The Sugarcane ScPetC Gene Improves Water-Deficit and Oxidative Stress Tolerance in Transgenic Tobacco Plants
Abstract
:1. Introduction
2. Materials and Methods
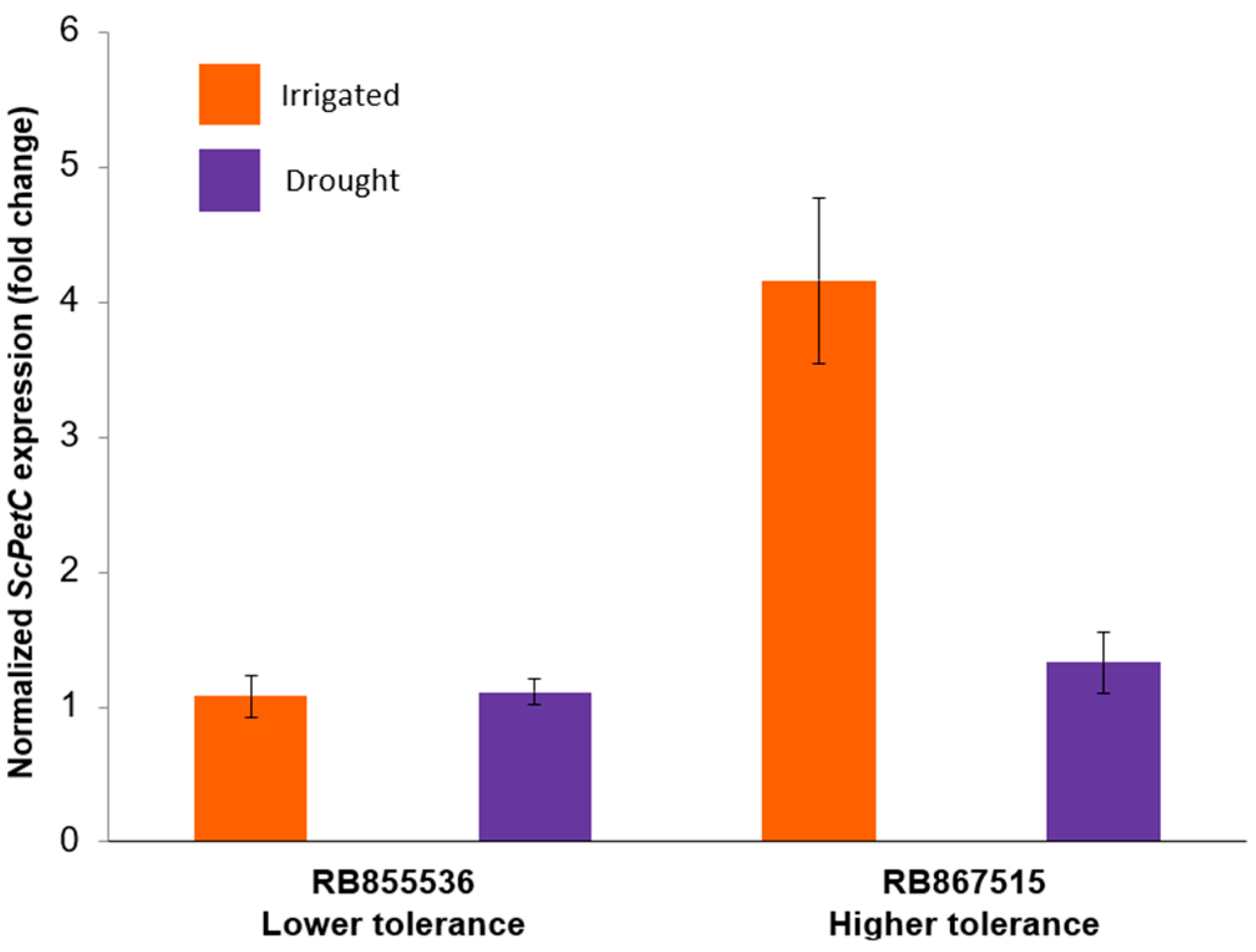
2.1. Gene Expression in Field-Grown Sugarcane Plants
2.2. ScPetC Protein Sequence Analysis
2.3. Construction of the ScPetC Overexpression Vector
2.4. Plant Material and Growth Conditions
2.5. Transformation of Tobacco Plants
2.6. Measurement of Gas Exchange, Chlorophyll Fluorescence, and P700 Parameters
2.7. Quantification of Chlorophyll Degradation under Oxidative Stress and Water Deficit
2.8. Statistical Analysis
3. Results
3.1. The Sugarcane ScPetC Gene
3.2. The Development of ScPetC-Overexpressing Plants Is Less Affected by Water Deficit
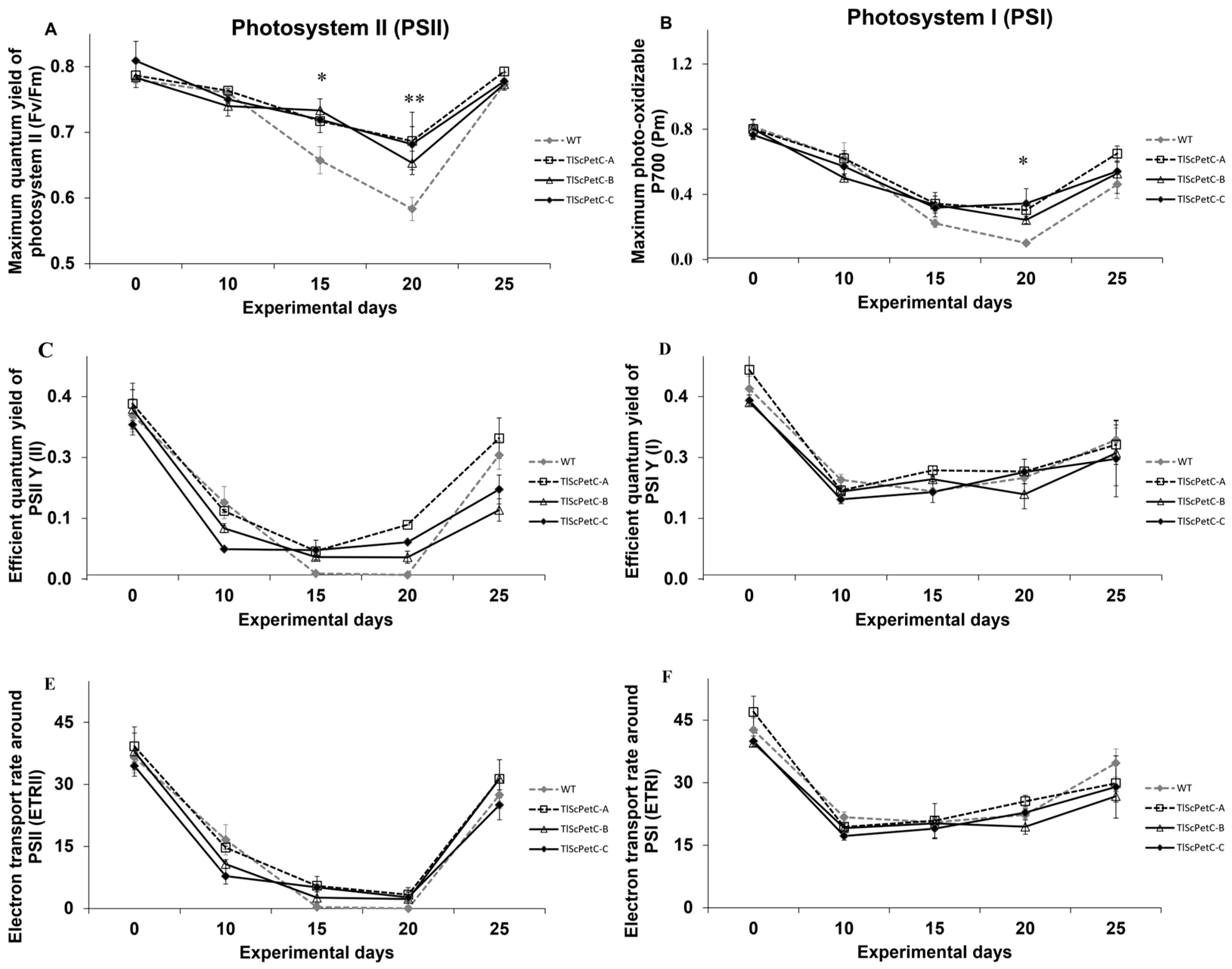
3.3. ScPetC Protects PSII and PSI Activities in Response to Water Deficit
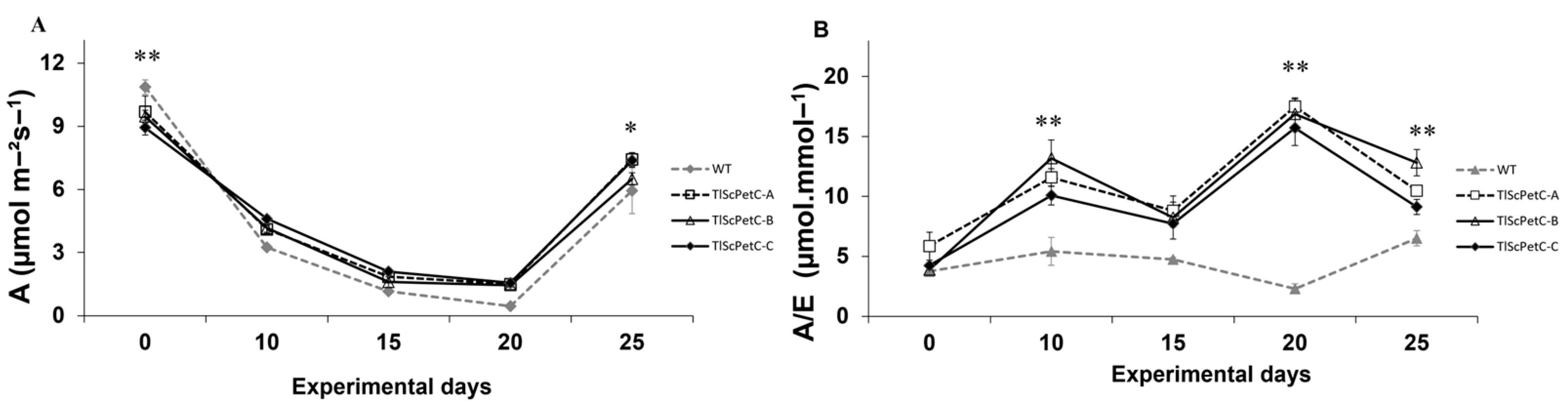
3.4. Effect of Water Deficit on Photosynthesis and Water Use Efficiency
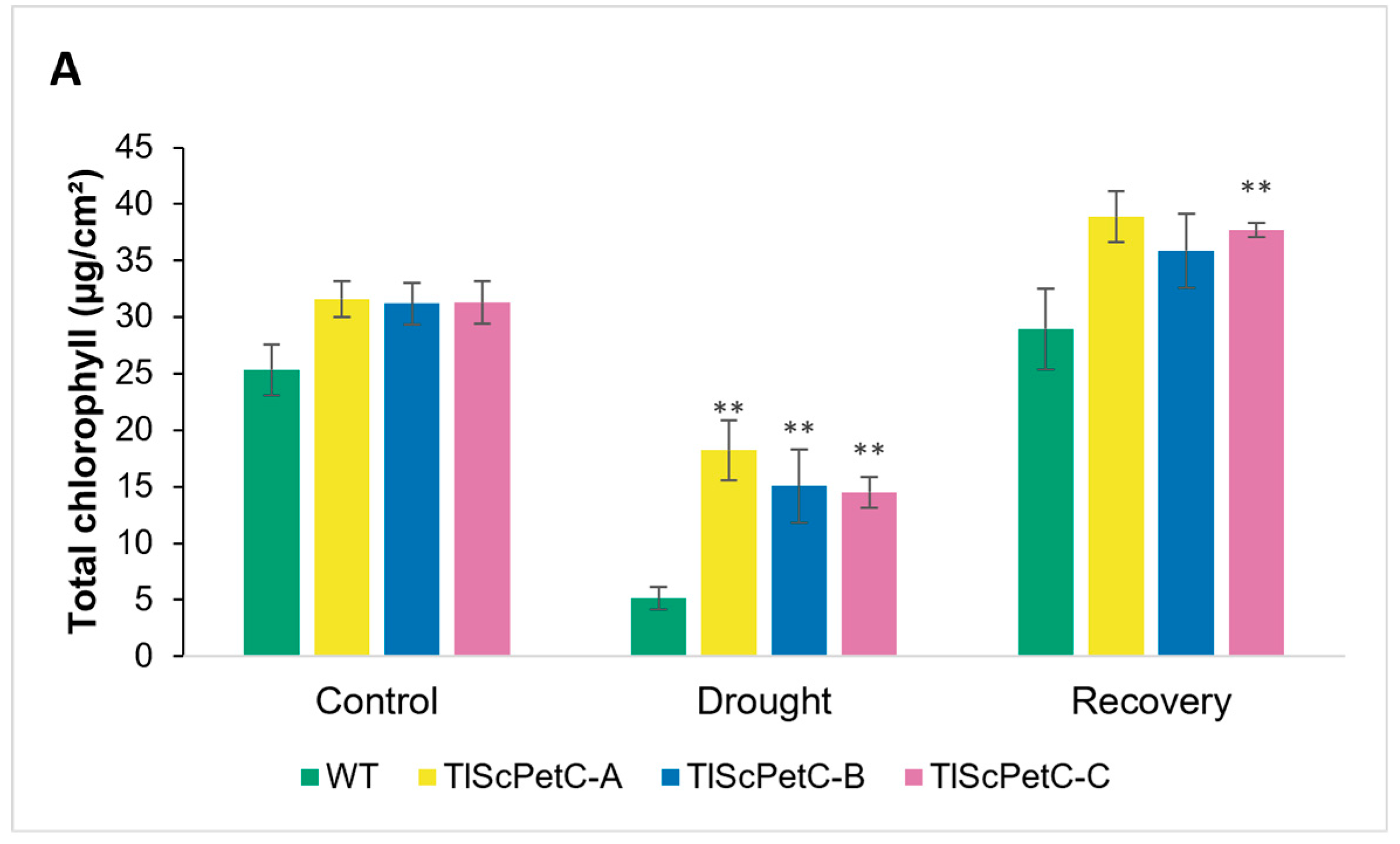
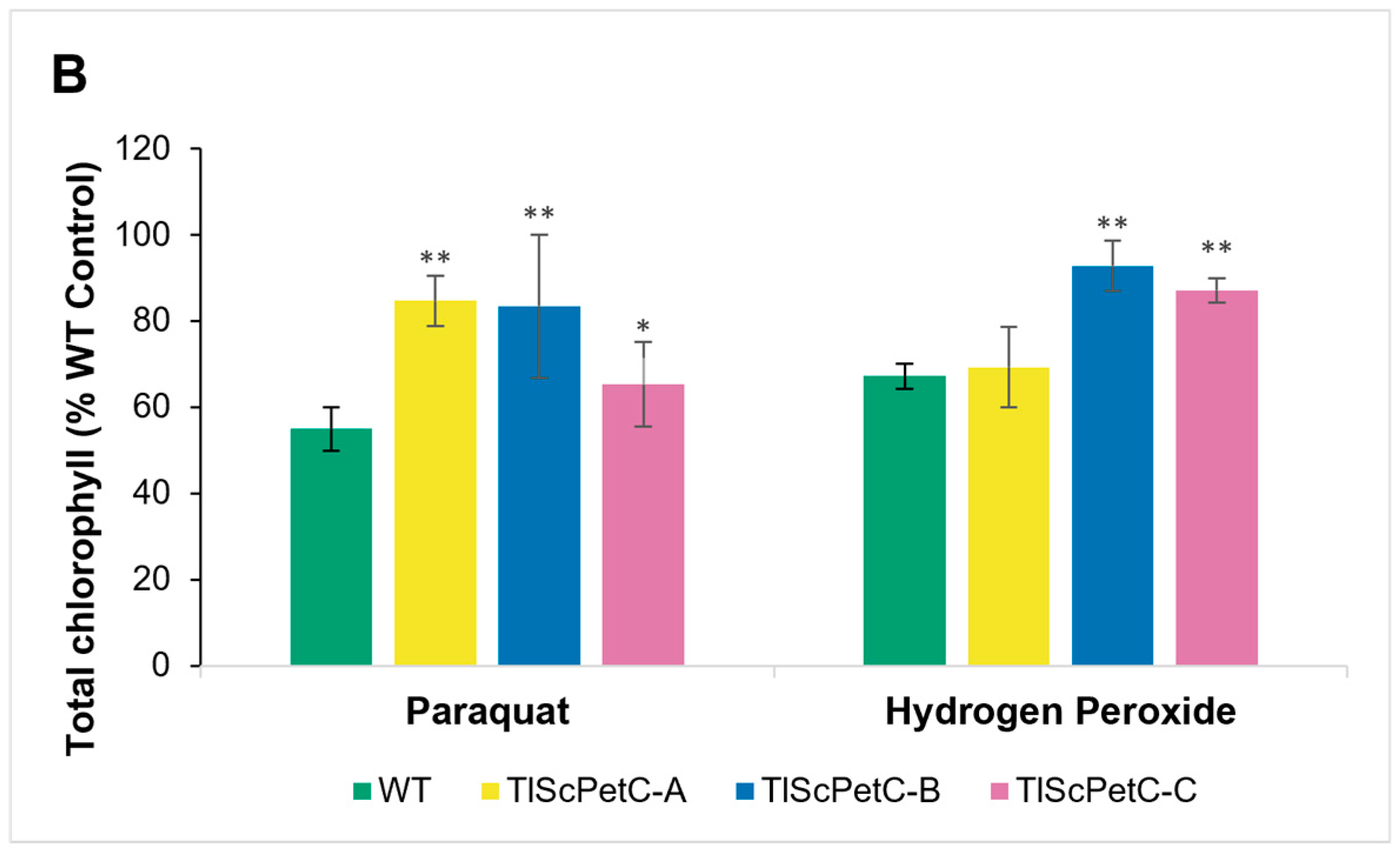
3.5. ScPetC Overexpression Protects Chlorophyll and Reduces Oxidative Stress Damage
4. Discussion
4.1. Effect of ScPetC on Plant Development under Water Deficit
4.2. Photosystems (PSI and PSII)
4.3. Gas Exchange
4.4. Water and Oxidative Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bull, T.; Glasziou, K. The Evolutionary Significance of Sugar Accumulation in Saccharum. Aust. J. Biol. Sci. 1963, 16, 737. [Google Scholar] [CrossRef]
- Rudorff, B.F.T.; Aguiar, D.A.; Silva, W.F.; Sugawara, L.M.; Adami, M.; Moreira, M.A. Studies on the Rapid Expansion of Sugarcane for Ethanol Production in São Paulo State (Brazil) Using Landsat Data. Remote Sens. 2010, 2, 1057–1076. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic Strategies for Improving Crop Yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araújo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Gonçalves, V.R.; Lakshmanan, P.; Menossi, M. Sugarcane Water Stress Tolerance Mechanisms and Its Implications on Developing Biotechnology Solutions. Front. Plant Sci. 2017, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Gentile, A.; Dias, L.I.; Mattos, R.S.; Ferreira, T.H.; Menossi, M. MicroRNAs and Drought Responses in Sugarcane. Front. Plant Sci. 2015, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Basnayake, J.; Jackson, P.A.; Inman-Bamber, N.G.; Lakshmanan, P. Sugarcane for Water-Limited Environments. Genetic Variation in Cane Yield and Sugar Content in Response to Water Stress. J. Exp. Bot. 2012, 63, 6023–6033. [Google Scholar] [CrossRef]
- Gentile, A.; Ferreira, T.H.; Mattos, R.S.; Dias, L.I.; Hoshino, A.A.; Carneiro, M.S.; Souza, G.M.; Calsa, T.; Nogueira, R.M.; Endres, L.; et al. Effects of Drought on the Microtranscriptome of Field-Grown Sugarcane Plants. Planta 2013, 237, 783–798. [Google Scholar] [CrossRef]
- Dos Santos, C.M.; De Almeida Silva, M. Physiological and Biochemical Responses of Sugarcane to Oxidative Stress Induced by Water Deficit and Paraquat. Acta Physiol. Plant 2015, 37, 172. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of Decreased Photosynthetic Rate and Metabolic Capacity in Water-Deficient Leaf Cells: A Critical Evaluation of Mechanisms and Integration of Processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Huang, W.; Fu, P.-L.; Jiang, Y.-J.; Zhang, J.-L.; Zhang, S.-B.; Hu, H.; Cao, K.-F. Differences in the Responses of Photosystem I and Photosystem II of Three Tree Species Cleistanthus Sumatranus, Celtis Philippensis and Pistacia Weinmannifolia Exposed to a Prolonged Drought in a Tropical Limestone Forest. Tree Physiol. 