Isolation and Characterization of Native Isolates of Metarhizium sp. as a Biocontrol Agent of Hypothenemus hampei in Rodríguez de Mendoza Province—Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Isolates of Metarhizium sp.
2.2. Isolation of Native Isolates of Metarhizium sp.
2.3. Morphological and Physiological Characterization of the Isolates
2.3.1. Macroscopic Morphology
2.3.2. Microscopic Morphology
Microculture of Native Isolates of Metarhizium sp.
2.3.3. Physiological Characterization of the Isolates
Radial Growth of Isolates of Metarhizium sp.
Evaluation of Conidial Concentration in Isolates of Metarhizium sp.
Evaluation of the Percentage Germination of Metarhizium sp. Spores
2.4. Determination of Pathogenicity and Mycelial Growth of Native Isolates of Metarhizium sp.
Experimental Design
2.5. Data Analysis
3. Results
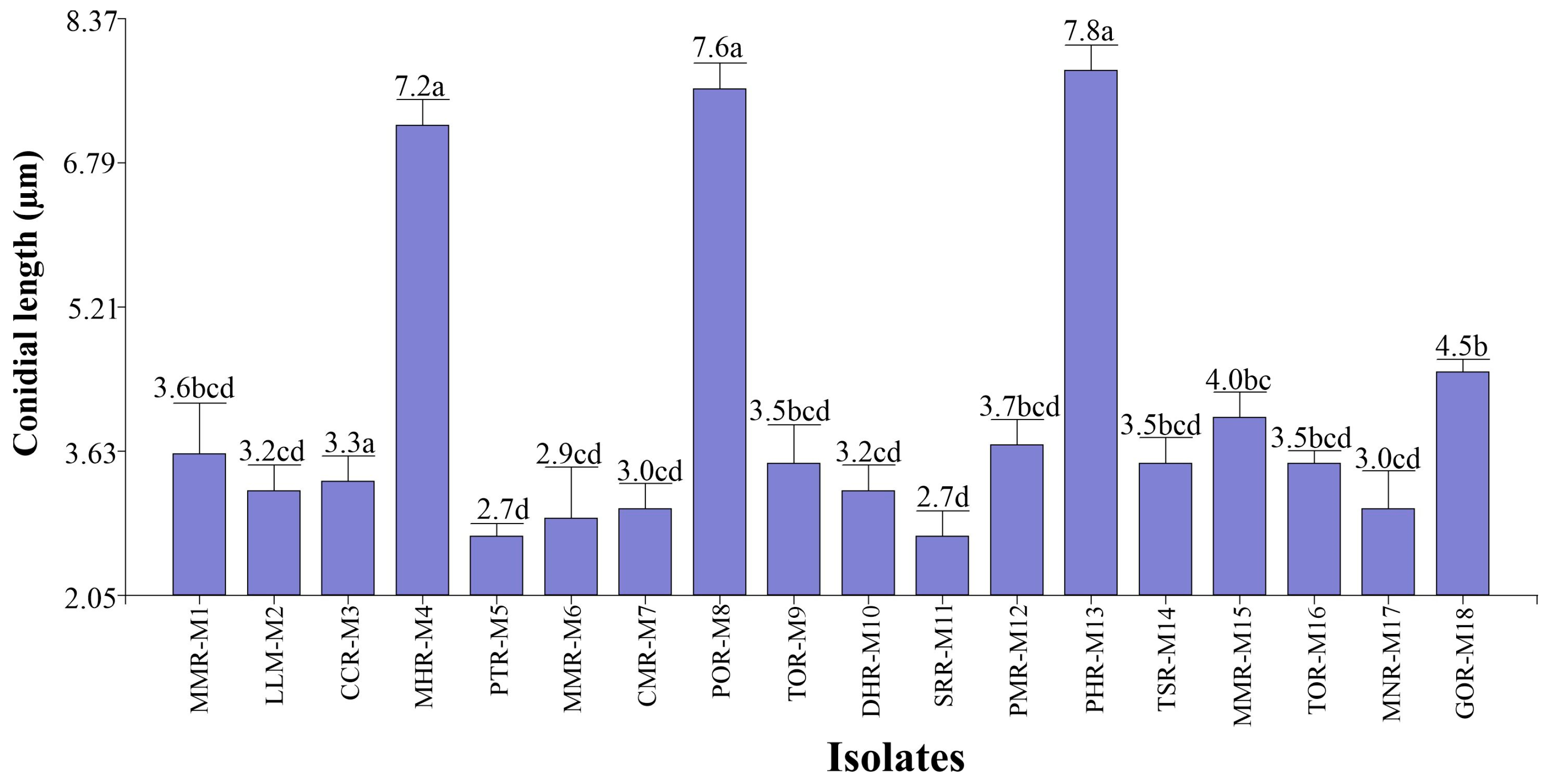
