The Effect of Exogenous Selenium Supplementation on the Nutritional Value and Shelf Life of Lettuce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation and Treatment of Lettuce
2.2. Determination of Growth Indicators
2.3. Determination of Antioxidant Indicators
2.4. Determination of Total Se Content and Identification of Se Species
2.5. Determination of Color Difference
2.6. Transcriptome Sequencing and Data Analysis
2.7. Statistical Analysis and Graph Plotting
3. Results
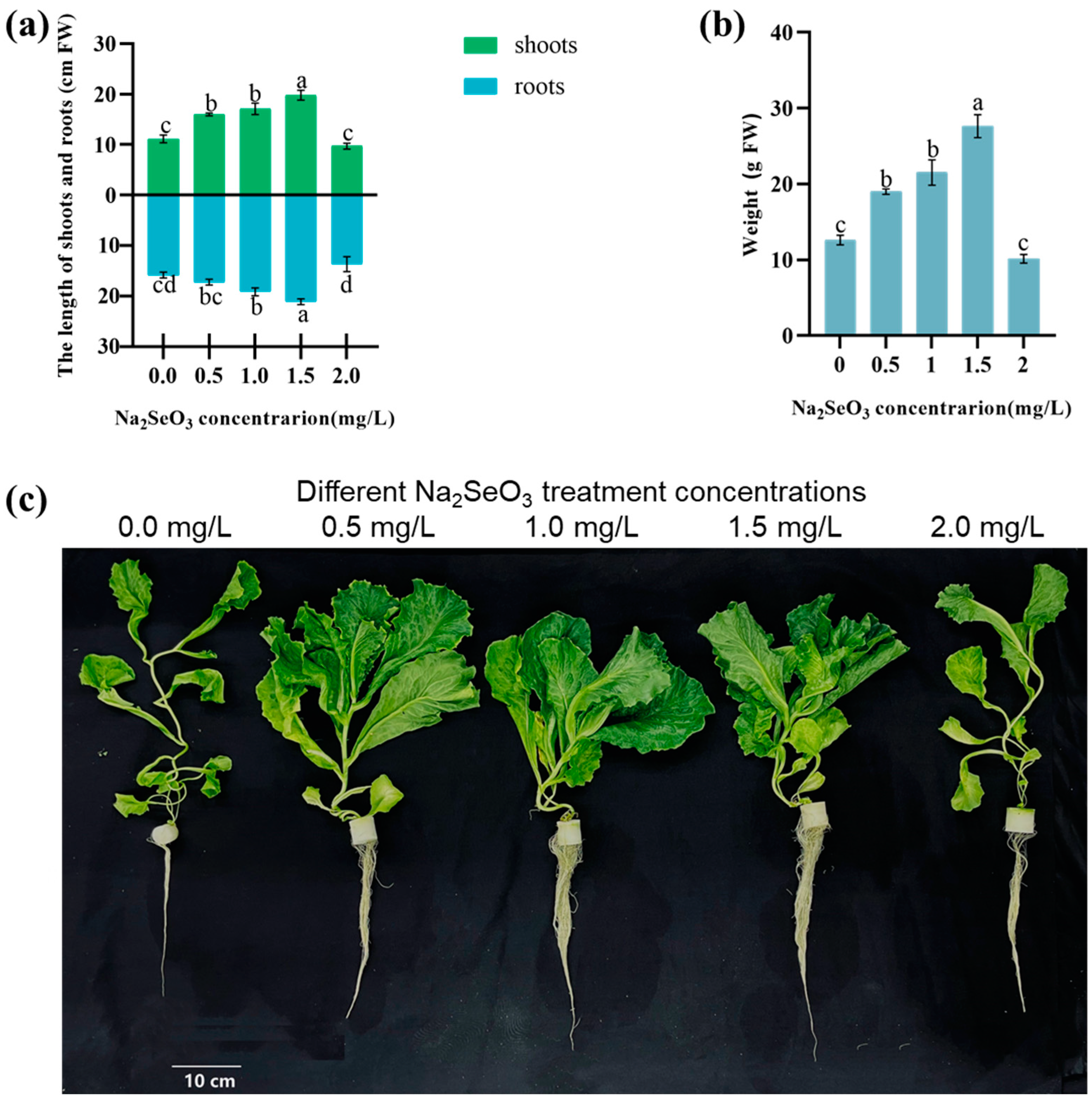