2013, 33, 211–220. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, R.S.; Machado, E.C.; Machado, D.F.S.P.; Magalhães Filho, J.R.; Landell, M.G.A. Revealing drought-resistance and productive patterns in sugarcane genotypes by evaluating both physiological responses and stalk yield. Exp. Agric. 2013, 49, 212–224. [Google Scholar] [CrossRef]
- Yamori, W.; Makino, A.; Shikanai, T. A Physiological Role of Cyclic Electron Transport around Photosystem I in Sustaining Photosynthesis under Fluctuating Light in Rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.-J.; Hu, H.; Zhang, S.-B. Different Roles of Cyclic Electron Flow around Photosystem I under Sub-Saturating and Saturating Light Intensities in Tobacco Leaves. Front. Plant Sci. 2015, 6, 923. [Google Scholar] [CrossRef] [PubMed]
- Cruz De Carvalho, M.H. Drought Stress and Reactive Oxygen Species: Production, Scavenging and Signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in Plants: A New Light on Photosystem II Damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Greer, K.L.; Golden, S.S. Conserved Relationship between psbH and petBD Genes: Presence of a Shared Upstream Element in Prochlorothrix hollandica. Plant Mol. Biol. 1992, 19, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Krieger-Liszkay, A. Regulation of Photosynthetic Electron Transport and Photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Takahashi, S.; Makino, A.; Price, G.D.; Badger, M.R.; Von Caemmerer, S. The Roles of ATP Synthase and the Cytochrome b 6/f Complexes in Limiting Chloroplast Electron Transport and Determining Photosynthetic Capacity. Plant Physiol. 2011, 155, 956–962. [Google Scholar] [CrossRef]
- Schöttler, M.A.; Tóth, S.Z.; Boulouis, A.; Kahlau, S. Photosynthetic Complex Stoichiometry Dynamics in Higher Plants: Biogenesis, Function, and Turnover of ATP Synthase and the Cytochrome B6f Complex. J. Exp. Bot. 2015, 66, 2373–2400. [Google Scholar] [CrossRef]