3.1. Morphological and Physiological Characterization of the Isolates
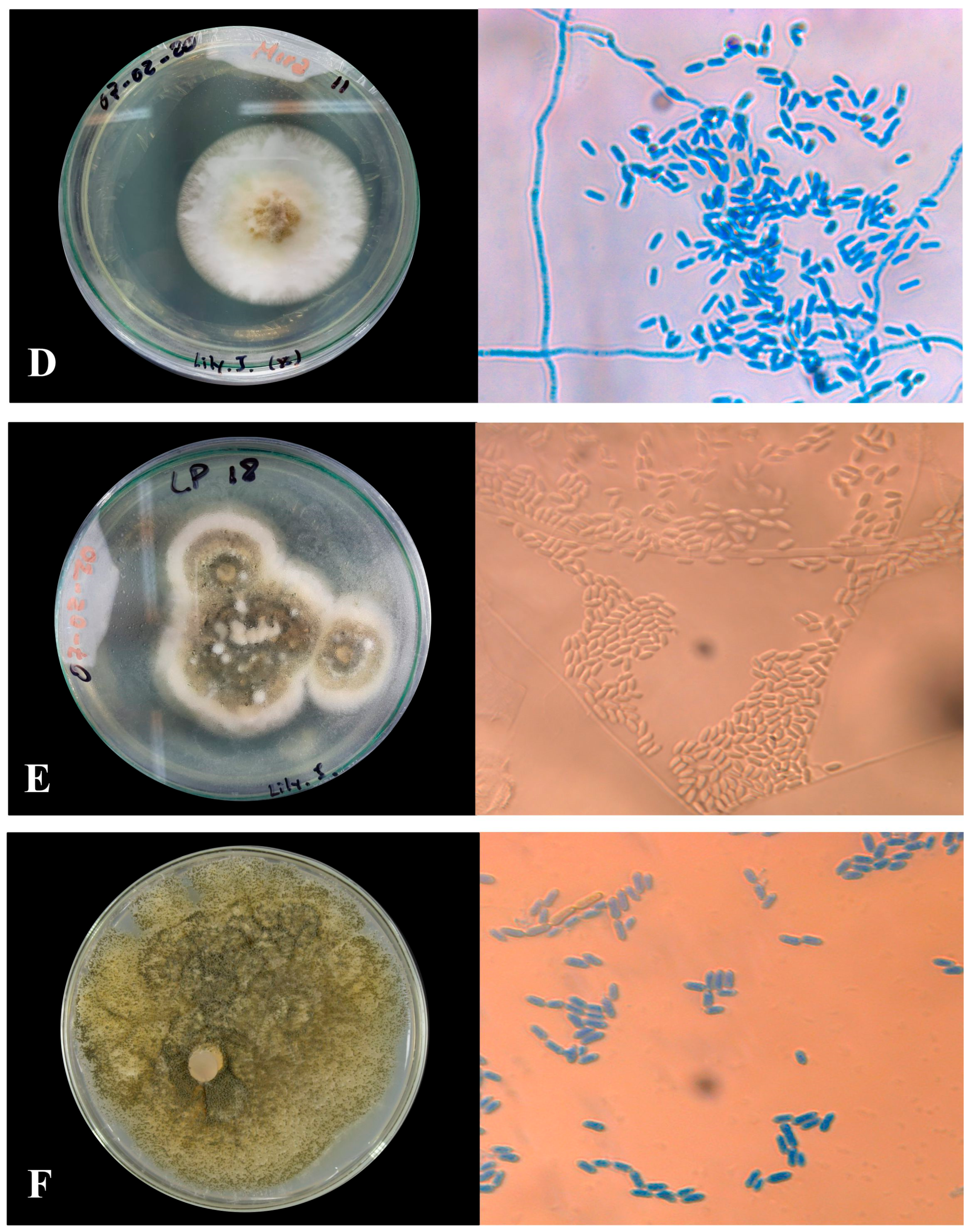
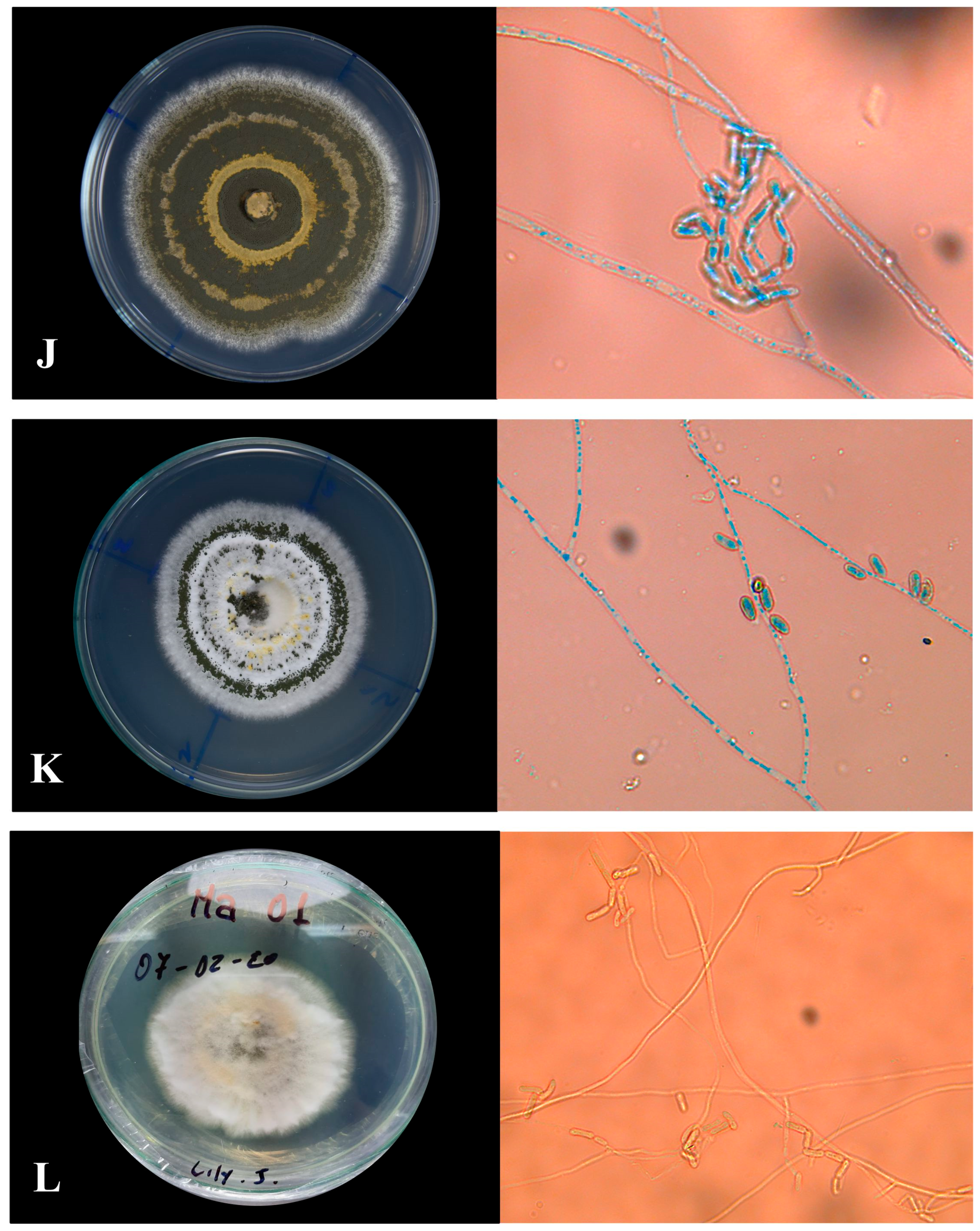
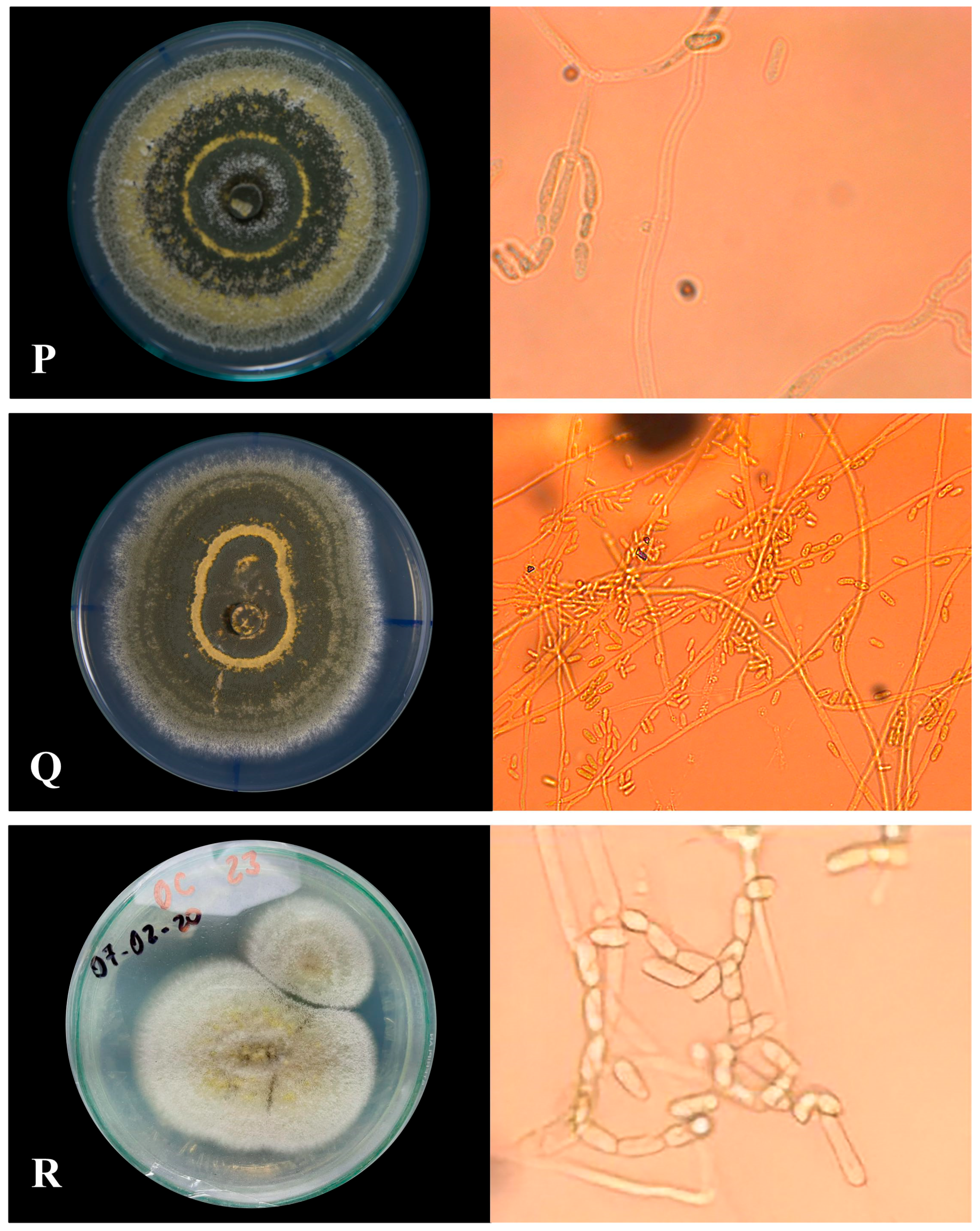
3.1.1. Macroscopic Characteristics of Metarhizium sp.
3.1.2. Microscopic Characterization
- (A)
- Conidial length
- (B) Conidial width
3.1.3. Physiological Characterization of Isolates of Metarhizium sp.
- (a)
- Radial growth of isolates of Metarhizium sp.
- (b)
- Conidial concentration in isolates of Metarhizium sp.
