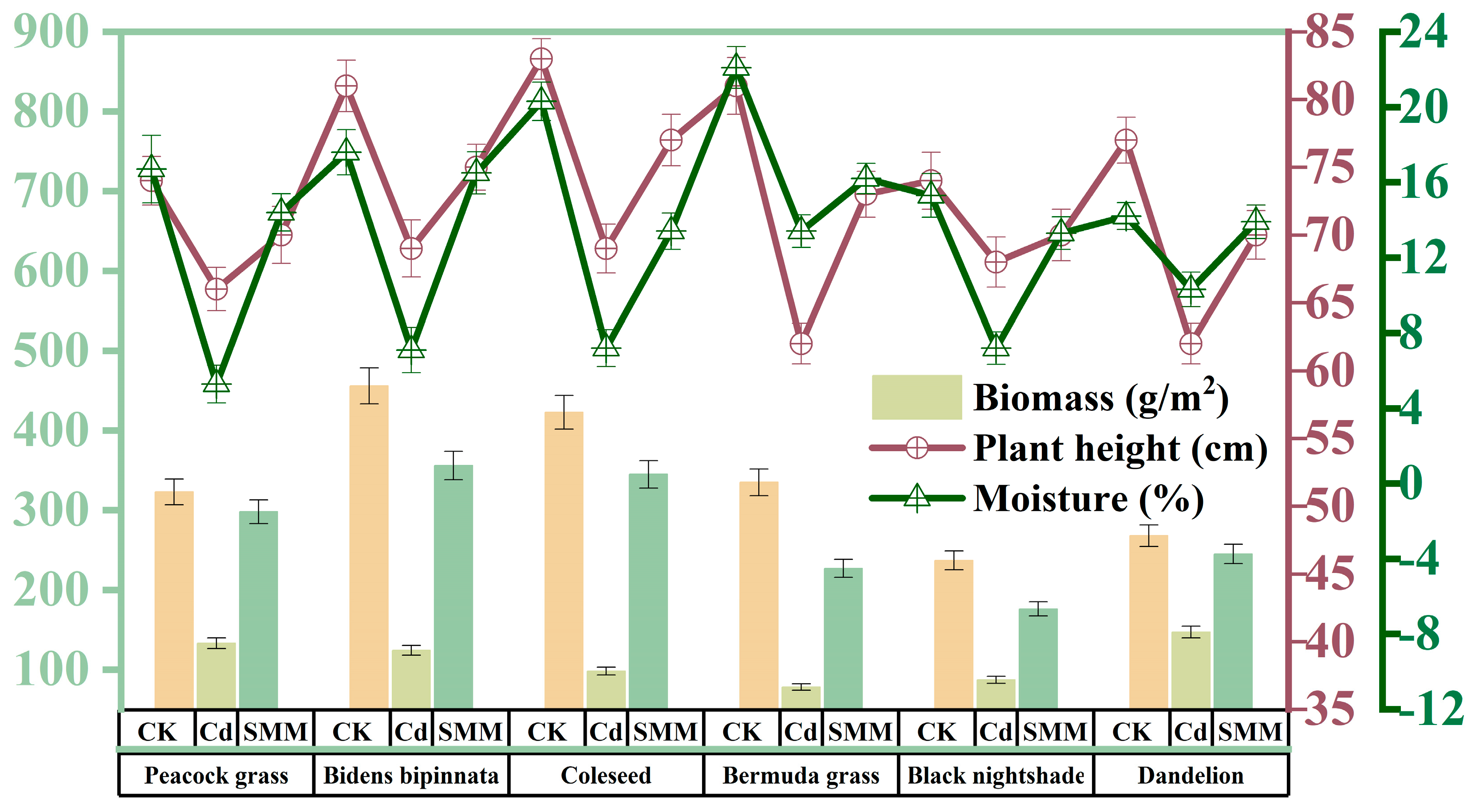
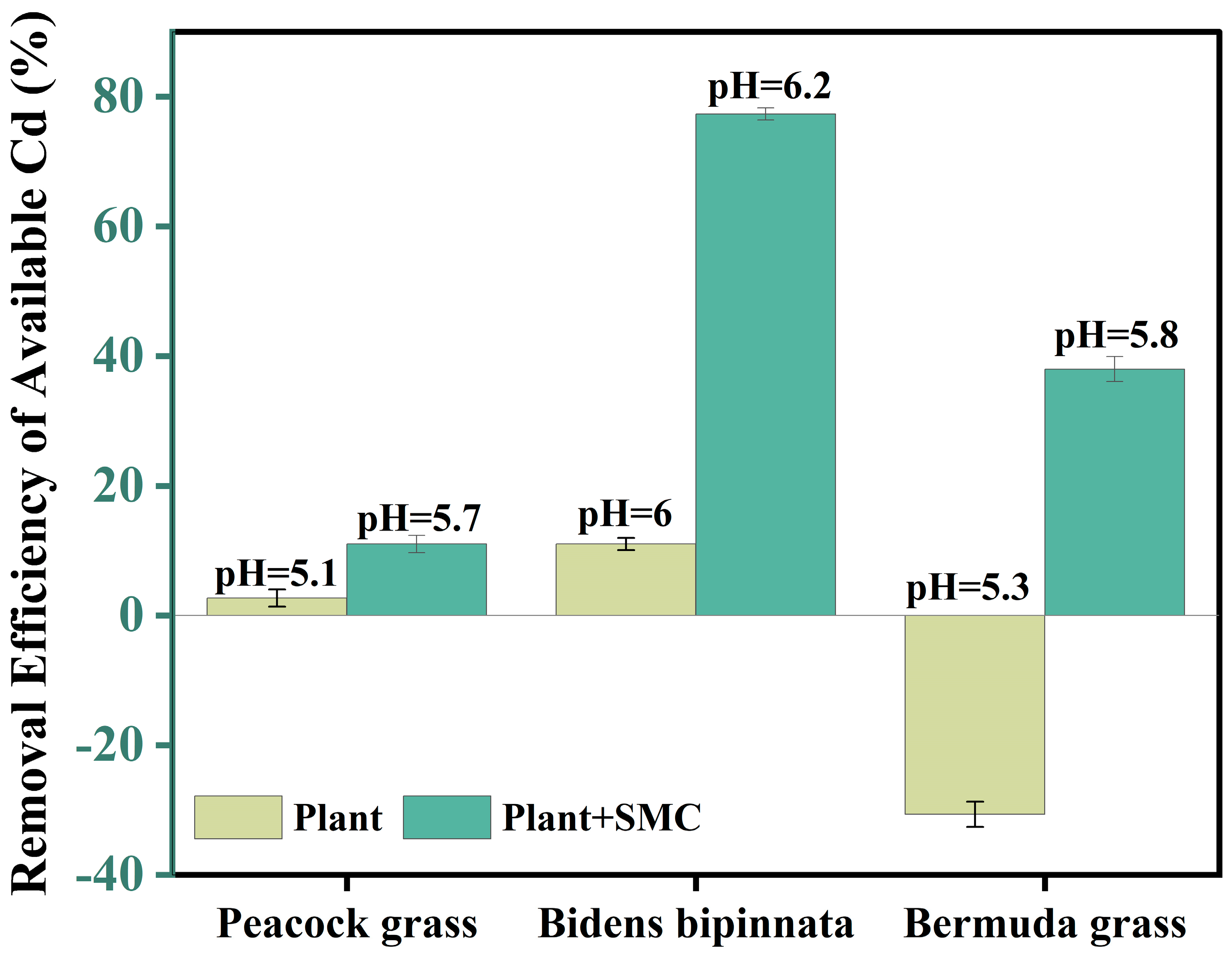
3.4. Screening of Remediation Plants by Potted Experiment
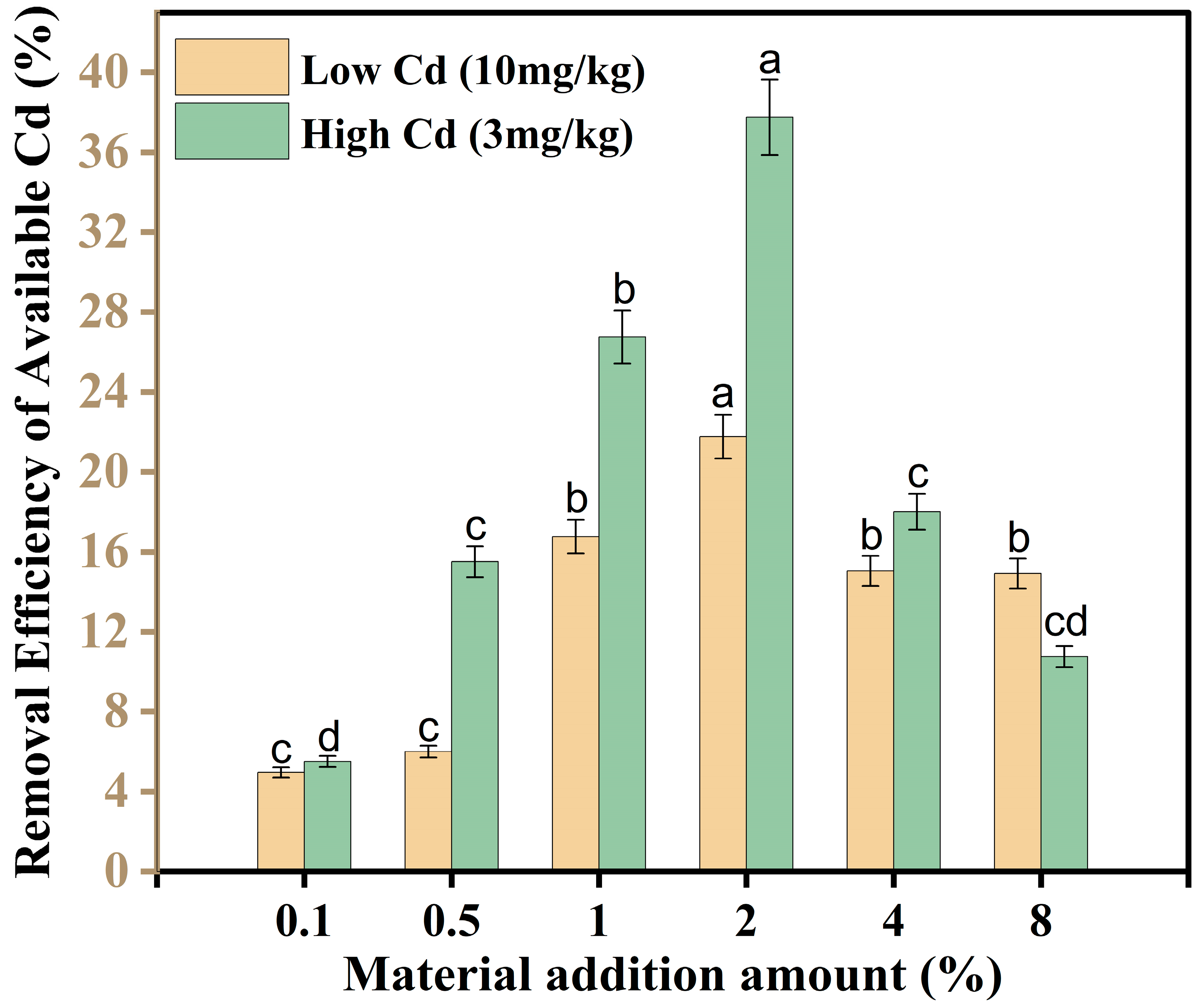
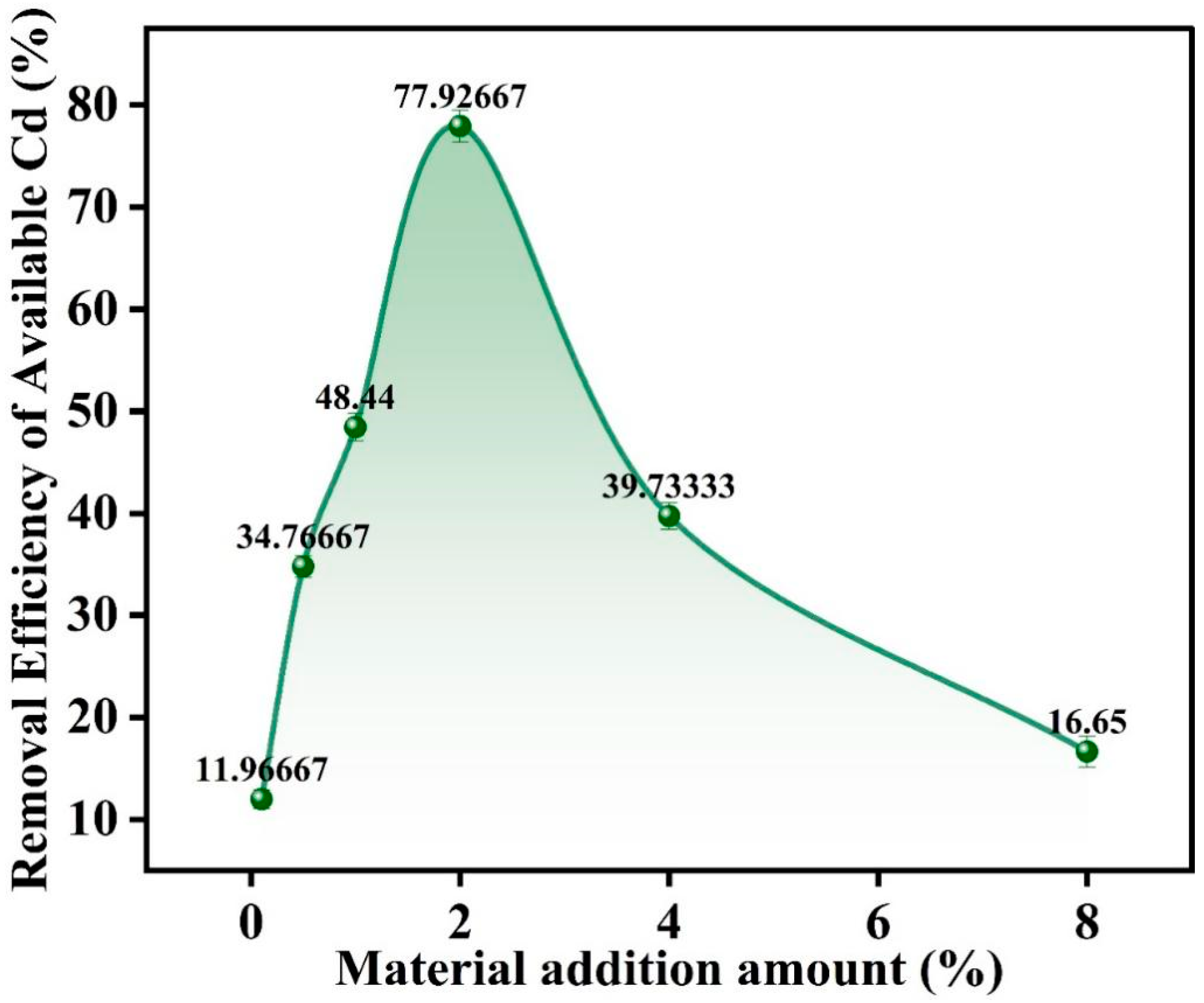
3.5. Screening of SMC Dosage by Potted Experiments
3.6. Application of SMC-Bidens bipinnata Synergistic Remediation Technology in Field
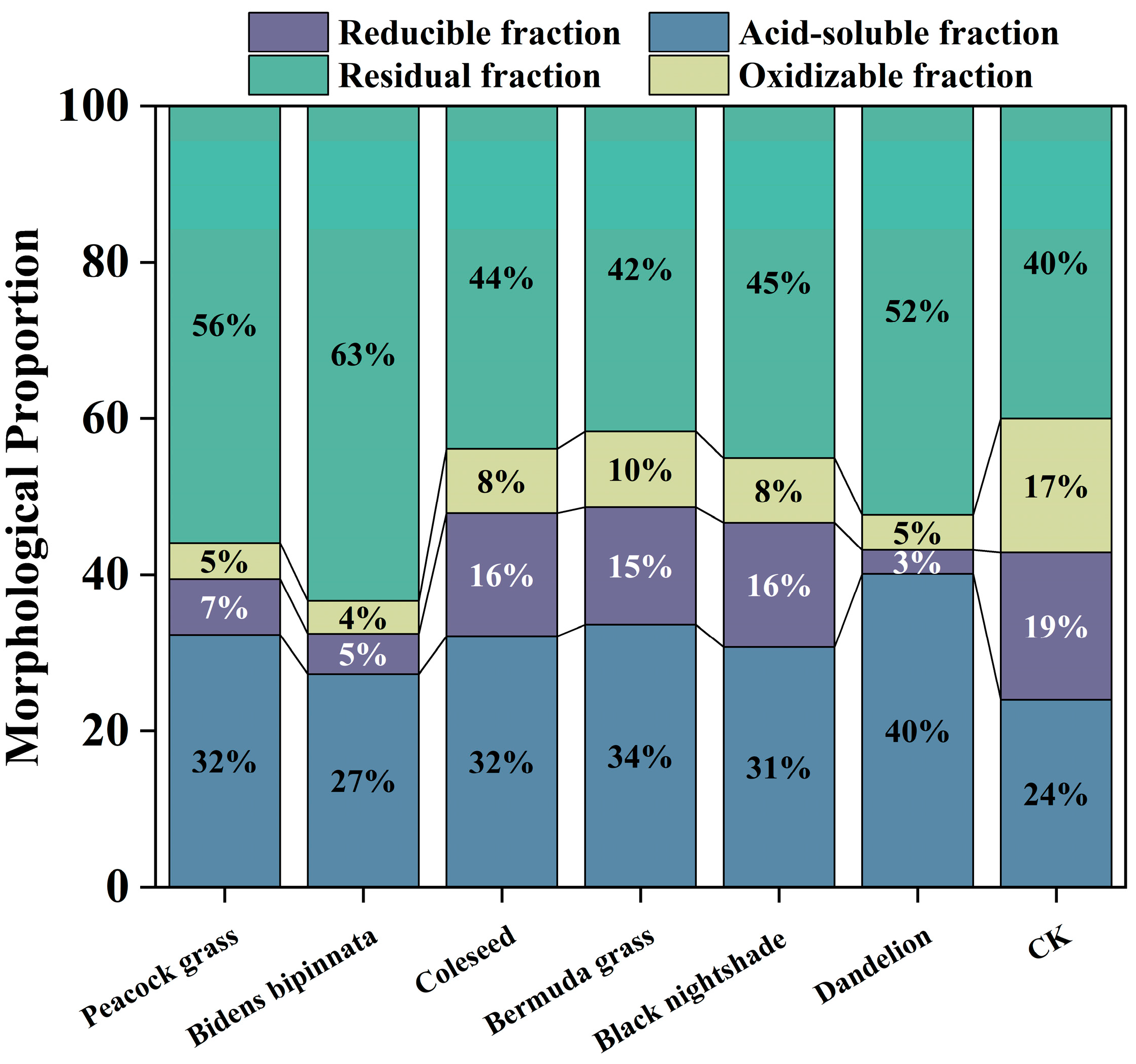
3.7. Changes in Soil BCR Fraction Components
3.8. TCLP Toxicity Leaching Experiment
4. Conclusions
- Remediation material was synthesized using natural montmorillonite and starch, and the remediation plant was screened from native plants. Hence, this synergistic remediation technique is high in environmental safety. Meanwhile, the reduction rate of the available Cd content in the remediated soil in the pot experiment was able to reach 77.92% and the total Cd concentration in soil in field experiments could be reduced to 0.29 mg/kg. Hence, this synergistic remediation technique is of high efficiency.
- In this synergistic remediation technique, the synergistic effect is pronounced. Starch-modified montmorillonite significantly enhances the transport capacity of plants for cadmium. On the other hand, beneficial microorganisms were released in the plant growth process, which significantly promoted the solidification effect of starch-modified montmorillonite towards Cd in soil.
- For cadmium-contaminated soil, this synergistic remediation technique has great application potential.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Ma, J.; Chen, F.; Zhang, Q.; Wang, Y.; Bian, Z. Effective remediation of electronic waste contaminated soil by the combination of metal immobilization and phytoremediation. J. Environ. Chem. Eng. 2022, 10, 107410. [Google Scholar] [CrossRef]
