The Effects of the Co-Application of MCPA Herbicide and Urea on Grass Rhizosphere Microcosms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Soil Properties
2.3. Greenhouse Experiment
2.4. pH Determination in Rhizosphere Soils
2.5. Estimation of N-NH4+ and N-NO3−
2.6. Enzymatic Activity of Rhizosphere Soil
2.7. Residual MCPA Analysis
2.8. Statistical Analysis
3. Results
3.1. pH of Rhizosphere Soils
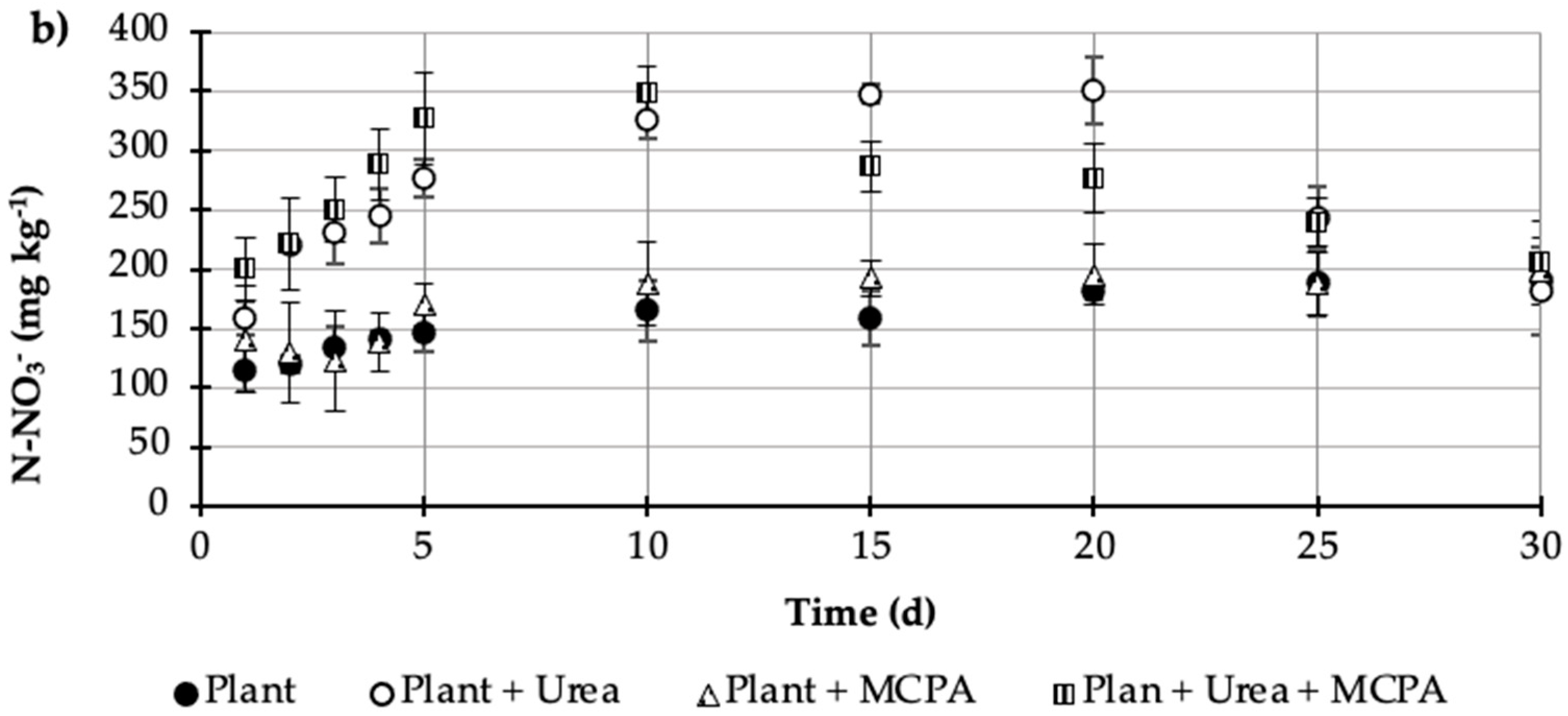
3.2. Mineralization of Urea into N-NH4+ and N-NO3− in Rhizosphere Soil Samples
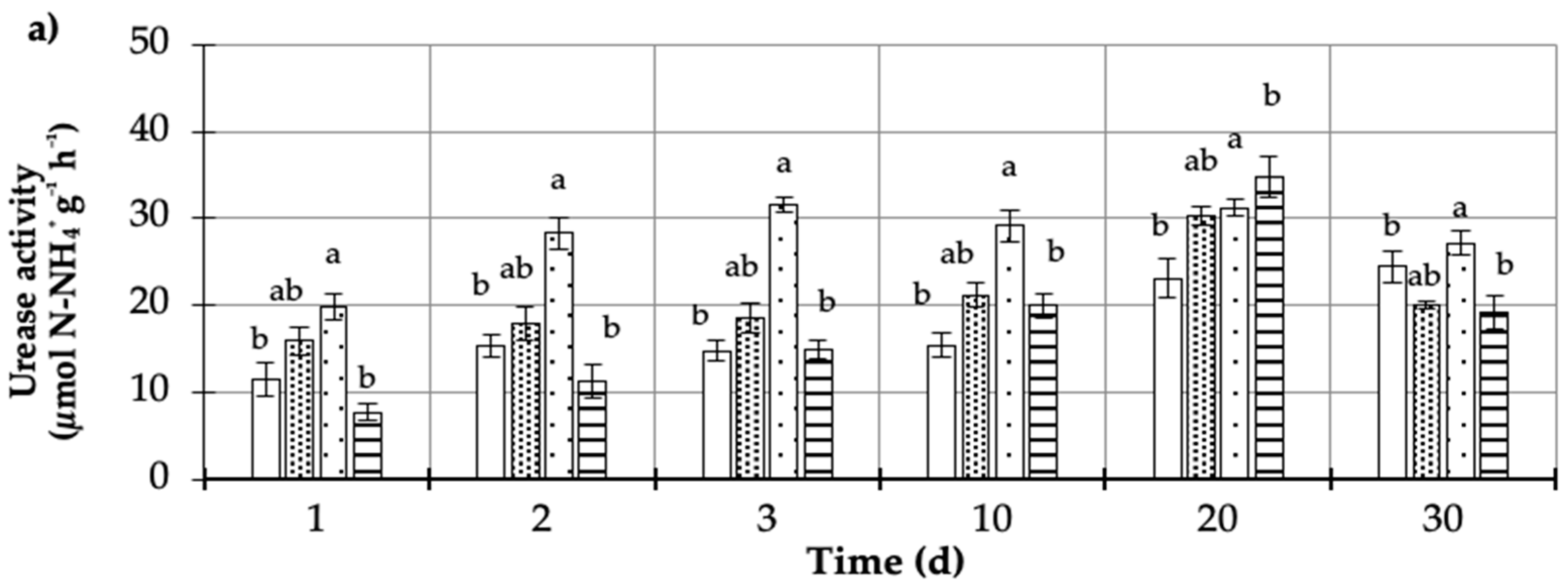
3.3. Enzymatic Activities of Rhizosphere Soil Samples
3.4. Dissipation of MCPA in Rhizosphere Soil Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zúñiga, F.; Ivelic-sáez, J.; López, I.; Huygens, D.; Dörner, F.J. Temporal Dynamics of the Physical Quality of an Andisol under a Grazing System Subjected to Different Pasture Improvement Strategies. Soil Tillage Res. 2015, 145, 233–241. [Google Scholar] [CrossRef]
- Ordóñez, I.; López, I.F.; Kemp, P.D.; Descalzi, C.A.; Horn, R. Effect of Pasture Improvement Managements on Physical Properties and Water Content Dynamics of a Volcanic Ash Soil in Southern Chile. Soil Tillage Res. 2018, 178, 55–64. [Google Scholar] [CrossRef]
- Devi, P.I.; Manjula, M.; Bhavani, R.V. Agrochemicals, Environment, and Human Health. Annu. Rev. Environ. Resour. 2022, 47, 399–421. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Chapter 16—Properties and Uses of Herbicides. In Fundamentals of Weed Science, 5th ed.; Zimdahl, R.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 463–499. ISBN 978-0-12-811143-7. [Google Scholar]