- (c)
- Germination percentage of isolates of Metarhizium sp.
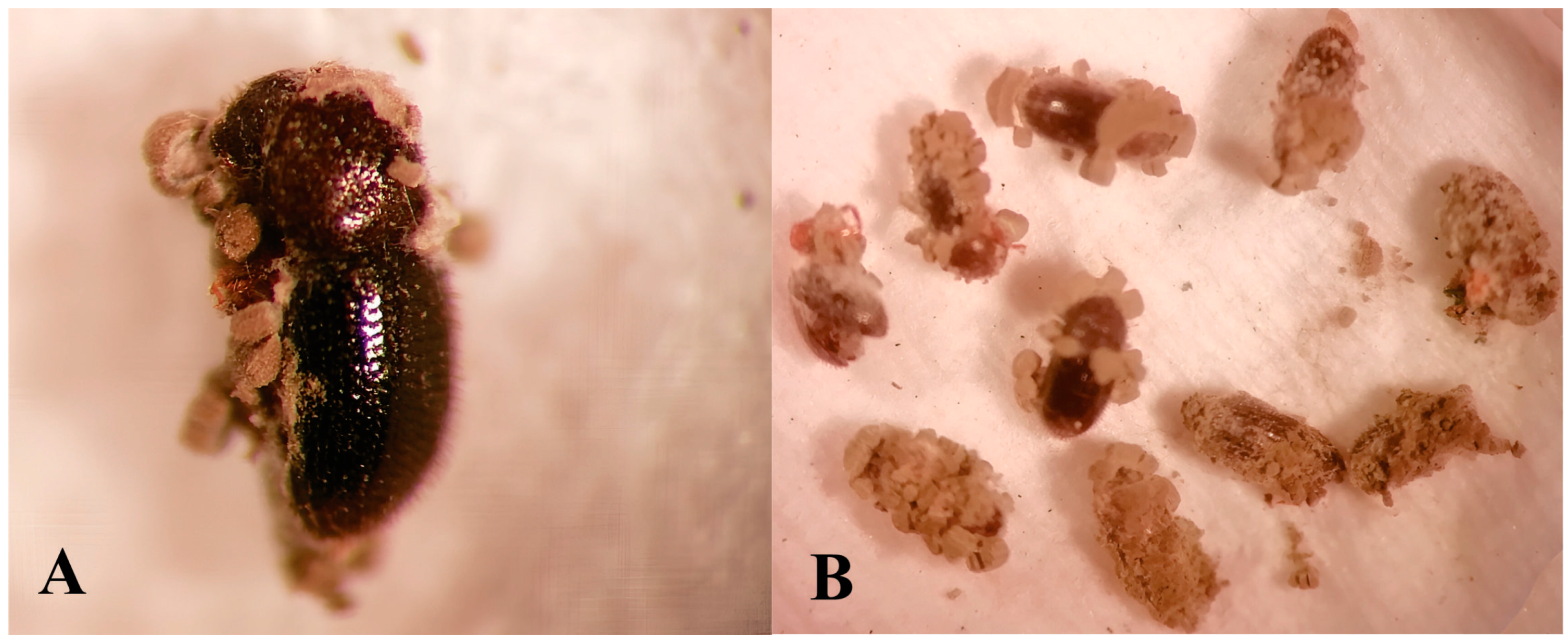
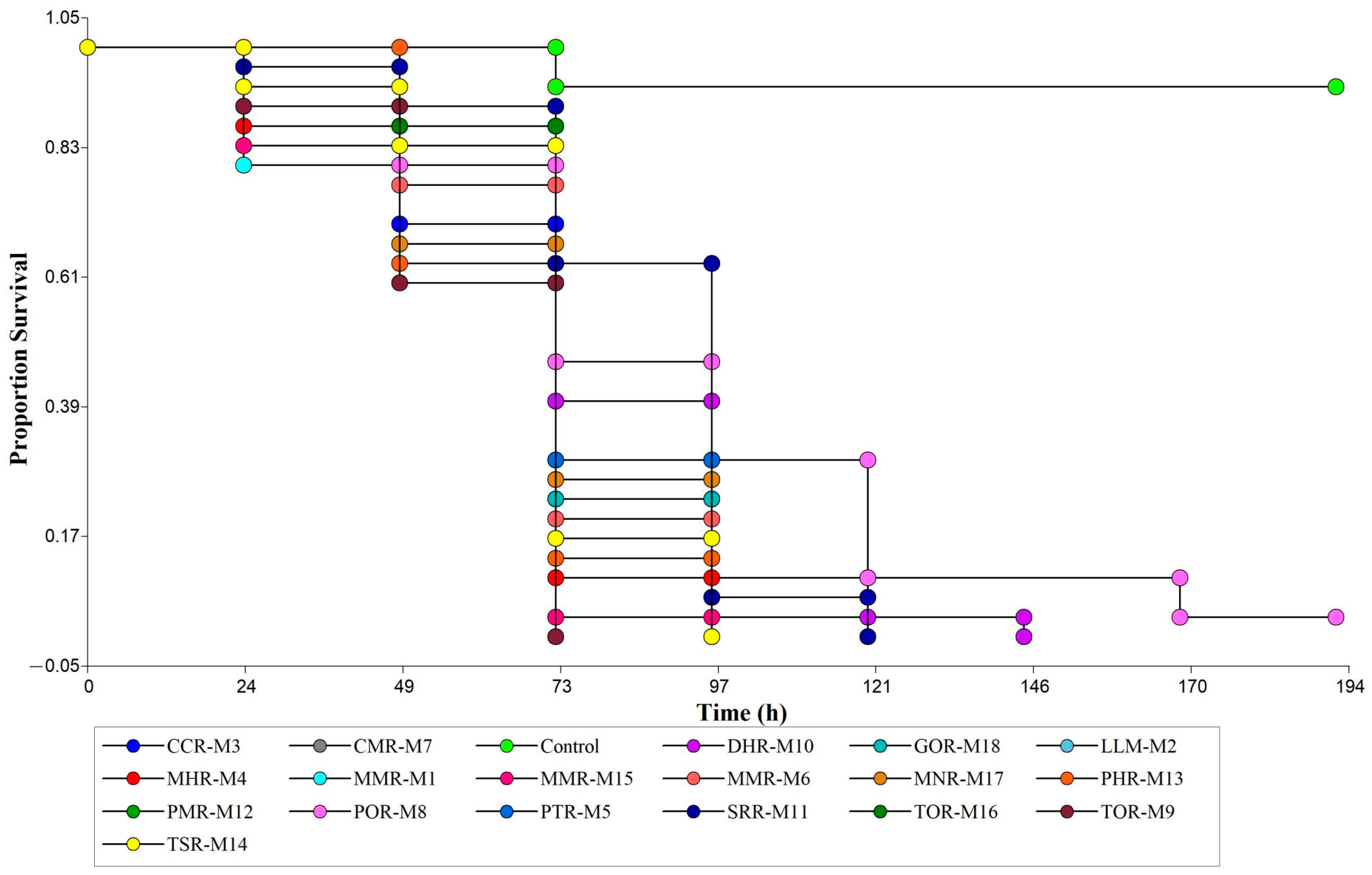
3.2. Pathogenicity and Mycelial Growth of Native Isolates of Metarhizium sp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Coffee Organization. Trade Statistics International Coffee Organization. Available online: http://www.ico.org/trade_statistics.asp (accessed on 7 December 2021).
