Soil Microbial Functions Linked Fragrant Rice 2-Acetyl-1-Pyrroline with Soil Active Carbon Pool: Evidence from Soil Metagenomic Sequencing of Tillage Practices
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Soil Samples Collection and Physiochemical Properties Analysis
2.3. Soil DNA Extraction, 16S, Internal Transcribed Spacer, and Metagenomic Sequencing
2.4. Detection of 2-Acetyl-1-Pyrroline and Yield of Aromatic Rice
2.5. Data Analysis
3. Results
3.1. Soil Basic Properties, Microbial Diversity, and Composition
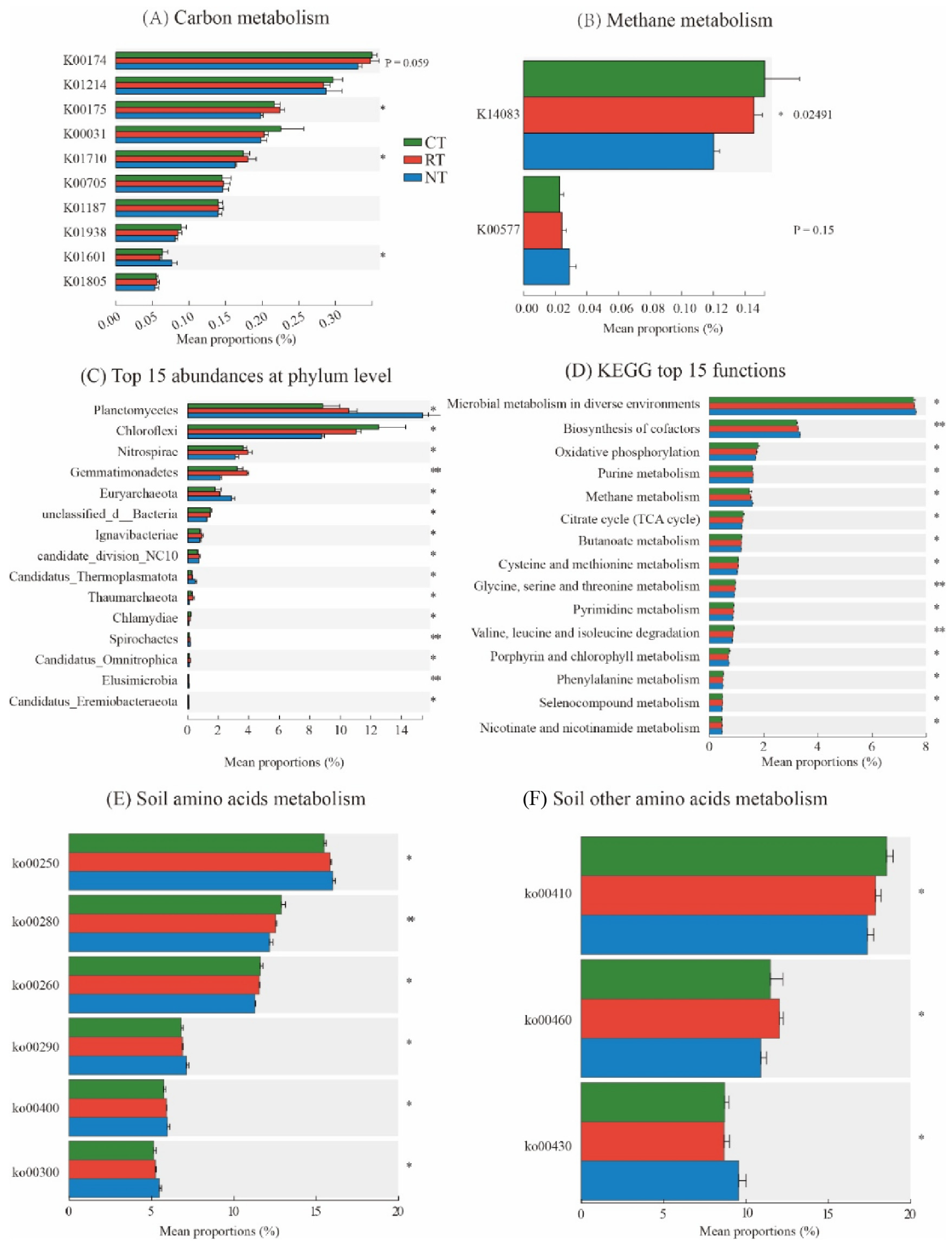
3.2. Soil Carbon and Methane Metabolism
3.3. Soil Alkaline Amino Acids Content and Total Carbon Content of Amino Acids
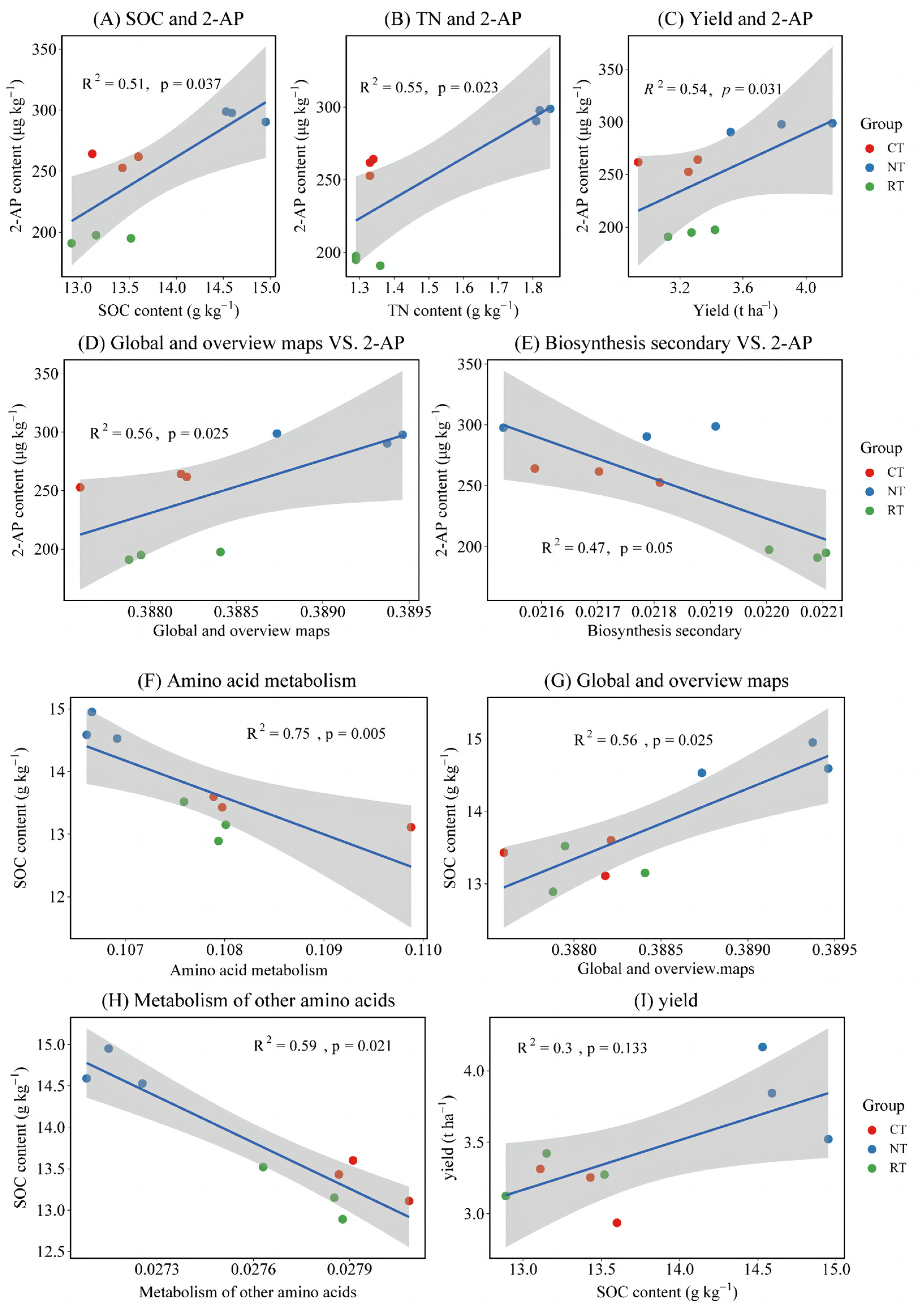
3.4. Fragrant Rice Yield, 2-Acetyl-1-Pyrroline, and Related Amino Acids Content
4. Discussions
4.1. Effects of Tillage Practices on Soil Chemical and Microbial Properties
4.2. Effects of Tillage Practices on KEGG Functions in Soil
4.3. Effects of Tillage Practices on Yield and 2-Acetyl-1-Pyrroline of Fragrant Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full name | Abbreviations | Full name |
| SOC | Soil organic carbon | C | Carbon |
| TN | Total nitrogen | N | Nitrogen |
| NT | No tillage | RT | Reduced tillage |
| CT | Conventional tillage |
References
- Niu, S.; Song, L.; Wang, J.; Luo, Y.; Yu, G. Dynamic Carbon-Nitrogen Coupling under Global Change. Sci. China-Life Sci. 2023, 66, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.J.C.; Smith, P. Carbon Losses from Soil and Its Consequences for Land-Use Management. Sci. Total Environ. 2007, 382, 165–190. [Google Scholar] [CrossRef] [PubMed]
- de Tombeur, F.; Sohy, V.; Chenu, C.; Colinet, G.; Cornelis, J.-T. Effects of Permaculture Practices on Soil Physicochemical Properties and Organic Matter Distribution in Aggregates: A Case Study of the Bec-Hellouin Farm (France). Front. Environ. Sci. 2018, 6, 116. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Pineiro, G. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. In Annual Review of Ecology, Evolution, and Systematics; Futuyma, D.J., Ed.; Annual Reviews: Palo Alto, CA, USA, 2017; Volume 48, pp. 419–445. ISBN 978-0-8243-1448-4. [Google Scholar]
- Schimel, J.P.; Schaeffer, S.M. Microbial Control over Carbon Cycling in Soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Yang, H.; Zhang, S.; Huang, S.; Zhao, S.; Xu, X.; He, P.; Zhou, W.; Zhao, Y.; Yan, N.; et al. Carbon Storage in an Arable Soil Combining Field Measurements, Aggregate Turnover Modeling and Climate Scenarios. Catena 2023, 220, 106708. [Google Scholar] [CrossRef]