- Palma, G.; Jorquera, M.; Demanet, R.; Elgueta, S.; Briceño, G.; de la Luz Mora, M. Urea Fertilizer and PH Influence on Sorption Process of Flumetsulam and MCPA Acidic Herbicides in a Volcanic Soil. J. Environ. Qual. 2016, 45, 323–330. [Google Scholar] [CrossRef]
- Ćwieląg-Piasecka, I.; Debicka, M.; Fleszar, A. Influence of SOM Composition, Clay Minerals, and PH on 2,4-D and MCPA Retention in Peri-Urban Soils. Sustainability 2023, 15, 12525. [Google Scholar] [CrossRef]
- Palma, G.; Spuler, M.J.; Jorquera, M.; Briceño, G. Effects of the Combined Application of Nitrogen Fertilizer and 2,4-D on Nitrification Ammonia Oxidizers and Herbicide Bioavailability in a Volcanic Soil. A Microcosm Study. J. Soil Sci. Plant Nutr. 2023, 23, 4309–4317. [Google Scholar] [CrossRef]
- Fontes, J.C.; Cameira, M.R.; Borba, L.G.; Amado, E.D.; Pereira, L.S. Nitrogen Dynamics in Volcanic Soils under Permanent Pasture. Geoderma 2011, 160, 384–393. [Google Scholar] [CrossRef]
- Swify, S.; Mažeika, R.; Baltrusaitis, J.; Drapanauskait, D. Review: Modified Urea Fertilizers and Their Effects on Improving Nitrogen Use Efficiency (NUE). Sustainability 2024, 16, 188. [Google Scholar] [CrossRef]
- Klimczyk, M.; Siczek, A.; Schimmelpfennig, L. Improving the Efficiency of Urea-Based Fertilization Leading to Reduction in Ammonia Emission. Sci. Total Environ. 2021, 771, 145483. [Google Scholar] [CrossRef]
- Singh, J.; Kunhikrishnan, A.; Bolan, N.S.; Saggar, S. Impact of Urease Inhibitor on Ammonia and Nitrous Oxide Emissions from Temperate Pasture Soil Cores Receiving Urea Fertilizer and Cattle Urine. Sci. Total Environ. 2013, 465, 56–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Yao, H. Urea-based nitrogen fertilization in agriculture: A key source of N2O emissions and recent development in mitigating strategies. Arch. Agron. Soil Sci. 2023, 69, 663–678. [Google Scholar] [CrossRef]