- Pintado, M. El rol de la pequeña y mediana agricultura en las agroexportaciones: El caso del café. La Rev. Agrar. 2018, 187, 15. [Google Scholar]
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The impact of climate change and variability on coffee production: A systematic review. Clim. Chang. 2019, 156, 609–630. [Google Scholar] [CrossRef]
- Vega, F.; Brown, S.; Chen, H.; Shen, E.; Nair, M.; Ceja-Navarro, J.; Brodie, E.; Infante, F.; Dowd, P.; Pain, A. Draft genome of the most devastating insect pest of coffee worldwide: The coffee berry borer, Hypothenemus hampei. Sci. Rep. 2015, 5, 12525. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.R.; Perfecto, I. Testing the potential for ant predation of immature coffee berry borer (Hypothenemus hampei) life stages. Agric. Ecosyst. Environ. 2016, 233, 224–228. [Google Scholar] [CrossRef]
- Monzón, A.; Guharay, F.; Klingen, I. Natural occurrence of Beauveria bassiana in Hypothenemus hampei (Coleoptera: Curculionidae) populations in unsprayed coffee fields. J. Invertebr. Pathol. 2008, 97, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Cure, J.; Rodríguez, D.; Gutierrez, A.; Ponti, L. The coffee agroecosystem: Bio-economic analysis of coffee berry borer control (Hypothenemus hampei). Sci. Rep. 2020, 10, 12262. [Google Scholar] [CrossRef]
- Leiva-Espinoza, S.; Oliva-Cruz, M.; Rubio-Rojas, K.; Maicelo-Quintana, J.; Milla-Pino, M. Uso de trampas de colores y atrayentes alcohólicos para la captura de la broca del café (Hypothenemus hampei) en plantaciones de café altamente infestadas. Rev. Colomb. Entomol. 2019, 45, e8537. [Google Scholar] [CrossRef]
- Garner, O.; Goulnik, M.; Muller, C. Sustainable Coffee for San Martin: Reinventing the Agricultural Value Chain through Cooperatives and Agroforestry; Virtual Field Trip; London’s Global University: London, UK, 2020; p. 30. [Google Scholar]
- Venzon, M. Agro-Ecological Management of Coffee Pests in Brazil. Front. Sustain. Food Syst. 2021, 5, 721117. [Google Scholar] [CrossRef]
- Donga, T.; Meadow, R.; Meyling, N.; Klingen, I. Natural Occurrence of Entomopathogenic Fungi as Endophytes of Sugarcane (Saccharum officinarum) and in Soil of Sugarcane Fields. Insects 2021, 12, 160. [Google Scholar] [CrossRef]
- Becerra, V.; Paredes, M.; Rojo, C.; France, A.; Franco, J. Intraspecific differentiation of Chilean isolates of the entomopathogenic fungi Metarhizium anisopliae var. anisopliae as revealed by RAPD, SSR and ITS markers. Genet. Mol. Biol. 2007, 30, 89–99. [Google Scholar]
- Nishi, O.; Shimizu, S.; Sato, H. Metarhizium bibionidarum and M. purpureogenum: New species from Japan. Mycol. Prog. 2017, 16, 987–998. [Google Scholar]
- Wang, B.; Kang, Q.; Lu, Y.; Bai, L.; Wang, C. Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc. Natl. Acad. Sci. USA 2012, 109, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.; Gorreti, M.; Lizcano, R. Aislamiento de Trichoderma sp., en las unidades productivas agrícolas del centro de formación agroindustrial la angostura del campoalegre (Huila). Cent. Form. Agroindus. Angos. 2014, 17, 15–20. [Google Scholar]
- Zimmermann, G. The “Galleria bait method” for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Cañedo, V.; Ames, T. Manual de Laboratorio para el Manejo de Hongos Entomopatógenos; Centro Internacional de la Papa (CIP): Lima, Perú, 2004. [Google Scholar]
- Obando, J.; Bustillo, A.; Castro, U.; Mesa, N. Selección de cepas de Metarhizium anisopliae para el control de Aeneolamia varia (Hemiptera: Cercopidae). Rev. Colomb. Entomol. 2019, 39, 26–33. [Google Scholar]
- Padilla, G.; Bernal, M.; Vélez, P.; Montoya, E. Caracterización patogénica y morfológica de aislamientos de Metarhizium anisopliae obtenidos de diferentes ordenes insectiles. Cenicafé 2000, 51, 28–40. [Google Scholar]
- Torres, M.; Cortez, H.; Ortiz, C.F.; Capello, S.; De La Cruz, A. Caracterización de aislamientos nativos de Metarhizium anisopliae y su patogenicidad hacia Aeneolamia postica, en Tabasco, México. Rev. Colomb. Entomol. 2013, 39, 40–46. [Google Scholar]
- Torres, M.; Ortiz, C.; Bautista, C.; Ramírez, J.; Ávalos, N.; Cappello, S.; De la Cruz, A. Diversidad de Trichoderma en el agroecosistema cacao del estado de Tabasco, México. Rev. Mex. Biodivers. 2015, 86, 947–961. [Google Scholar] [CrossRef]