- Semenov, A.M.; Dukic, D.A. The Role of Microbial Communities in Soil Formation and Soil Ecosystem Health. Paleontol. J. 2020, 54, 843–852. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, F.; Li, X.; Li, C.; Zhao, Y.; Gao, Y.; Liu, J. Effects of Plants and Soil Microorganisms on Organic Carbon and the Relationship between Carbon and Nitrogen in Constructed Wetlands. Environ. Sci. Pollut. Res. 2023, 30, 62249–62261. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.E.; Egan, R.; Foster, B.; Eloe-Fadrosh, E.A.; Buckley, D.H. Genomic Features Predict Bacterial Life History Strategies in Soil, as Identified by Metagenomic Stable Isotope Probing. MBIO 2023, 14, e03584-22. [Google Scholar] [CrossRef]
- Hernandez-Guzman, M.; Perez-Hernandez, V.; Gomez-Acata, S.; Jimenez-Bueno, N.; Verhulst, N.; Catalina Munoz-Arenas, L.; Navarro-Noya, Y.E.; Luna-Guido, M.L.; Dendooven, L. Application of Young Maize Plant Residues Alters the Microbiome Composition and Its Functioning in a Soil under Conservation Agriculture: A Metagenomics Study. Arch. Microbiol. 2022, 204, 458. [Google Scholar] [CrossRef]
- Zhong, C.; Chen, C.; Wang, L.; Ning, K. Integrating Pan-Genome with Metagenome for Microbial Community Profiling. Comput. Struct. Biotechnol. J. 2021, 19, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, M.E.; Delmont, T.O.; Eren, A.M.; Meyer, K.M.; Guo, J.; Khan, K.; Rodrigues, J.L.M.; Bohannan, B.J.M.; Tringe, S.G.; Borges, C.D.; et al. New Biological Insights Into How Deforestation in Amazonia Affects Soil Microbial Communities Using Metagenomics and Metagenome-Assembled Genomes. Front. Microbiol. 2018, 9, 1635. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Wang, Q.; Zhang, Y.; Xu, Z.; Wang, R.; Wang, X.; Jia, G.; Zhang, X. The Effects of Eight Years of Conservation Tillage on the Soil Physicochemical Properties and Bacterial Communities in a Rain-Fed Agroecosystem of the Loess Plateau, China. Land Degrad. Dev. 2020, 31, 2475–2489. [Google Scholar] [CrossRef]
- Morugan-Coronado, A.; Perez-Rodriguez, P.; Insolia, E.; Soto-Gomez, D.; Fernandez-Calvino, D.; Zornoza, R. The Impact of Crop Diversification, Tillage and Fertilization Type on Soil Total Microbial, Fungal and Bacterial Abundance: A Worldwide Meta-Analysis of Agricultural Sites. Agric. Ecosyst. Environ. 2022, 329, 107867. [Google Scholar] [CrossRef]