- Barreras-Urbina, C.G.; Rodr, F.; Luis, C.; Plascencia-jatomea, M.; Manuel, P.; Ledesma-osuna, A.I.; Denisse, D. Effect of a Prolonged-Release System of Urea on Nitrogen Losses and Microbial Population Changes in Two Types of Agricultural Soil. ACS Omega 2023, 8, 42319–42328. [Google Scholar] [CrossRef] [PubMed]
- Czarny, J.; Piotrowska-Cyplik, A.; Lewicki, A.; Zgoła-Grześkowiak, A.; Wolko, Ł.; Galant, N.; Syguda, A.; Cyplik, P. The Toxic Effect of Herbicidal Ionic Liquids on Biogas-Producing Microbial Community. Int. J. Environ. Res. Public Health 2019, 16, 916. [Google Scholar] [CrossRef]
- Schellenberger, S.; Drake, H.L.; Kolb, S. Impairment of Cellulose- and Cellobiose-Degrading Soil Bacteria by Two Acidic Herbicides. FEMS Microbiol. Lett. 2012, 327, 60–65. [Google Scholar] [CrossRef]
- Kour, D.; Kour, H.; Khan, S.S.; Khan, R.T.; Bhardwaj, M.; Kailoo, S.; Kumari, C.; Rasool, S.; Yadav, A.N.; Sharma, Y.P. Biodiversity and Functional Attributes of Rhizospheric Microbiomes: Potential Tools for Sustainable Agriculture. Curr. Microbiol. 2023, 80, 192. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of Continuous Nitrogen Fertilizer Application on the Diversity and Composition of Rhizosphere Soil Bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.; Perruchon, C.; Karas, P.A.; Karavasilis, D.; Diez, M.C.; Karpouzas, D.G. Bioaugmentation and Rhizosphere-Assisted Biodegradation as Strategies for Optimization of the Dissipation Capacity of Biobeds. J. Environ. Manag. 2017, 187, 103–110. [Google Scholar] [CrossRef]