- Simkin, A.J.; McAusland, L.; Lawson, T.; Raines, C.A. Overexpression of the RieskeFeS Protein Increases Electron Transport Rates and Biomass Yield. Plant Physiol. 2017, 175, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, M.; Lopez-Calcagno, P.E.; Raines, C.A.; Furbank, R.T.; Von Caemmerer, S. Overexpression of the Rieske FeS Protein of the Cytochrome B6f Complex Increases C4 Photosynthesis in Setaria viridis. Commun. Biol. 2019, 2, 314. [Google Scholar] [CrossRef] [PubMed]
- Sanda, S.; Yoshida, K.; Kuwano, M.; Kawamura, T.; Munekage, Y.N.; Akashi, K.; Yokota, A. Responses of the Photosynthetic Electron Transport System to Excess Light Energy Caused by Water Deficit in Wild Watermelon. Physiol. Plant. 2011, 142, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Ostrowska, A.; Gadzinowska, J.; Grzesiak, M.T.; Dziurka, K.; Dubas, E. Rieske Iron-Sulfur Protein of Cytochrome-B6f Is Involved in Plant Recovery after Drought Stress. Environ. Exp. Bot. 2018, 156, 228–239. [Google Scholar] [CrossRef]
- Rocha, F.R.; Papini-Terzi, F.S.; Nishiyama, M.Y.; Vêncio, R.Z.; Vicentini, R.; Duarte, R.D.; De Rosa, V.E.; Vinagre, F.; Barsalobres, C.; Medeiros, A.H.; et al. Signal Transduction-Related Responses to Phytohormones and Environmental Challenges in Sugarcane. BMC Genom. 2007, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Diniz, A.L.; Da Silva, D.I.R.; Lembke, C.G.; Costa, M.D.-B.L.; ten-Caten, F.; Li, F.; Vilela, R.D.; Menossi, M.; Ware, D.; Endres, L.; et al. Amino Acid and Carbohydrate Metabolism Are Coordinated to Maintain Energetic Balance during Drought in Sugarcane. Int. J. Mol. Sci. 2020, 21, 9124. [Google Scholar] [CrossRef] [PubMed]