- Gómez, H.; Soberanis, W.; Tenorio, M.; Torres, E. Manual de Producción y uso de Hongos Antagonistas; Laboratorio de antagonistas SCB–Senasa: La Molina, Perú, 2013; p. 24. [Google Scholar]
- Lipa, J.; Slizynzki, K. Wskazówki metodyczne I terminologia dowyznaczani snedniej dawki swiertelnej (LD50) W Patologii Owadow i toksykologii. Pr. Nauk. Instytotu Ochr. Rosl. 1973, 15, 59–83. [Google Scholar]
- Acuña, M.; García, C.; Rosas, N.; López, M.; Sainz, J. Formulación de Metarhizium anisopliae (metschnikoff) sorokin con polímeros biodegradables y su virulencia contra Heliothis virescens (fabricius). Rev. Int. Contam. Ambient. México. 2015, 31, 219–226. [Google Scholar]
- González, M.; Posada, F.; Bustillo, A. Bioensayo para evaluar la patogenicidad de Beauveria bassiana (Bals.) sobre la broca del café, Hypothenemus hampei (Ferrari). Rev. Colomb. Entomol. 1993, 19, 123–130. [Google Scholar] [CrossRef]
- Kassa, A.G.; Zimmermann, D.S.; Vidal, S. Susceptibility of Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae) and Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) to Entomopathogenic Fungi from Ethiopia. Biocontrol Sci. Technol. 2002, 12, 727–736. [Google Scholar]
- Shoaib, F.; Feng-Liang, J.; Shun-Xiang, R. Determination of genetic variability among the isolates of Metarhizium anisopliae var. anisopliae from different geographical origins. World J. Microbiol. Biotechnol. 2010, 27, 359–370. [Google Scholar]
- Bustillo, A.; Cárdenas, R.; Posada, F.J. Natural Enemies and Competitors of Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae) in Colombia. Neotrop. Entomol. 2002, 31, 635–639. [Google Scholar] [CrossRef]
- Valle-Ramírez, S.B.; Torres-Gutiérrez, R.; Caicedo-Quinche, W.O.; Abril-Santos, R.V.; Sucoshañay-Villalba, D.J. Isolation and characterization of Metarhizium spp. of sugar cane crops and their pathogenicity against Mahanarva andigena (Hemiptera: Cercopidae). Cienc. Tecnol. Agropecu. 2022, 23, e2631. [Google Scholar] [CrossRef]
- Milner, R.; Samson, P.; Bullard, G. FI-1045: A Profile of a Commercially Useful Isolate of Metarhizium anisopliae var. anisopliae. Biocontrol Sci. Technol. 2002, 12, 43–58. [Google Scholar]
- Dimbi, S.; Maniania, N.K.; Lux, S.A.; Mueke, J.M. Effect of constant temperatures on germination, radial growth and virulence of Metarhizium anisopliae to three species of African tephritid fruit flies. BioControl 2004, 49, 83–94. [Google Scholar] [CrossRef]
- Villamizar, L.F.; Barrera, G.; Hurst, M.; Glare, T.R. Characterization of a new strain of Metarhizium novozealandicum with potential to be developed as a biopesticide. Mycology 2021, 12, 261–278. [Google Scholar] [CrossRef]
- Ghayedi, S.; Abdollahi, M. Biocontrol potential of Metarhizium anisopliae (Hypocreales: Clavicipitaceae), isolated from suppressive soils of The Boyer-Ahmad region, Iran, Against J2s of Heterodera avenae. J. Plant Prot. Res. 2013, 53, 165–171. [Google Scholar] [CrossRef]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Phenotypic, molecular, and virulence characterization of entomopathogenic fungi, Beauveria bassiana (Balsam) Vuillemin, and Metarhizium anisopliae (Metschn.) Sorokin from soil samples of Ethiopia for the development of mycoinsecticide. Heliyon 2021, 7, e07091. [Google Scholar] [CrossRef]
- Rachappa, V.; Lingappa, S.; Patil, R. Growth characteristics and bio-efficacy of different isolates of Metarhizium anisopliae (Metschnikoff) Sorokin against certain key insect pests. J. Biol. Control 2009, 23, 271–276. [Google Scholar]
- Sepúlveda, M.; Vargas, M.; Gerding, M.; Ceballos, R.; Oyarzúa, P. Molecular, morphological and pathogenic characterization of six strains of Metarhizium spp. (Deuteromycotina: Hyphomycetes) for the control of Aegorhinus superciliosus (Coleoptera: Curculionidae). Chil. J. Agric. Res. 2016, 76, 77–83. [Google Scholar] [CrossRef]
- Aynalem, B.; Muleta, D.; Venegas, J.; Assefa, F. Morphological, molecular, and pathogenicity characteristics of the native isolates of Metarhizium anisopliae against the tomato leafminer, Tuta absoluta (Meyrick 1917) (Lepidoptera: Gelechiidae) in Ethiopia. Egypt J. Biol. Pest Con. 2020, 30, 59. [Google Scholar]