- Riah, W.; Laval, K.; Laroche-Ajzenberg, E.; Mougin, C.; Latour, X.; Trinsoutrot-Gattin, I. Effects of Pesticides on Soil Enzymes: A Review. Environ. Chem. Lett. 2014, 12, 257–273. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nahar, K.; Ali, M.; Sultana, N.; Minnatul, M. Effect of Long—Term Pesticides and Chemical Fertilizers Application on the Microbial Community Specifically Anammox and Denitrifying Bacteria in Rice Field Soil of Jhenaidah and Kushtia District, Bangladesh. Bull. Environ. Contam. Toxicol. 2020, 104, 828–833. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbò, P.; Rainio, M.; Saikkonen, K.; Helander, M. Ecosystem Consequences of Herbicides: The Role of Microbiome. Trends Ecol. Evol. 2023, 38, 35–43. [Google Scholar] [CrossRef]
- Liu, H. Co-Existence of Pesticides and Fertilizers in Agricultural Soil Environment. Agric. Res. Technol. 2022, 27, 2–5. [Google Scholar] [CrossRef]
- Zhang, P.W.; Wang, S.Y.; Huang, C.L.; Fu, J.T.; Huang, R.L.; Li, Z.H. Dissipation and Residue of Clothianidin in Granules and Pesticide Fertilizers Used in Cabbage and Soil under Field Conditions. Environ. Sci. Pollut. Res. 2018, 25, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Raza, A.; Rafique, M.; Ullah, S.; Mehmood, A.; Muhammad, D.; Hussain, J. Investigating the Degradation Behavior of Cypermethrin (CYP) and Chlorpyrifos (CPP) in Peach Orchard Soils Using Organic/Inorganic Amendments. Saudi J. Biol. Sci. 2021, 28, 5890–5896. [Google Scholar] [CrossRef]
- Sadzawka, A.; Carrasco, M.A.; Grez, R.; Mora, M.L.M. Métodos de Análisis Recomendados Para Los Suelos Chilenos; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2004. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Keeney, D.; Nelson, D. Nitrogen-Inorganic Forms. In Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; Page, A., Miller, R., Keeney, D., Eds.; American Society of Agronomy Madison: Washington, DC, USA, 1982; pp. 643–698. ISBN 9780849335860. [Google Scholar]