- Zhong, S.; Zeng, H. Long-Term Interactions of Reduced Tillage and Different Amounts of Residue Retaining Improved Soil Environment in a Semi-Arid Tropical Climate. Chil. J. Agric. Res. 2020, 80, 197–208. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Xie, J.; Coulter, J.A.; Zhang, R.; Luo, Z.; Cai, L.; Wang, L.; Gopalakrishnan, S. Soil Bacterial Diversity and Potential Functions Are Regulated by Long-Term Conservation Tillage and Straw Mulching. Microorganisms 2020, 8, 836. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Singh, D.; Hathi, Z.; Purohit, H.J.; Philip, A.; Uthup, T.K.; Singh, L. Soil Microbiome Dynamics Associated with Conversion of Tropical Forests to Different Rubber Based Land Use Management Systems. Appl. Soil Ecol. 2023, 188, 104933. [Google Scholar] [CrossRef]
- Huang, M. The Decreasing Area of Hybrid Rice Production in China: Causes and Potential Effects on Chinese Rice Self-Sufficiency. Food Secur. 2022, 14, 267–272. [Google Scholar] [CrossRef]
- Diversity of Global Rice Markets and the Science Required for Consumer-Targeted Rice Breeding-All Databases. Available online: https://webofscience.clarivate.cn/wos/alldb/full-record/WOS:000329925800022 (accessed on 1 June 2024).
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice Quality: How Is It Defined by Consumers, Industry, Food Scientists, and Geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef]
- Suwannaporn, P.; Linnemann, A.; Chaveesuk, R. Consumer Preference Mapping for Rice Product Concepts. Br. Food J. 2008, 110, 595–606. [Google Scholar] [CrossRef]
- Imran, M.; Shafiq, S.; Ashraf, U.; Qi, J.; Mo, Z.; Tang, X. Biosynthesis of 2-Acetyl-1-Pyrroline in Fragrant Rice: Recent Insights into Agro-Management, Environmental Factors, and Functional Genomics. J. Agric. Food Chem. 2023, 71, 4201–4215. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the Grain Filling Period Increases 2-Acetyl-1-Pyrroline Content in Fragrant Rice. Rice 2015, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Starkenmann, C.; Niclass, Y.; Vuichoud, B.; Schweizer, S.; He, X. Occurrence of 2-Acetyl-1-Pyrroline and Its Nonvolatile Precursors in Celtuce (Lactuca Sativa L. Var. Augustana). J. Agric. Food Chem. 2019, 67, 11710–11717. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Sun, Q.; Methven, L.; Elmore, J.S. Comparison of the Sensory Properties of Fragrant and Non-Fragrant Rice (Oryza Saliva), Focusing on the Role of the Popcorn-like Aroma Compound 2-Acetyl-1-Pyrroline. Food Chem. 2021, 339, 128077. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Luo, H.; Wu, F.; He, L.; Lai, R.; Tang, X. Organic Cultivation Induced Regulation in Yield Formation, Grain Quality Attributes, and Volatile Organic Compounds of Fragrant Rice. Food Chem. 2023, 405, 134845. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.; Hu, F.; Tang, X.; Du, B. Zinc Supplementation and Light Intensity Affect 2-Acetyl-1-Pyrroline (2AP) Formation in Fragrant Rice. BMC Plant Biol. 2023, 23, 194. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhang, T.; Zheng, A.; He, L.; Lai, R.; Liu, J.; Xing, P.; Tang, X. Exogenous Proline Induces Regulation in 2-Acetyl-1-Pyrroline (2-AP) Biosynthesis and Quality Characters in Fragrant Rice (Oryza sativa L.). Sci. Rep. 2020, 10, 13971. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.W.; Xing, P.P.; Liu, J.H.; Lai, R.F.; He, L.X.; Zhang, T.T.; Tang, X.R. Application of Ornithine-Induced Regulation in Yield Formation, Grain Quality and Aroma of Fragrant Rice. Cereal Res. Commun. 2020, 48, 485–492. [Google Scholar] [CrossRef]
- Du, B.; He, L.; Lai, R.; Luo, H.; Zhang, T.; Tang, X. Fragrant Rice Performances in Response to Continuous Zero-Tillage in Machine-Transplanted Double-Cropped Rice System. Sci. Rep. 2020, 10, 8326. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.-Y.; Yao, X.-B.; Lu, J.; He, L.-X.; Cao, J.-L.; Kan, Z.-R.; Wang, X.; Pan, S.-G.; Tang, X.-R. A 40% Paddy Surface Soil Organic Carbon Increase after 5-Year No-Tillage Is Linked with Shifts in Soil Bacterial Composition and Functions. Sci. Total Environ. 2023, 859, 160206. [Google Scholar] [CrossRef]
- Li, D.; Wu, C.; Wu, J. Soil Fungal Community Has Higher Network Stability than Bacterial Community in Response to Warming and Nitrogen Addition in a Subtropical Primary Forest. Appl. Environ. Microbiol. 2024, e00001-24. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Ye, S.; Wang, S. Large Macro-Aggregate Formation Improves Soil Bacterial Metabolic Activity and Diversity in a Chronosequence of Chinese Fir Plantations. J. Soil Sci. Plant Nutr. 2024, 1–13. [Google Scholar] [CrossRef]
- Liu, L.; Liu, D.; Ding, X.; Chen, M.; Zhang, S. Straw Incorporation and Nitrogen Fertilization Enhance Soil Carbon Sequestration by Altering Soil Aggregate and Microbial Community Composition in Saline-Alkali Soil. Plant Soil 2024, 498, 341–356. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Wang, S.; Xie, H.; Jiang, N.; Chen, Z.; Wei, K.; Bao, X.; Song, X.; Bai, Z. Stover and Biochar Can Improve Soil Microbial Necromass Carbon, and Enzymatic Transformation at the Genetic Level. Glob. Chang. Biol. Bioenergy 2022, 14, 1082–1096. [Google Scholar] [CrossRef]
- Khmelevtsova, L.E.; Sazykin, I.S.; Azhogina, T.N.; Sazykina, M.A. Influence of Agricultural Practices on Bacterial Community of Cultivated Soils. Agriculture 2022, 12, 371. [Google Scholar] [CrossRef]
- Mackay, J.E.; Bernhardt, L.T.; Smith, R.G.; Ernakovich, J.G. Tillage and Pesticide Seed Treatments Have Distinct Effects on Soil Microbial Diversity and Function. Soil Biol. Biochem. 2023, 176, 108860. [Google Scholar] [CrossRef]