- Fernandes, E.; Keyser, C.; Chong, J.; Rangel, D.; Miller, M.; Roberts, D. Characterization of Metarhizium species and varieties based on molecular analysis, heat tolerance and cold activity. J. Appl. Microbiol. 2010, 108, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Prakash, R. Infectivity of Ten Metarhizium anisopliae Isolates to the Coffee Berry Borer (Coleoptera: Curculionidae). J. Entomol. Zool. Stud. 2014, 2, 246–249. [Google Scholar]
- Mesquita, E.; Marciano, A.; Corval, A.; Fiorotti, J.; Corrêa, T.; Quinelato, S.; Golo, P.S. Efficacy of a native isolate of the entomopathogenic fungus Metarhizium anisopliae against larval tick outbreaks under semifield conditions. BioControl 2020, 65, 353–362. [Google Scholar] [CrossRef]
- Apriyanto, D.; Nadrawati. Laboratory Evaluation of Local Isolates of Beauveria bassiana and Metarhizium anisopliae against Coffee Berry Borer, Hyphotenemus hampei, using spryaing method. J. Trop. Plant Pests Dis. 2019, 2, 93–100. [Google Scholar]
- Samuels, R.I.; Pereira, R.C.; Gava, C. Infection of the Coffee Berry Borer Hypothenemus hampei (Coleoptera: Scolytidae) by Brazilian Isolates of the Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae (Deuteromycotina: Hyphomycetes). Biocontrol Sci. Technol. 2002, 12, 631–635. [Google Scholar] [CrossRef]
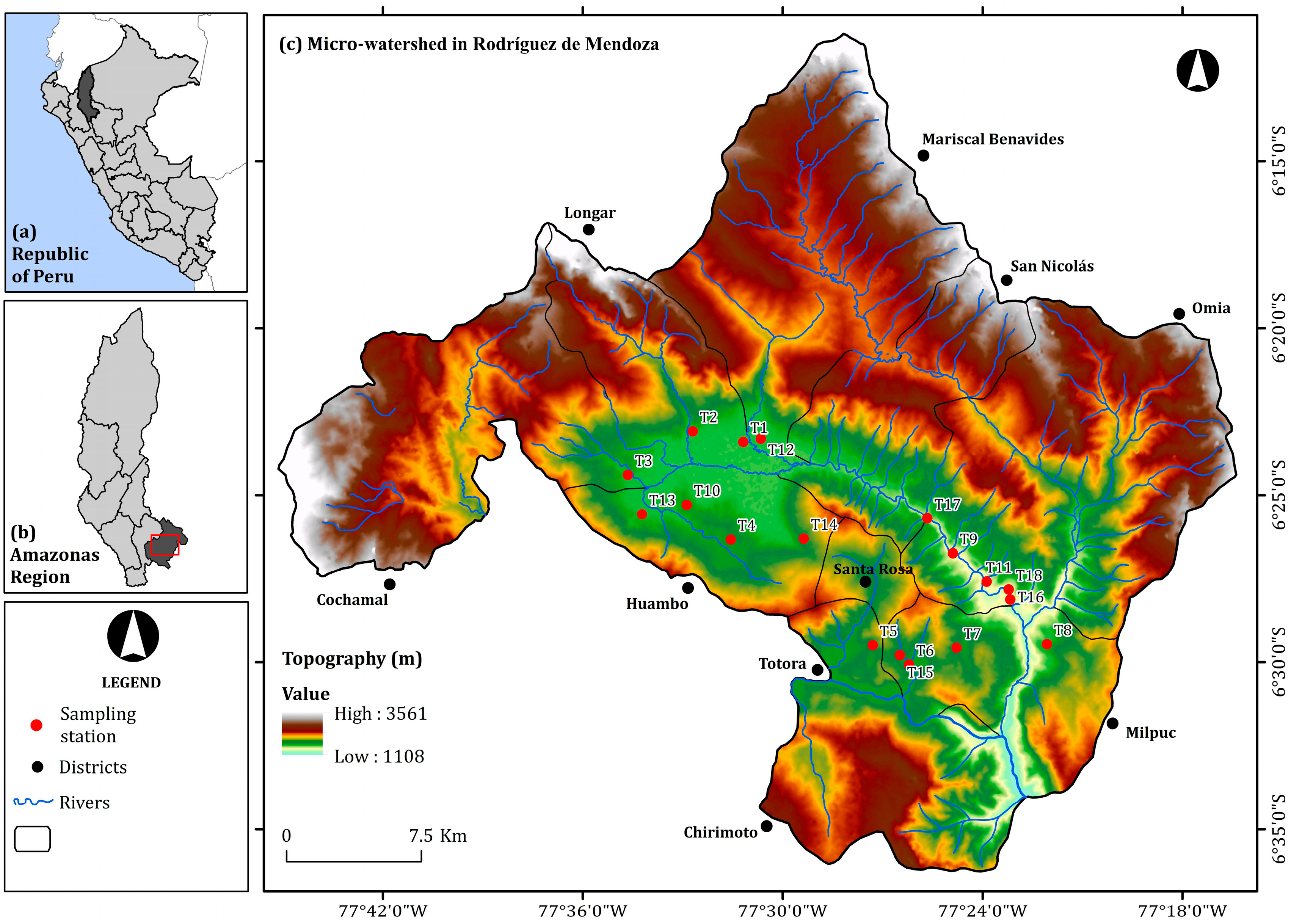





| Collection Points | Code | District (Village) | UTM Coordinates | Altitude (Masl) | |
|---|---|---|---|---|---|
| East | North | ||||
| T1 | MMR-M1 | Mariscal Benavides (Michina) | 221265 | 9292992 | 1565 |
| T2 | LLM-M2 | Longar (Longar) | 218471 | 9293560 | 1586 |
| T3 | CCR-M3 | Cochamal (Cochama) | 214885 | 9291139 | 1598 |
| T4 | MHR-M4 | Huambo (Miraflores) | 220603 | 9287599 | 1661 |
| T5 | PTR-M5 | Totora (La Perla) | 228487 | 9281802 | 1757 |
| T6 | MMR-M6 | Milpuc (Milpuc) | 230505 | 9280744 | 1662 |
| T7 | CMR-M7 | Milpuc (Chontapampa) | 233125 | 9281689 | 1689 |
| T8 | POR-M8 | Omia (Pumamarca) | 238132 | 9281904 | 1614 |
| T9 | TOR-M9 | Omia (Tuemal) | 232896 | 9286902 | 1431 |
| T10 | DHR-M10 | Huambo (Dos Cruces) | 218155 | 9289509 | 1645 |
| T11 | SRR-M11 | Omia (Limón) | 234775 | 9285337 | 1391 |
| T12 | PMR-M12 | Mariscal Benavides (Pilancon) | 222237 | 9293179 | 1585 |
| T13 | PHR-M13 | Huambo (Puquio) | 215684 | 9288975 | 1588 |
| T14 | TSR-M14 | Santa Rosa (Trancaguaico) | 224648 | 9287667 | 1729 |
| T15 | MMR-M15 | Milpuc (Milpuc) | 229996 | 9281264 | 1656 |
| T16 | TOR-M16 | Omia (Gebil) | 236088 | 9284363 | 1369 |
| T17 | MNR-M17 | San Nicolás (Mito) | 231472 | 9288826 | 1478 |
| T18 | GOR-M18 | Omia (Gebil) | 235997 | 9284919 | 1391 |
| Labeled Sample | Type of Sporulation | Colony Edge | Colony Texture | Colors | Pigmentation |
|---|---|---|---|---|---|
| MMR-M1 | Rings in the whole colony | Wavy | Cottony | Dark olive gray | Reddish yellow |
| LLM-M2 | Rings in the whole colony | Wavy | Powdery | White | White |
| CCR-M3 | Rings in the whole colony | Wavy | Powdery | Dark olive gray | Red |
| MHR-M4 | Rings in the whole colony | Feathery | Cottony | Dark olive gray | Pale yellow |
| PTR-M5 | Rings in the whole colony | Wavy | Powdery | Reddish yellow | Reddish yellow |
| MMR-M6 | Rings in the whole colony | Wavy | Cottony | Dark olive gray | Yellowish brown |
| CMR-M7 | Uniform with central rings | Feathery | Powdery | Dark olive gray | Yellow |
| POR-M8 | Uniform with terminal rings | Feathery | Cottony | Dark olive gray | White |
| TOR-M9 | Rings in the whole colony | Wavy | Powdery | Dark olive gray | Red |
| DHR-M10 | Rings in the whole colony | Wavy | Powdery | Pale yellow | Pale yellow |
| SRR-M11 | Uniform with central rings | Feathery | Cottony | Dark olive gray | Pale yellow |
| PMR-M12 | Rings in the whole colony | Feathery | Cottony | Dark olive gray | Pale yellow |
| PHR-M13 | Uniform with central rings | Feathery | Powdery | Dark olive gray | Red |
| TSR-M14 | Rings in the whole colony | Wavy | Powdery | Dark olive gray | Pale yellow |
| MMR-M15 | Rings in the whole colony | Feathery | Powdery | Dark olive gray | Pale yellow |