- Doane, T.A.; Horwáth, W.R. Spectrophotometric Determination of Nitrate with a Single Reagent Spectrophotometric Determination of Nitrate. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry/Edited by Kassem Alef and Paolo Nannipieri; Academic Press: London, UK, 1995; ISBN 0125138407. [Google Scholar]
- Garcia, C.; Hernandez, T.; Costa, F. Potential Use of Dehydrogenase Activity as an Index of Microbial Activity in Degraded Soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Gao, R.; Duan, Y.; Zhang, J.; Ren, Y. Effects of Long-Term Application of Organic Manure and Chemical Fertilizer on Soil Properties and Microbial Communities in the Agro-Pastoral Ecotone of North. Front. Environ. Sci. 2022, 10, 993973. [Google Scholar] [CrossRef]
- Chen, J.; Zhuang, X.; Xie, H.; Bai, Z.; Qi, H.; Zhang, H. Associated Impact of Inorganic Fertilizers and Pesticides on Microbial Communities in Soils. World J. Mcrobiol. Biotechnol. 2007, 23, 23–29. [Google Scholar] [CrossRef]
- Tong, D.; Xu, R. Effects of Urea and (NH4)2SO4 on Nitrification and Acidification of Ultisols from Southern China. J. Environ. Sci. 2012, 24, 682–689. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.A.; Demanet, R. Urease Activity and Nitrogen Mineralization Kinetics as Affected by Temperature and Urea. J. Soil Sci. Plant Nutr. 2009, 9, 69–82. [Google Scholar]