- Cui, J.; Yang, B.; Xu, X.; Ai, C.; Zhou, W. Long-Term Maize-Soybean Rotation in Northeast China: Impact on Soil Organic Matter Stability and Microbial Decomposition. Plant Soil 2024, 1–18. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Fang, Y.; Vancov, T.; Jin, X.; Gao, Q.; Dong, W.; Du, Z. No-Tillage Farming for Two Decades Increases Plant- and Microbial-Biomolecules in the Topsoil Rather than Soil Profile in Temperate Agroecosystem. Soil Tillage Res. 2024, 241, 106108. [Google Scholar] [CrossRef]
- Du, P.; Luo, H.; He, J.; Mao, T.; Du, B.; Hu, L. Different Tillage Induces Regulation in 2-Acetyl-1-Pyrroline Biosynthesis in Direct-Seeded Fragrant Rice. BMC Plant Biol. 2019, 19, 308. [Google Scholar] [CrossRef]
- Renuka, N.; Barvkar, V.T.; Ansari, Z.; Zhao, C.; Wang, C.; Zhang, Y.; Nadaf, A.B. Co-Functioning of 2AP Precursor Amino Acids Enhances 2-Acetyl-1-Pyrroline under Salt Stress in Aromatic Rice (Oryza Sativa L.) Cultivars. Sci. Rep. 2022, 12, 3911. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental Evaluation of Methods to Quantify Dissolved Organic Nitrogen (DON) and Dissolved Organic Carbon (DOC) in Soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct de Bruijn Graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic Gene Recognition and Translation Initiation Site Identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for Clustering the next-Generation Sequencing Data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, C.; Li, Y.; Lam, T.-W.; Yiu, S.-M.; Kristiansen, K.; Wang, J. SOAP2: An Improved Ultrafast Tool for Short Read Alignment. Bioinformatics 2009, 25, 1966–1967. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Reuter, K.; Drost, H.-G. Sensitive Protein Alignments at Tree-of-Life Scale Using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef]
- Mo, Z.; Lei, S.; Ashraf, U.; Khan, I.; Li, Y.; Pan, S.; Duan, M.; Tian, H.; Tang, X. Silicon Fertilization Modulates 2-Acetyl-1-Pyrroline Content, Yield Formation and Grain Quality of Aromatic Rice. J. Cereal Sci. 2017, 75, 17–24. [Google Scholar] [CrossRef]
- Cai, L.; Guo, Z.; Zhang, J.; Gai, Z.; Liu, J.; Meng, Q.; Liu, X. No Tillage and Residue Mulching Method on Bacterial Community Diversity Regulation in a Black Soil Region of Northeastern China. PLoS ONE 2021, 16, e0256970. [Google Scholar] [CrossRef]
- Buckley, D.H.; Huangyutitham, V.; Nelson, T.A.; Rumberger, A.; Thies, J.E. Diversity of Planctomycetes in Soil in Relation to Soil History and Environmental Heterogeneity. Appl. Environ. Microbiol. 2006, 72, 4522–4531. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Kulichevskaya, I.S.; Merkel, A.Y.; Toshchakov, S.V.; Dedysh, S.N. High Diversity of Planctomycetes in Soils of Two Lichen-Dominated Sub-Arctic Ecosystems of Northwestern Siberia. Front. Microbiol. 2016, 7, 2065. [Google Scholar] [CrossRef]