- Endres, L.; Dos Santos, C.M.; Silva, J.V.; Barbosa, G.V.D.S.; Silva, A.L.J.; Froehlich, A.; Teixeira, M.M. Inter-relationship between Photosynthetic Efficiency, Δ 13 C, Antioxidant Activity and Sugarcane Yield under Drought Stress in Field Conditions. J. Agron. Crop Sci. 2019, 205, 433–446. [Google Scholar] [CrossRef]
- Papini-Terzi, F.S. Transcription Profiling of Signal Transduction-Related Genes in Sugarcane Tissues. DNA Res. 2005, 12, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Tournayre, J.; Reichstadt, M.; Parry, L.; Fafournoux, P.; Jousse, C. “Do My qPCR Calculation”, a Web Tool. Bioinformation 2019, 15, 369–372. [Google Scholar] [CrossRef]
- Nishiyama, M.Y., Jr.; Vicente, F.F.R.; Lembke, C.G.; Sato, P.M.; Dal-Bianco, M.L.; Fandiño, R.A.; Hotta, C.T.; Souza, G.M. The SUCEST-FUN Regulatory Network Database: Designing an Energy Grass. Proc. Int. Sugar Cane Technol. 2010, 27, 1–8. [Google Scholar]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and Sequence Analysis Tools Services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, J.; Gao, Q.; Xing, S.; Li, F.; Wang, W. Drought Tolerance through Overexpression of Monoubiquitin in Transgenic Tobacco. J. Plant Physiol. 2008, 165, 1745–1755. [Google Scholar] [CrossRef]
- Brandalise, M.; Maia, I.G.; Borecký, J.; Vercesi, A.E.; Arruda, P. Overexpression of Plant Uncoupling Mitochondrial Protein in Transgenic Tobacco Increases Tolerance to Oxidative Stress. J. Bioenerg. Biomembr. 2003, 35, 203–209. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. ISBN 978-0-12-182048-0. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 5 September 2023).
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond P Values: Data Analysis with Estimation Graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef]