- Rose, M.T.; Ling, E.; Han, Z.; Wood, R.; Rose, T.J.; Zwieten, L. Van Minor Effects of Herbicides on Microbial Activity in Agricultural Soils Are Detected by N-Transformation but Not Enzyme Activity Assays. Eur. J. Soil Biol. 2018, 87, 72–79. [Google Scholar] [CrossRef]
- Martens, J.M.; Bremner, D.A. Effects of Preemergence and Postemergence Herbicides on Urea Hydrolysis and Nitrification of Urea Nitrogen in Soil. Biol. Fertil. Soils 1994, 17, 309–313. [Google Scholar] [CrossRef]
- Yuan, T.; Ren, W.; Wang, Z.; Fry, E.L.; Tang, S.; Yin, J.; Zhang, J.; Jia, Z. How Does the Pattern of Root Metabolites Regulating Bene Fi Cial Microorganisms Change with Different Grazing Pressures? Front. Plant Sci. 2023, 14, 1180576. [Google Scholar] [CrossRef]
- Crouzet, O.; Poly, F.; Bonnemoy, F.; Bru, D.; Batisson, I.; Bohatier, J.; Philippot, L.; Mallet, C. Functional and Structural Responses of Soil N-Cycling Microbial Communities to the Herbicide Mesotrione: A Dose-Effect Microcosm Approach. Environ. Sci. Pollut. Res. Int. 2016, 23, 4207–4217. [Google Scholar] [CrossRef] [PubMed]
- Abdo, A.I.; Xu, Y.; Shi, D.; Li, J.; Li, H.; El-sappah, A.H.; Elrys, A.S.; Almwarai, S.; Zhou, C.; Wang, L.; et al. Nitrogen Transformation Genes and Ammonia Emission from Soil under Biochar and Urease Inhibitor Application. Soil Tillage Res. 2022, 223, 105491. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Hu, C.; Liu, B. Long-Term Urea Fertilization Alters the Composition and Increases the Abundance of Soil Ureolytic Bacterial Communities in an Upland Soil. FEMS Microbiol. Ecol. 2019, 95, fiz044. [Google Scholar] [CrossRef]