- Kang, J.W. Removing environmental organic pollutants with bioremediation and phytoremediation. Biotechnol. Lett. 2014, 36, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Katoh, M.; Hashimoto, K.; Sato, T. Lead and Antimony Removal from Contaminated Soil by Phytoremediation Combined with an Immobilization Material. Clean-Soil Air Water 2016, 44, 1717–1724. [Google Scholar] [CrossRef]
- Jain, S.; Tembhurkar, A.R. Growth, remediation, and yield assessment of Jatropha curcas, Millettia pinnata, and Helianthus annus on fly ash amended soil: A comparative study. Acta Physiol. Plant. 2023, 45, 35. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, E.Y.; Park, H.; Yun, J.; Kim, J.G. In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 2011, 161, 1–7. [Google Scholar] [CrossRef]
- Wang, S.; Chen, N.; Liu, X.; Fu, Y.; Liu, H. Utilization of municipal sludge passivated by coal ash on yellow garden soil. Fujian J. Agric. Sci. 2018, 33, 1097–1103. [Google Scholar]
- Jiang, K.; Xiang, A.H.; Liu, K.; Peng, Q. Potential of montmorillonite and humus-like substances modified montmorillonite for remediation of Pb and Zn-contaminated soils. Appl. Clay Sci. 2023, 234, 106853. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, J.; Knudsen, T.S.; Liu, J.; Zhu, X.; Ma, B.; Li, H.; Cao, Y.; Liu, B. Performance and mechanisms for Cd(II) and As(III) simultaneous adsorption by goethite-loaded montmorillonite in aqueous solution and soil. J. Environ. Manag. 2023, 330, 117163. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Xu, J.; Jiang, X.; Liu, C.; McCall, W.; Lu, J. Stabilization of heavy metals in soil using two organo-bentonites. Chemosphere 2017, 184, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, X.; Xu, Y.; Qin, X.; Huang, Q.; Wang, L.; Sun, Y. Remediation of Heavy Metal-Polluted Agricultural Soils Using Clay Minerals: A Review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Liu, C. Chromium immobilization in soil using quaternary ammonium cations modified montmorillonite: Characterization and mechanism. J. Hazard. Mater. 2017, 321, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Naidu, R.; Rahman, M.M.; Megharaj, M.; Xi, Y. Organoclays reduce arsenic bioavailability and bioaccessibility in contaminated soils. J. Soils Sediments 2012, 12, 704–712. [Google Scholar] [CrossRef]
- Liu, X.; Laipan, M.; Zhang, C.; Zhang, M.; Wang, Z.; Yuan, M.; Guo, J. Microbial weathering of montmorillonite and its implication for Cd(II) immobilization. Chemosphere 2024, 349, 140850. [Google Scholar] [CrossRef] [PubMed]
- Yost, J.L.; Hartemink, A.E. How deep is the soil studied–an analysis of four soil science journals. Plant Soil 2020, 452, 5–18. [Google Scholar] [CrossRef]
- El Fadili, H.; Ali, M.B.; Touach, N.; El Mahi, M. Ecotoxicological and pre-remedial risk assessment of heavy metals in municipal solid wastes dumpsite impacted soil in Morocco. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100640. [Google Scholar] [CrossRef]
- Yu, G.; Ullah, H.; Lin, H.; Sunahara, G.I.; Zhang, X.; Chen, B.; Yu, H.; Shahab, A.; Liu, L.; Liu, J. Long-term phytoextraction potential and mechanism of Celosia argentea on soils with different levels of Cd and Mn co-contamination. J. Environ. Chem. Eng. 2024, 12, 112125. [Google Scholar] [CrossRef]
- Shi, G.; Hu, J.; Zhang, S.; Ni, G.; Shi, W.; Hu, C.; Zhao, X. The application of exogenous Se improved the remediation efficiency of Lolium multiflorum Lam grown in nonylphenol-Cd Co-contaminated soil. J. Environ. Chem. Eng. 2022, 10, 108962. [Google Scholar] [CrossRef]