| TOR-M16 | Rings in the whole colony | Wavy | Powdery | Dark olive gray | Pale yellow |
| MNR-M17 | Uniform with central rings | Wavy | Powdery | Pale yellow | Pale yellow |
| GOR-M18 | Uniform with central rings | Wavy | Powdery | Dark olive gray | Yellow |
| F.V. | SC | gl | CM | F | p-Value |
|---|---|---|---|---|---|
| Model | 186,499.42 | 56 | 3330.35 | 32.54 | <0.0001 |
| Isolate | 29,210.53 | 18 | 1622.81 | 15.86 | <0.0001 |
| Time | 139,945.03 | 2 | 69,972.51 | 683.73 | <0.0001 |
| Isolate * time | 17,343.86 | 36 | 481.77 | 4.71 | <0.0001 |
| Error | 11,666.67 | 114 | 102.34 | ||
| Total | 198,166.08 | 170 |
| Isolates | Time | Mortality Percentage (Media ± SD) | Isolates | Time | Mortality Percentage (Media ± SD) |
|---|---|---|---|---|---|
| MMR-M1 | 24 h | 20.00 ± 10.00 g–j | SRR-M11 | 24 h | 3.33 ± 5.77 ij |
| MMR-M1 | 48 h | 36.67 ± 15.28 e–i | SRR-M11 | 48 h | 10.00 ± 0.00 h–j |
| MMR-M1 | 72 h | 100.00 ± 0 a | SRR-M11 | 72 h | 36.67 ± 15.28 e–i |
| LLM-M2 | 24 h | 13.33 ± 5.77 h–j | PMR-M12 | 24 h | 6.67 ± 5.77 h–j |
| LLM-M2 | 48 h | 33.33 ± 5.77 f–j | PMR-M12 | 48 h | 40.00 ± 0.00 d–h |
| LLM-M2 | 72 h | 70.00 ± 10.00 a–e | PMR-M12 | 72 h | 83.33 ± 11.55 a–c |
| CCR-M3 | 24 h | 6.67 ± 11.55 h–j | PHR-M13 | 24 h | 0.00 j |
| CCR-M3 | 48 h | 30.00 ± 10.00 f–j | PHR-M13 | 48 h | 36.67 ± 5.77 e–i |
| CCR-M3 | 72 h | 80.00 ± 10.00 a–c | PHR-M13 | 72 h | 86.67 ± 5.77 a–c |
| MHR-M4 | 24 h | 13.33 ± 5.77 h–j | TSR-M14 | 24 h | 6.67 ± 5.77 h–j |
| MHR-M4 | 48 h | 40.00 ± 0 d–h | TSR-M14 | 48 h | 16.67 ± 20.82 h–j |
| MHR-M4 | 72 h | 90.00 ± 0 ab | TSR-M14 | 72 h | 83.33 ± 11.55 a–c |
| PTR-M5 | 24 h | 16.67 ± 5.77 h–j | MMR-M15 | 24 h | 16.67 ± 5.77 h–j |
| PTR-M5 | 48 h | 70.00 ± 10.00 a–e | MMR-M15 | 48 h | 40.00 ± 0.00 d–h |
| PTR-M5 | 72 h | 100.00 ± 0.00 a | MMR-M15 | 72 h | 96.67 ± 5.77 a |
| MMR-M6 | 24 h | 3.33 ± 5.77 ij | TOR-M16 | 24 h | 6.67 ± 5.77 h–j |
| MMR-M6 | 48 h | 23.33 ± 20.82 g–j | TOR-M16 | 48 h | 13.33 ± 5.77 h–j |
| MMR-M6 | 72 h | 80.00 ± 26.46 a–c | TOR-M16 | 72 h | 100.00 ± 0.00 a |
| CMR-M7 | 24 h | 10.00 ± 10.00 h–j | MNR-M17 | 24 h | 10.00 ± 0.00 h–j |
| CMR-M7 | 48 h | 33.33 ± 5.77 f–j | MNR-M17 | 48 h | 33.33 ± 11.55 f–j |
| CMR-M7 | 72 h | 96.67 ± 5.77 a | MNR-M17 | 72 h | 73.33 ± 23.09 a–d |
| POR-M8 | 24 h | 10.00 ± 10.00 h–j | GOR-M18 | 24 h | 20.00 ± 10.00 g–j |
| POR-M8 | 48 h | 20.00 ± 10.00 g–j | GOR-M18 | 48 h | 23.33 ± 5.77 g–j |
| POR-M8 | 72 h | 53.33 ± 20.82 c–g | GOR-M18 | 72 h | 76.67 ± 5.77 a–c |
| TOR-M9 | 24 h | 10.00 ± 0.00 h–j | Control | 24 h | 0.00 j |
| TOR-M9 | 48 h | 40.00 ± 0.00 d–h | Control | 48 h | 0.00 j |
| TOR-M9 | 72 h | 100.00 ± 0.00 a | Control | 72 h | 6.67 ± 5.77 h–j |
| DHR-M10 | 24 h | 3.33 ± 5.77 ij | |||
| DHR-M10 | 48 h | 23.33 ± 5.77 g–j | |||
| DHR-M10 | 72 h | 60.00 ± 26.46 b–f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva-Cruz, M.; Altamirano-Tantalean, M.A.; Chuquizuta-Torres, R.; Oliva-Cruz, C.; Maicelo-Quintana, J.L.; Leiva-Espinoza, S.T.; Culqui, L.; Mendez-Fasabi, L.D.; Rojas Ventura, H.M.; Corazon-Guivin, M.A.; et al. Isolation and Characterization of Native Isolates of Metarhizium sp. as a Biocontrol Agent of Hypothenemus hampei in Rodríguez de Mendoza Province—Peru. Agronomy 2024, 14, 1341. https://doi.org/10.3390/agronomy14071341
Oliva-Cruz M, Altamirano-Tantalean MA, Chuquizuta-Torres R, Oliva-Cruz C, Maicelo-Quintana JL, Leiva-Espinoza ST, Culqui L, Mendez-Fasabi LD, Rojas Ventura HM, Corazon-Guivin MA, et al. Isolation and Characterization of Native Isolates of Metarhizium sp. as a Biocontrol Agent of Hypothenemus hampei in Rodríguez de Mendoza Province—Peru. Agronomy. 2024; 14(7):1341. https://doi.org/10.3390/agronomy14071341
Chicago/Turabian StyleOliva-Cruz, Manuel, Miguel A. Altamirano-Tantalean, Reyna Chuquizuta-Torres, Carlos Oliva-Cruz, Jorge L. Maicelo-Quintana, Santo T. Leiva-Espinoza, Lorenzo Culqui, Lizette Daniana Mendez-Fasabi, Heidel Marcelo Rojas Ventura, Mike Anderson Corazon-Guivin, and et al. 2024. "Isolation and Characterization of Native Isolates of Metarhizium sp. as a Biocontrol Agent of Hypothenemus hampei in Rodríguez de Mendoza Province—Peru" Agronomy 14, no. 7: 1341. https://doi.org/10.3390/agronomy14071341