- Tejada, M.; García-martínez, A.M.; Gómez, I.; Parrado, J. Application of MCPA Herbicide on Soils Amended with Biostimulants: Short-Time Effects on Soil Biological Properties. Chemosphere 2010, 80, 1088–1094. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase Activity in the Soil Environment. In Dehydrogenases; Canuto, R.A., Ed.; InTechOpen: London, UK, 2012; pp. 183–209. ISBN 978-953-307-019-3. [Google Scholar]
- Järvan, M.; Edesi, L.; Adamson, A.; Võsa, T. Soil Microbial Communities and Dehydrogenase Activity Depending on Farming Systems. Plant Soil Environ. 2014, 60, 459–463. [Google Scholar] [CrossRef]
- Łozowicka, B.; Wołejko, E.; Kaczyński, P.; Konecki, R.; Iwaniuk, P.; Drągowski, W.; Łozowicki, J.; Tujtebajeva, G.; Wydro, U.; Jablońska-Trypuć, A. Effect of Microorganism on Behaviour of Two Commonly Used Herbicides in Wheat/Soil System. Appl. Soil Ecol. 2021, 162, 103879. [Google Scholar] [CrossRef]
- Tejada, M.; Toro, M.; Paneque, P.; Isidoro, G.; Parrado, J. Response of Biochemical Properties in Agricultural Soils Polluted with 4-Chloro-2-Methylphenoxyacetic Acid (MCPA) under Severe Drought Conditions. Agronomy 2023, 13, 478. [Google Scholar] [CrossRef]
- Mierzejewska, E.; Urbaniak, M.; Zagibajło, K.; Vangronsveld, J.; Thijs, S. The Effect of Syringic Acid and Phenoxy Herbicide 4-Chloro-2-Methylphenoxyacetic Acid (MCPA) on Soil, Rhizosphere, and Plant Endosphere Microbiome. Front. Plant Sci. 2022, 13, 882228. [Google Scholar] [CrossRef] [PubMed]
- Paszko, T. Modeling of PH-Dependent Adsorption and Leaching of MCPA in Profiles of Polish Mineral Soils. Sci. Total Environ. 2014, 494–495, 229–240. [Google Scholar] [CrossRef] [PubMed]
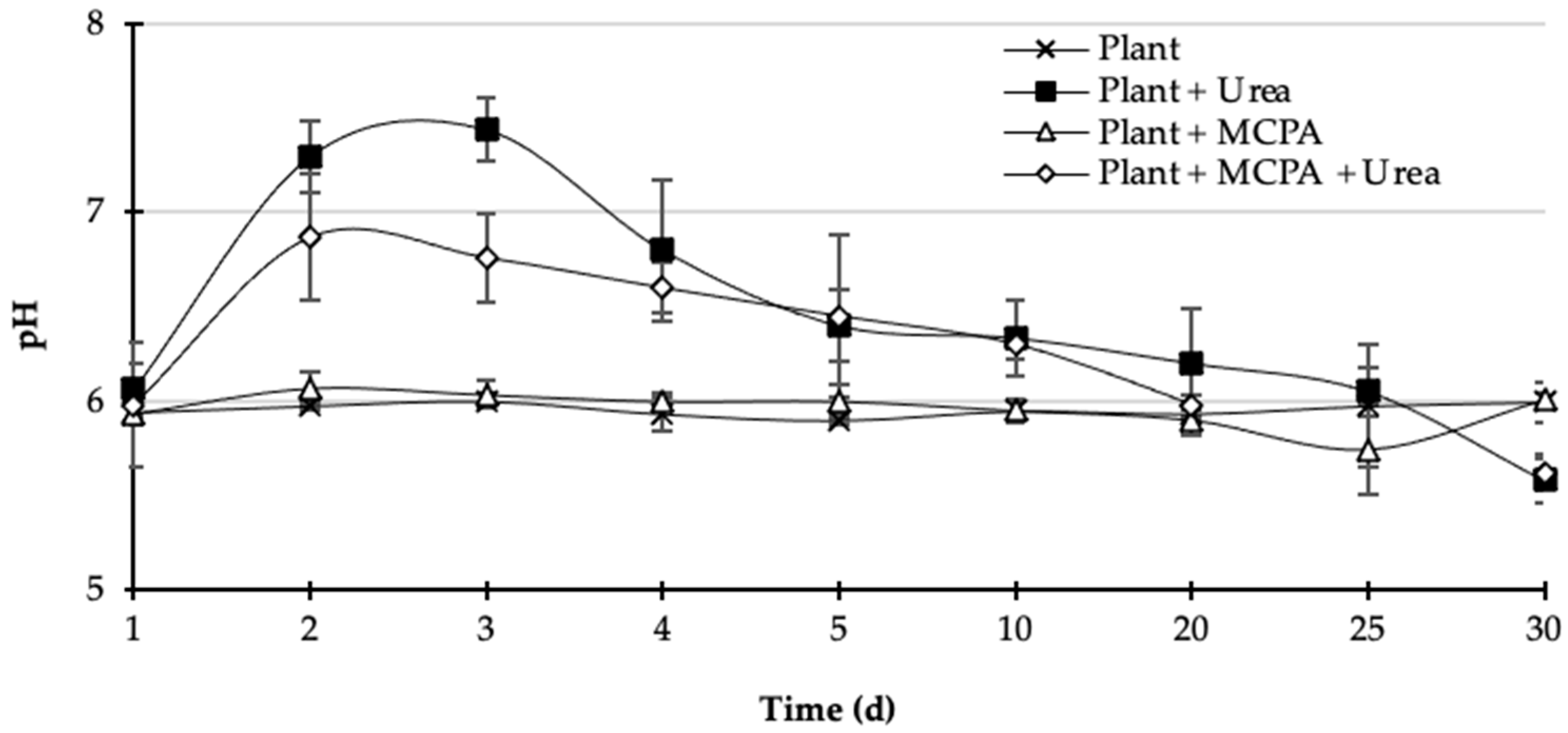
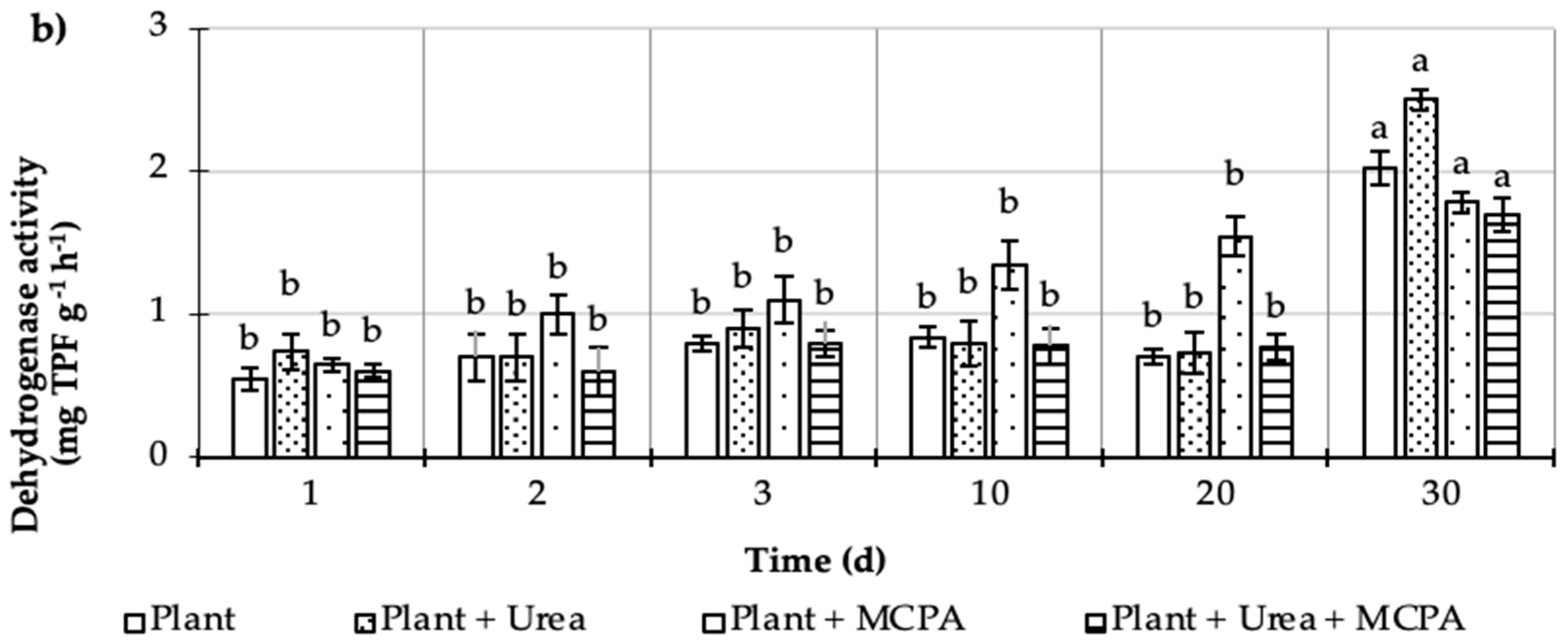
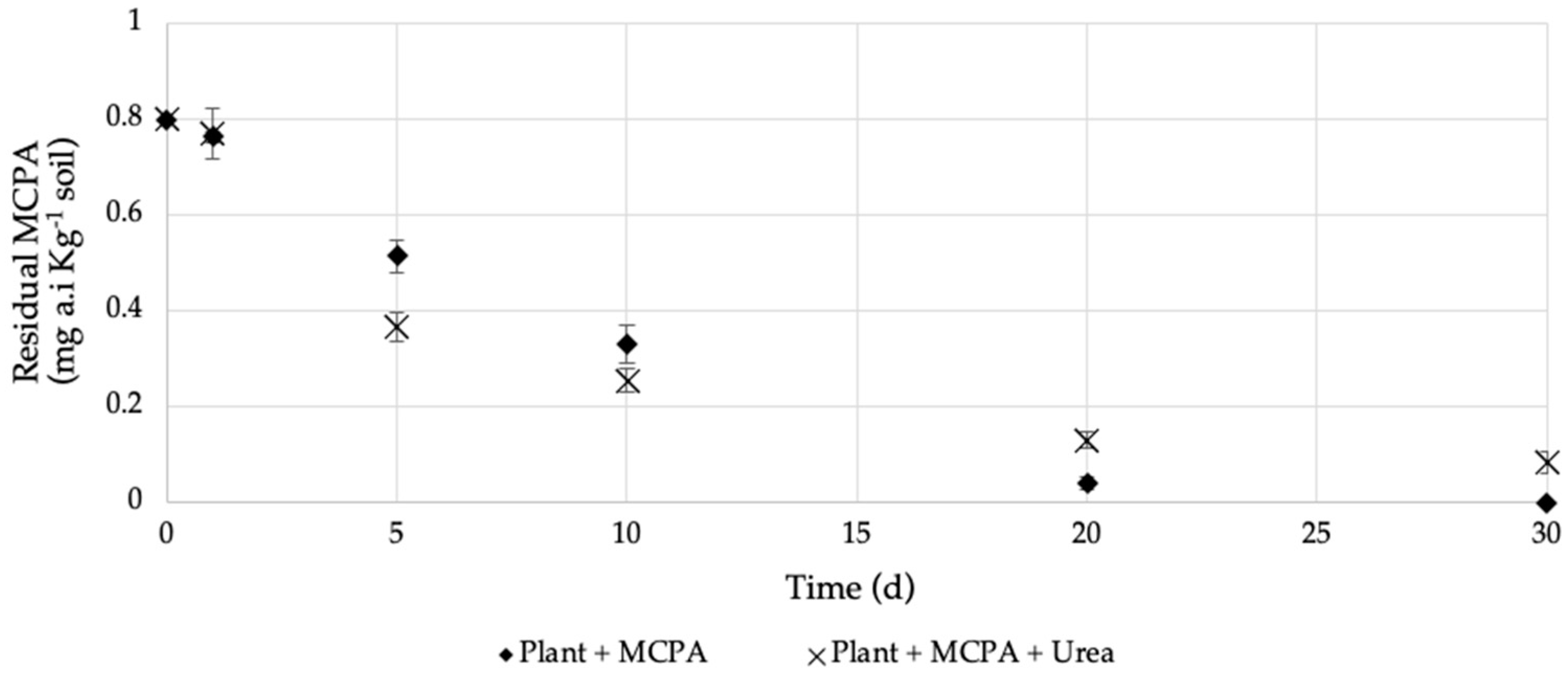
 = Plant + MCPA + Urea). Values represent the mean of three replicates (n = 3) ± standard deviation.