- Brunetti, G.; Kodešová, R.; Švecová, H.; Fér, M.; Nikodem, A.; Klement, A.; Grabic, R.; Šimůnek, J. On the use of mechanistic soil–plant uptake models: A comprehensive experimental and numerical analysis on the translocation of carbamazepine in green pea plants. Environ. Sci. Technol. 2021, 55, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Cai, J.; Liu, M.; Yu, Y.; Xiong, H.; Tang, S.; Ding, S. Structure and properties of starch/PVA/nano-SiO2 hybrid films. Carbohydr. Polym. 2011, 86, 1784–1789. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Moghanm, F.S.; Youssef, M.S.; El-Mohsnawy, E.; Haroun, S.A. Bioaccumulation and translocation of nine heavy metals by Eichhornia crassipes in Nile Delta, Egypt: Perspectives for phytoremediation. Int. J. Phytoremediation 2019, 21, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Rao, G.; Ashraf, U.; He, L.; Zhang, Z.; Zhang, H.; Mo, Z.; Pan, S.; Tang, X. Application of inorganic passivators reduced Cd contents in brown rice in oilseed rape-rice rotation under Cd contaminated soil. Chemosphere 2020, 259, 127404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Mu, L.; Zhang, C.; Fu, T.L.; He, T.B. Effect of amendments on bioavailability of cadmium in soil-rice system: A field experiment study. Environ. Sci. Pollut. Res. 2023, 30, 37659–37668. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sun, Z.; Yu, Y.; Li, X.; Li, T. Effects of modified carbon black nanoparticles on plant-microbe remediation of petroleum and heavy metal co-contaminated soils. Int. J. Phytoremediation 2019, 21, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sun, Z.; Li, X.; Yu, Y. Effects of modified nanoscale carbon black on plant growth, root cellular morphogenesis, and microbial community in cadmium-contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 18423–18433. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Song, F.; Lu, Y.; Zhuge, Y.; Niu, Y.; Lou, Y.; Pan, H.; Zhang, P.; Pang, L. Phytoremediation potential of wheat intercropped with different densities of Sedum plumbizincicola in soil contaminated with cadmium and zinc. Chemosphere 2021, 276, 130223. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Luo, Z.; Zhou, Y.; Hou, D.; Mao, Q.; Zhi, D.; Zhang, J.; Yang, Y.; Luo, L. Effect of RM-based-passivator for the remediation of two kinds of Cd polluted paddy soils and mechanism of Cd(II) adsorption. Environ. Technol. 2021, 42, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Ghosh, D.; Dash, A.; Bose, S. Arsenic Contamination in Soil and Sediment in India: Sources, Effects, and Remediation. Curr. Pollut. Rep. 2015, 1, 35–46. [Google Scholar] [CrossRef]
- Tang, X.; Li, Q.; Wu, M.; Lin, L.; Scholz, M. Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to China. J. Environ. Manag. 2016, 181, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Dani, P.; Daverey, A. Phytoremediation of Heavy Metal Contaminated Soil Using Bidens pilosa: Effect of Varying Concentrations of Sophorolipids. Appl. Biochem. Biotechnol. 2023, 196, 2399–2413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Fang, L.; Hong, R.; Gao, J.; Gu, C. Degradation of bisphenol A by nano-sized manganese dioxide synthesized using montmorillonite as templates. Appl. Clay Sci. 2016, 132, 155–160. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality: Agricultural Land Soil Pollution Risk Control Standards. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Bashir, S.; Ali, U.; Shaaban, M.; Gulshan, A.B.; Iqbal, J.; Khan, S.; Husain, A.; Ahmed, N.; Mehmood, S.; Kamran, M.; et al. Role of sepiolite for cadmium (Cd) polluted soil restoration and spinach growth in wastewater irrigated agricultural soil. J. Environ. Manag. 2020, 258, 110020. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Li, W.; Xiong, W.; Ren, Y.; Liu, Y.; Miao, Y.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Diversity-triggered deterministic bacterial assembly constrains community functions. Nat. Commun. 2019, 10, 3833. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Lu, T.; Qian, H.; Liu, Y. Machine learning models reveal how biochar amendment affects soil microbial communities. Biochar 2023, 5, 89. [Google Scholar] [CrossRef]
- Diao, Y.; Zhou, L.; Ji, M.; Wang, X.; Dan, Y.; Sang, W. Immobilization of Cd and Pb in soil facilitated by magnetic biochar: Metal speciation and microbial community evolution. Environ. Sci. Pollut. Res. 2022, 29, 71871–71881. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Fan, Q.; Wang, S.; Zhang, J.; Zhang, G.; Zheng, H.; Zhao, Y.; Chang, S.; Hou, F. Bacillus subtilis field spray on alpine meadows promotes digestibility in Tibetan sheep via increasing the nutrient quality of herbage and enhancing rumen bacterial populations. Anim. Feed Sci. Technol. 2024, 310, 115920. [Google Scholar] [CrossRef]
- Awolope, O.K.; O’Driscoll, N.H.; Di Salvo, A.; Lamb, A.J. The complete genome sequence of Hafnia alvei A23BA; a potential antibiotic-producing rhizobacterium. BMC Res. Notes 2021, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Boros-Lajszner, E.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Energetic value of Elymus elongatus L. and Zea mays L. grown on soil polluted with Ni2+, Co2+, Cd2+, and sensitivity of rhizospheric bacteria to heavy metals. Energies 2021, 14, 4903. [Google Scholar] [CrossRef]