- Endres, L.; Santos, C.M.D.; Souza, G.V.D.; Menossi, M.; Santos, J.C.M.D. Morphological Changes Recorded in Different Phenophases of Sugarcane Plants Subjected to Water Stress in Tropical Field Conditions. Aust. J. Crop Sci. 2018, 12, 1041–1050. [Google Scholar] [CrossRef]
- Begcy, K.; Mariano, E.D.; Gentile, A.; Lembke, C.G.; Zingaretti, S.M.; Souza, G.M.; Menossi, M. A Novel Stress-Induced Sugarcane Gene Confers Tolerance to Drought, Salt and Oxidative Stress in Transgenic Tobacco Plants. PLoS ONE 2012, 7, e44697. [Google Scholar] [CrossRef]
- Macková, H.; Hronková, M.; Dobrá, J.; Turečková, V.; Novák, O.; Lubovská, Z.; Motyka, V.; Haisel, D.; Hájek, T.; Prášil, I.T.; et al. Enhanced Drought and Heat Stress Tolerance of Tobacco Plants with Ectopically Enhanced Cytokinin Oxidase/Dehydrogenase Gene Expression. J. Exp. Bot. 2013, 64, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.-T.; Sun, B.; Niu, J.-Q.; Tan, Q.-L.; Li, J.; Yang, L.-T.; Li, Y.-R. Overexpression of Sugarcane Gene SoSnRK2.1 Confers Drought Tolerance in Transgenic Tobacco. Plant Cell Rep. 2016, 35, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Begcy, K.; Mariano, E.D.; Lembke, C.G.; Zingaretti, S.M.; Souza, G.M.; Araújo, P.; Menossi, M. Overexpression of an Evolutionarily Conserved Drought-Responsive Sugarcane Gene Enhances Salinity and Drought Resilience. Ann. Bot. 2019, 124, 691–700. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zhao, H.; Deng, X.; Su, Y.; Li, R.; Chen, B. Overexpression of Sugarcane ScDIR Genes Enhances Drought Tolerance in Nicotiana Benthamiana. Int. J. Mol. Sci. 2022, 23, 5340. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Dall’Osto, L. Dissipation of Light Energy Absorbed in Excess: The Molecular Mechanisms. Annu. Rev. Plant Biol. 2021, 72, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A.; Shimakawa, G. Regulation of the Generation of Reactive Oxygen Species during Photosynthetic Electron Transport. Biochem. Soc. Trans. 