- Fuerst, J.A.; Sagulenko, E. Beyond the Bacterium: Planctomycetes Challenge Our Concepts of Microbial Structure and Function. Nat. Rev. Microbiol. 2011, 9, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Op den Camp, H.J.M.; Islam, T.; Stott, M.B.; Harhangi, H.R.; Hynes, A.; Schouten, S.; Jetten, M.S.M.; Birkeland, N.-K.; Pol, A.; Dunfield, P.F. Environmental, Genomic and Taxonomic Perspectives on Methanotrophic Verrucomicrobia. Environ. Microbiol. Rep. 2009, 1, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Q.; Li, F.; Li, Z.; Qiao, Y.; Du, K.; Yue, Z.; Tian, C.; Leng, P.; Cheng, H.; et al. Soil CO2 Emission Reduction with No-Tillage and Medium Nitrogen Fertilizer Applications in Semi-Humid Maize Cropland in North China Plain. Eur. J. Agron. 2023, 147, 126838. [Google Scholar] [CrossRef]
- Wacker, T.S.; Jensen, L.S.; Thorup-Kristensen, K. Conservation Agriculture Affects Soil Organic Matter Distribution, Microbial Metabolic Capacity and Nitrogen Turnover under Danish Field Conditions. Soil Tillage Res. 2022, 224, 105508. [Google Scholar] [CrossRef]
- Doyeni, M.O.; Suproniene, S.; Versuliene, A.; Meskauskiene, L.; Kadziene, G. Influence of the Long-Term Application of Management Practices (Tillage, Cover Crop and Glyphosate) on Greenhouse Gas Emissions and Soil Physical Properties. Sustainability 2024, 16, 2859. [Google Scholar] [CrossRef]
- Ndour, P.M.S.; Bargaz, A.; Rchiad, Z.; Pawlett, M.; Clark, I.M.; Mauchline, T.H.; Harris, J.; Lyamlouli, K. Microbial Catabolic Activity: Methods, Pertinence, and Potential Interest for Improving Microbial Inoculant Efficiency. Microb. Ecol. 2023, 86, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Harish, M.N.; Choudhary, A.K.; Kumar, S.; Dass, A.; Singh, V.K.; Sharma, V.K.; Varatharajan, T.; Dhillon, M.K.; Sangwan, S.; Dua, V.K.; et al. Double Zero Tillage and Foliar Phosphorus Fertilization Coupled with Microbial Inoculants Enhance Maize Productivity and Quality in a Maize-Wheat Rotation. Sci. Rep. 2022, 12, 3161. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, Y.; Wan, Y.; Shi, S.; Li, Y.; Wang, H.; Gao, Q.; Ma, X.; Liu, S.; Liu, Y.; et al. Relationships between Methane Emissions and Soil Microorganisms in a Double-Rice Field in Southern Subtropical China. J. Integr. Environ. Sci. 2012, 9, 97–111. [Google Scholar] [CrossRef][Green Version]
- Yoshihashi, T.; Huong, N.T.T.; Inatomi, H. Precursors of 2-Acetyl-1-Pyrroline, a Potent Flavor Compound of an Aromatic Rice Variety. J. Agric. Food Chem. 2002, 50, 2001–2004. [Google Scholar] [CrossRef]