= Plant + MCPA + Urea). Values represent the mean of three replicates (n = 3) ± standard deviation.
 = Plant + MCPA + Urea). Values represent the mean of three replicates (n = 3) ± standard deviation.
= Plant + MCPA + Urea). Values represent the mean of three replicates (n = 3) ± standard deviation.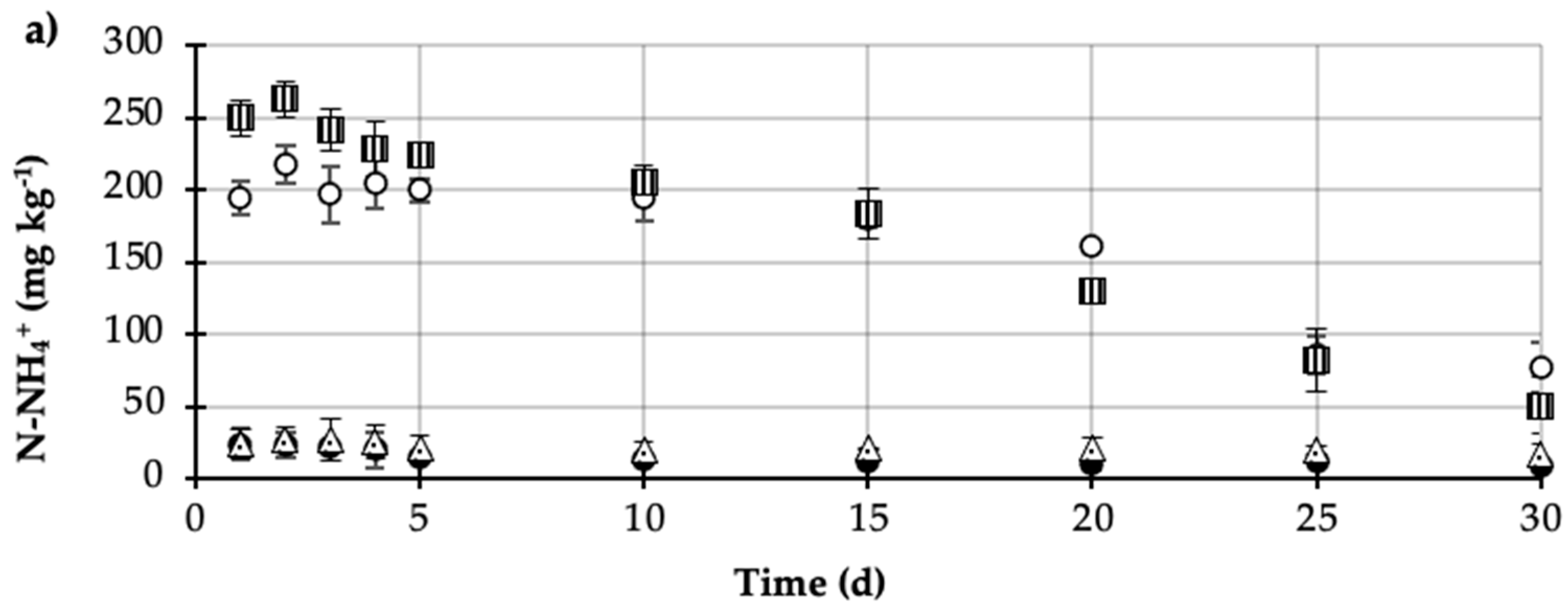
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, M.A.; Palma, G.; Faundez, C.; Elgueta, S. The Effects of the Co-Application of MCPA Herbicide and Urea on Grass Rhizosphere Microcosms. Agronomy 2024, 14, 1366. https://doi.org/10.3390/agronomy14071366
Campos MA, Palma G, Faundez C, Elgueta S. The Effects of the Co-Application of MCPA Herbicide and Urea on Grass Rhizosphere Microcosms. Agronomy. 2024; 14(7):1366. https://doi.org/10.3390/agronomy14071366
Chicago/Turabian StyleCampos, Marco A., Graciela Palma, Carlos Faundez, and Sebastian Elgueta. 2024. "The Effects of the Co-Application of MCPA Herbicide and Urea on Grass Rhizosphere Microcosms" Agronomy 14, no. 7: 1366. https://doi.org/10.3390/agronomy14071366