2022, 50, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Lima-Melo, Y.; Kılıç, M.; Aro, E.-M.; Gollan, P.J. Photosystem I Inhibition, Protection and Signalling: Knowns and Unknowns. Front. Plant Sci. 2021, 12, 791124. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Krieger-Liszkay, A. Photosynthetic Regulatory Mechanisms for Efficiency and Prevention of Photo-Oxidative Stress. In Annual Plant Reviews Online; Roberts, J.A., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 273–306. ISBN 978-1-119-31299-4. [Google Scholar]
- Zhengbin, Z.; Ping, X.; Hongbo, S.; Mengjun, L.; Zhenyan, F.; Liye, C. Advances and Prospects: Biotechnologically Improving Crop Water Use Efficiency. Crit. Rev. Biotechnol. 2011, 31, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Siaca, L.G.; Głowacka, K.; Driever, S.M.; Salesse-Smith, C.E.; Lugassi, N.; Granot, D.; Long, S.P.; Kromdijk, J. Guard-Cell-Targeted Overexpression of Arabidopsis Hexokinase 1 Can Improve Water Use Efficiency in Field-Grown Tobacco Plants. J. Exp. Bot. 2022, 73, 5745–5757. [Google Scholar] [CrossRef]
- Lugassi, N.; Yadav, B.S.; Egbaria, A.; Wolf, D.; Kelly, G.; Neuhaus, E.; Raveh, E.; Carmi, N.; Granot, D. Expression of Arabidopsis Hexokinase in Tobacco Guard Cells Increases Water-Use Efficiency and Confers Tolerance to Drought and Salt Stress. Plants 2019, 8, 613. [Google Scholar] [CrossRef]
- Contiliani, D.F.; Nebó, J.F.C.D.O.; Ribeiro, R.V.; Landell, M.G.D.A.; Pereira, T.C.; Ming, R.; Figueira, A.; Creste, S. Drought-Triggered Leaf Transcriptional Responses Disclose Key Molecular Pathways Underlying Leaf Water Use Efficiency in Sugarcane (Saccharum Spp.). Front. Plant Sci. 2023, 14, 1182461. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll Hormesis: Are Chlorophylls Major Components of Stress Biology in Higher Plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef]