- Kede, M.L.F.; Correia, F.V.; Conceição, P.F.; Salles Junior, S.F.; Marques, M.; Moreira, J.C.; Pérez, D.V. Evaluation of mobility, bioavailability and toxicity of Pb and Cd in contaminated soil using TCLP, BCR and earthworms. Int. J. Environ. Res. Public Health 2014, 11, 11528–11540. [Google Scholar] [CrossRef]






| Items | Cu | Pb | Zn | Cd | Cr | As | Ni |
|---|---|---|---|---|---|---|---|
| Total heavy metal (mg/kg) | 52.2 | 90 | 126 | 1.91 | 98.6 | 53.9 | 36.3 |
| Agricultural Soil Risk Screening Standard Value (mg/kg) | 150 | 120 | 200 | 0.3 | 250 | 30 | 60 |
| Agricultural Soil Risk Control Standard Value (mg/kg) | - | 500 | - | 1.5 | 800 | 200 | - |
| Pi value | 0.348 | 0.75 | 0.73 | 6.36 | 0.394 | 1.79 | 0.605 |
| Pollution Level [17] | Clean | Relatively clean | Clean | Heavy pollution | Clean | Slight pollution | Clean |
| Material | Cu | Pb | Zn | Cd | Cr | As | Ni |
|---|---|---|---|---|---|---|---|
| Montmorillonite (%) | 74.33 | 64.37 | 50.31 | 50.45 | 33.45 | 28.07 | 39.66 |
| SMC (%) | 87.92 | 94.12 | 73.22 | 95.31 | 48.23 | 48.23 | 51.37 |
| Plant | Cd Content (mg/kg) | BF | TF | Plant | Cd Content (mg/kg) | BF | TF |
|---|---|---|---|---|---|---|---|
| 1 shoot | 0.0145 | 0.0076 | 0.3713 | 9 shoot | 0.0998 | 0.0522 | 0.2791 |
| 1 root | 0.0391 | 0.0205 | 9 root | 0.3575 | 0.1872 | ||
| 2 shoot | 0.0529 | 0.0277 | 0.6451 | 10 shoot | 0.0945 | 0.0495 | 0.1755 |
| 2 root | 0.0820 | 0.0430 | 10 root | 0.5385 | 0.2819 | ||
| 3 shoot | 0.1729 | 0.0905 | 0.6725 | 11 shoot | 0.1885 | 0.0987 | 0.2622 |
| 3 root | 0.2570 | 0.1346 | 11 root | 0.7189 | 0.3764 | ||