- Ren, Y.; Ashraf, U.; He, L.X.; Mo, Z.W.; Wang, F.; Wan, X.C.; Kong, H.; Ran, X.L.; Tang, X.R. Irrigation and Nitrogen Management Practices Affect Grain Yield and 2-Acetyl-1-Pyrroline Content in Aromatic Rice. Appl. Ecol. Environ. Res. 2017, 15, 1447–1460. [Google Scholar] [CrossRef]
- Mo, Z.; Ashraf, U.; Tang, Y.; Li, W.; Pan, S.; Duan, M.; Tian, H.; Tang, X. Nitrogen Application at the Booting Stage Affects 2-Acetyl-1-Pyrroline, Proline, and Total Nitrogen Contents in Aromatic Rice. Chil. J. Agric. Res. 2018, 78, 165–172. [Google Scholar] [CrossRef]




| Treatments | Clay (%) | pH | TP (g kg−1) | TK (g kg−1) | AK (mg kg−1) | AP (mg kg−1) | NH4-N (mg kg−1) | NO3-N (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| NT | 19.44 | 5.59 (0.06) | 0.77 (0.01) | 22.48 (0.07) | 20 (2) | 48.58 (0.19) | 4.30 (0.13) | 0.82 (0.05) |
| RT | 11.27 | 5.20 (0.03) | 0.68 (0.01) | 22.99 (0.35) | 17 (1) | 30.42 (1.34) | 1.75 (0.05) | 0.69 (0.03) |
| CT | 15.15 | 5.04 (0.03) | 0.69 (0.01) | 25.76 (0.44) | 20 (1) | 36.96 (0.71) | 2.38 (0.24) | 0.53 (0.04) |
| Biosynthesis Secondary | Global and Overview Maps | Metabolism of Cofactors and Vitamins | Metabolism of Other Amino Acids | Amino Acid Metabolism | |
|---|---|---|---|---|---|
| 2-AP | −0.683 * | 0.750 * | 0.650 | −0.467 | −0.517 |
| Yield | −0.217 | 0.767 * | 0.867 ** | −0.750 * | −0.483 |
| SOC | −0.383 | 0.750 * | 0.583 | −0.767 * | −0.867 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Lin, J.; Xie, Q.; Shi, J.; Du, X.; Pan, S.; Tang, X.; Qi, J. Soil Microbial Functions Linked Fragrant Rice 2-Acetyl-1-Pyrroline with Soil Active Carbon Pool: Evidence from Soil Metagenomic Sequencing of Tillage Practices. Agronomy 2024, 14, 1308. https://doi.org/10.3390/agronomy14061308
Huang X, Lin J, Xie Q, Shi J, Du X, Pan S, Tang X, Qi J. Soil Microbial Functions Linked Fragrant Rice 2-Acetyl-1-Pyrroline with Soil Active Carbon Pool: Evidence from Soil Metagenomic Sequencing of Tillage Practices. Agronomy. 2024; 14(6):1308. https://doi.org/10.3390/agronomy14061308
Chicago/Turabian StyleHuang, Xiangwen, Jiajun Lin, Qihuan Xie, Jingdan Shi, Xiaoxu Du, Shenggang Pan, Xiangru Tang, and Jianying Qi. 2024. "Soil Microbial Functions Linked Fragrant Rice 2-Acetyl-1-Pyrroline with Soil Active Carbon Pool: Evidence from Soil Metagenomic Sequencing of Tillage Practices" Agronomy 14, no. 6: 1308. https://doi.org/10.3390/agronomy14061308
APA StyleHuang, X., Lin, J., Xie, Q., Shi, J., Du, X., Pan, S., Tang, X., & Qi, J. (2024). Soil Microbial Functions Linked Fragrant Rice 2-Acetyl-1-Pyrroline with Soil Active Carbon Pool: Evidence from Soil Metagenomic Sequencing of Tillage Practices. Agronomy, 14(6), 1308. https://doi.org/10.3390/agronomy14061308