- Joshi, P.S.; Agarwal, P.; Agarwal, P.K. Overexpression of AlNAC1 from Recretohalophyte Aeluropus Lagopoides Alleviates Drought Stress in Transgenic Tobacco. Environ. Exp. Bot. 2021, 181, 104277. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.-H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef] [PubMed]
- López-Calcagno, P.E.; Brown, K.L.; Simkin, A.J.; Fisk, S.J.; Vialet-Chabrand, S.; Lawson, T.; Raines, C.A. Stimulating Photosynthetic Processes Increases Productivity and Water-Use Efficiency in the Field. Nat. Plants 2020, 6, 1054–1063. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.R.L.; de Souza, C.B.; dos Santos, C.M.; Floreste, B.S.; Zani, N.C.; Hoshino-Bezerra, A.A.; Bueno, G.C.; Chagas, E.B.R.; Menossi, M. The Sugarcane ScPetC Gene Improves Water-Deficit and Oxidative Stress Tolerance in Transgenic Tobacco Plants. Agronomy 2024, 14, 1371. https://doi.org/10.3390/agronomy14071371
Silva CRL, de Souza CB, dos Santos CM, Floreste BS, Zani NC, Hoshino-Bezerra AA, Bueno GC, Chagas EBR, Menossi M. The Sugarcane ScPetC Gene Improves Water-Deficit and Oxidative Stress Tolerance in Transgenic Tobacco Plants. Agronomy. 2024; 14(7):1371. https://doi.org/10.3390/agronomy14071371
Chicago/Turabian StyleSilva, Carolina Ribeiro Liberato, César Bueno de Souza, Claudiana Moura dos Santos, Bruno Spinassé Floreste, Nicholas Camargo Zani, Andrea Akemi Hoshino-Bezerra, Giane Carolina Bueno, Eder Bedani Ruiz Chagas, and Marcelo Menossi. 2024. "The Sugarcane ScPetC Gene Improves Water-Deficit and Oxidative Stress Tolerance in Transgenic Tobacco Plants" Agronomy 14, no. 7: 1371. https://doi.org/10.3390/agronomy14071371