| 4 shoot | 0.1541 | 0.0807 | 0.9862 | 12 shoot | 0.1003 | 0.0525 | 0.6524 |
| 4 root | 0.1563 | 0.0818 | 12 root | 0.1537 | 0.0805 | ||
| 5 shoot | 1.1768 | 0.6161 | 0.8376 | 13 shoot | 2.2833 | 1.1954 | 0.6982 |
| 5 root | 1.4049 | 0.7356 | 13 root | 3.2704 | 1.7123 | ||
| 6 shoot | 1.8418 | 0.9643 | 0.7760 | 14 shoot | 0.5077 | 0.2658 | 0.6873 |
| 6 root | 2.3735 | 1.2427 | 14 root | 0.7386 | 0.3867 | ||
| 7 shoot | 1.6964 | 0.8881 | 1.3830 | 15 shoot | 0.2916 | 0.1527 | 0.9119 |
| 7 root | 1.2266 | 0.6422 | 15 root | 0.3197 | 0.1674 | ||
| 8 shoot | 0.3021 | 0.1581 | 0.4652 | ||||
| 8 root | 0.6493 | 0.3399 |
| Items | Cd | As |
|---|---|---|
| Total heavy metal (mg/kg) | 0.29 | 28.4 |
| Agricultural Soil Risk Screening Standard Value (mg/kg) | 0.3 | 30 |
| Pi value | 0.94 | 0.94 |
| Pollution Level | Relatively clean | Relatively clean |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Xu, H.; Yin, F.; Cao, J.; Xu, X.; Li, C.; Huang, F.; Chen, F.; Mao, X.; Liao, Q. Synergistic Remediation of Cd-Contaminated Soil with Pure Natural Adsorption Material and Hyperaccumulator Plant. Agronomy 2024, 14, 1299. https://doi.org/10.3390/agronomy14061299
Guo J, Xu H, Yin F, Cao J, Xu X, Li C, Huang F, Chen F, Mao X, Liao Q. Synergistic Remediation of Cd-Contaminated Soil with Pure Natural Adsorption Material and Hyperaccumulator Plant. Agronomy. 2024; 14(6):1299. https://doi.org/10.3390/agronomy14061299
Chicago/Turabian StyleGuo, Jun, Honggen Xu, Fengxiang Yin, Jian Cao, Xuesheng Xu, Cong Li, Fengcun Huang, Fangwei Chen, Xiong Mao, and Qi Liao. 2024. "Synergistic Remediation of Cd-Contaminated Soil with Pure Natural Adsorption Material and Hyperaccumulator Plant" Agronomy 14, no. 6: 1299. https://doi.org/10.3390/agronomy14061299