3.1. The Influence of Na2SeO3 Hydroponics on the Plant Biomass of Lettuce
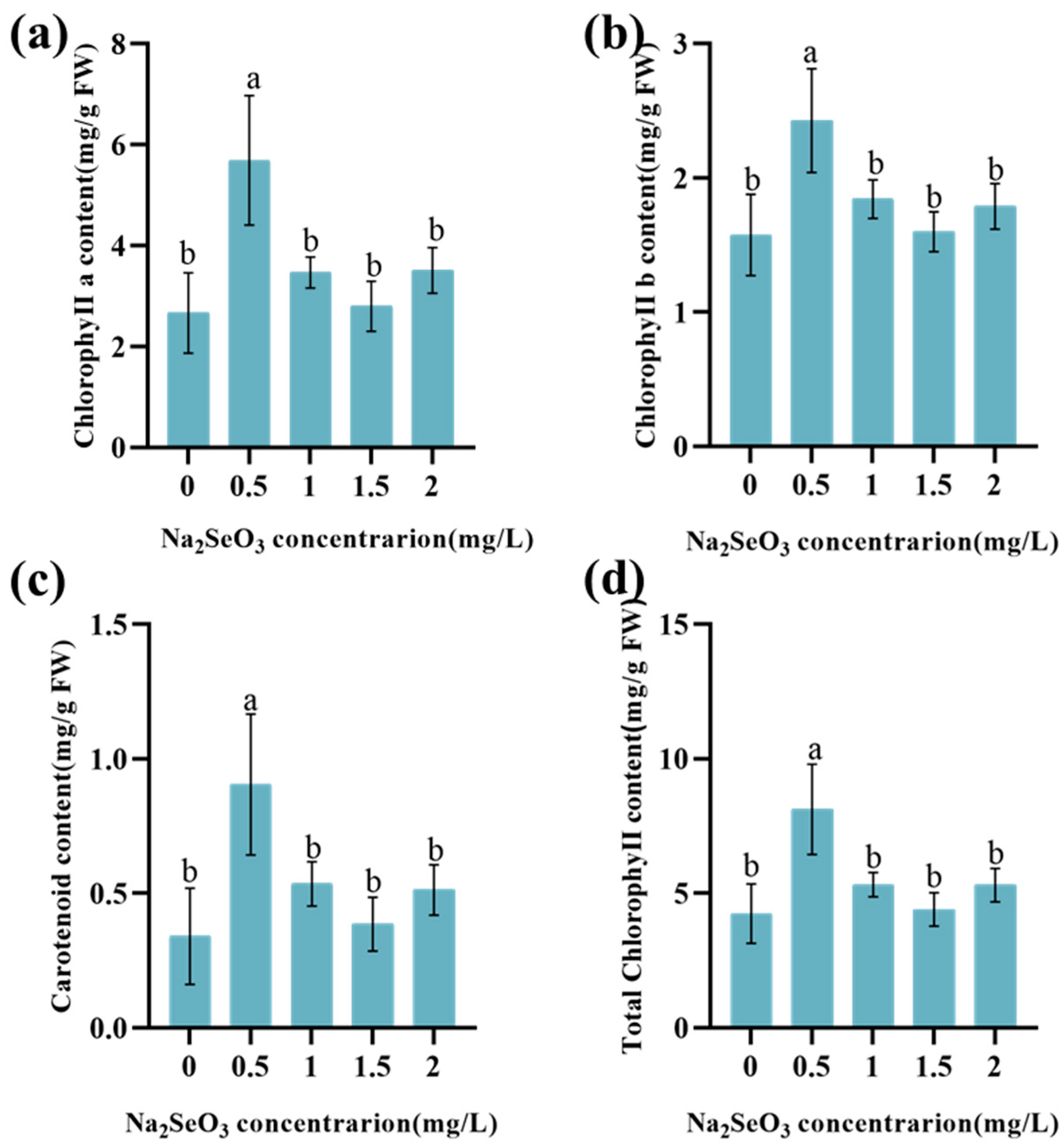
3.2. Effect of Na2SeO3 on the Content of Photosynthetic Pigments in Lettuce
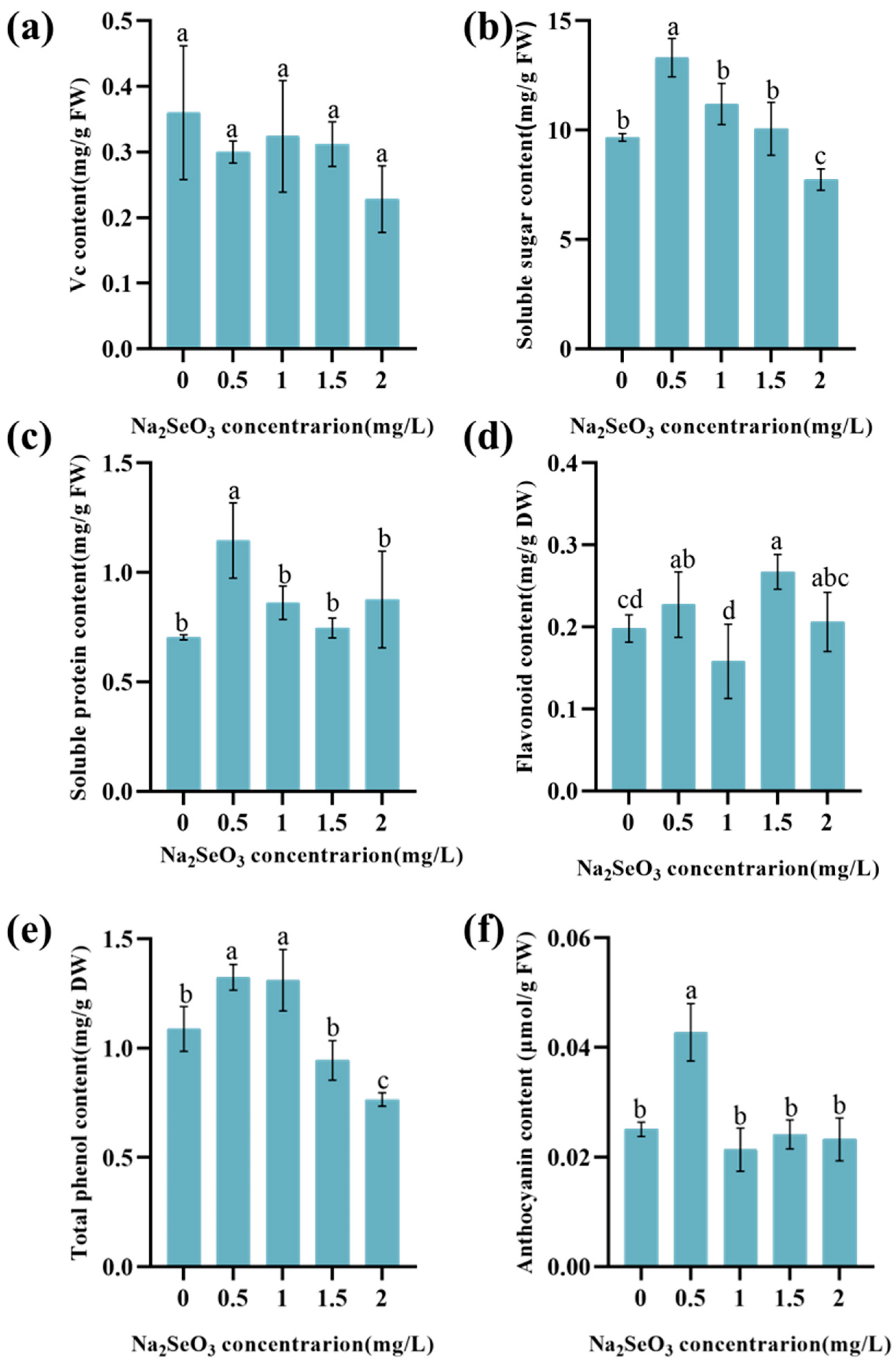
3.3. Effect of Na2SeO3 on the Nutritional Quality of Lettuce
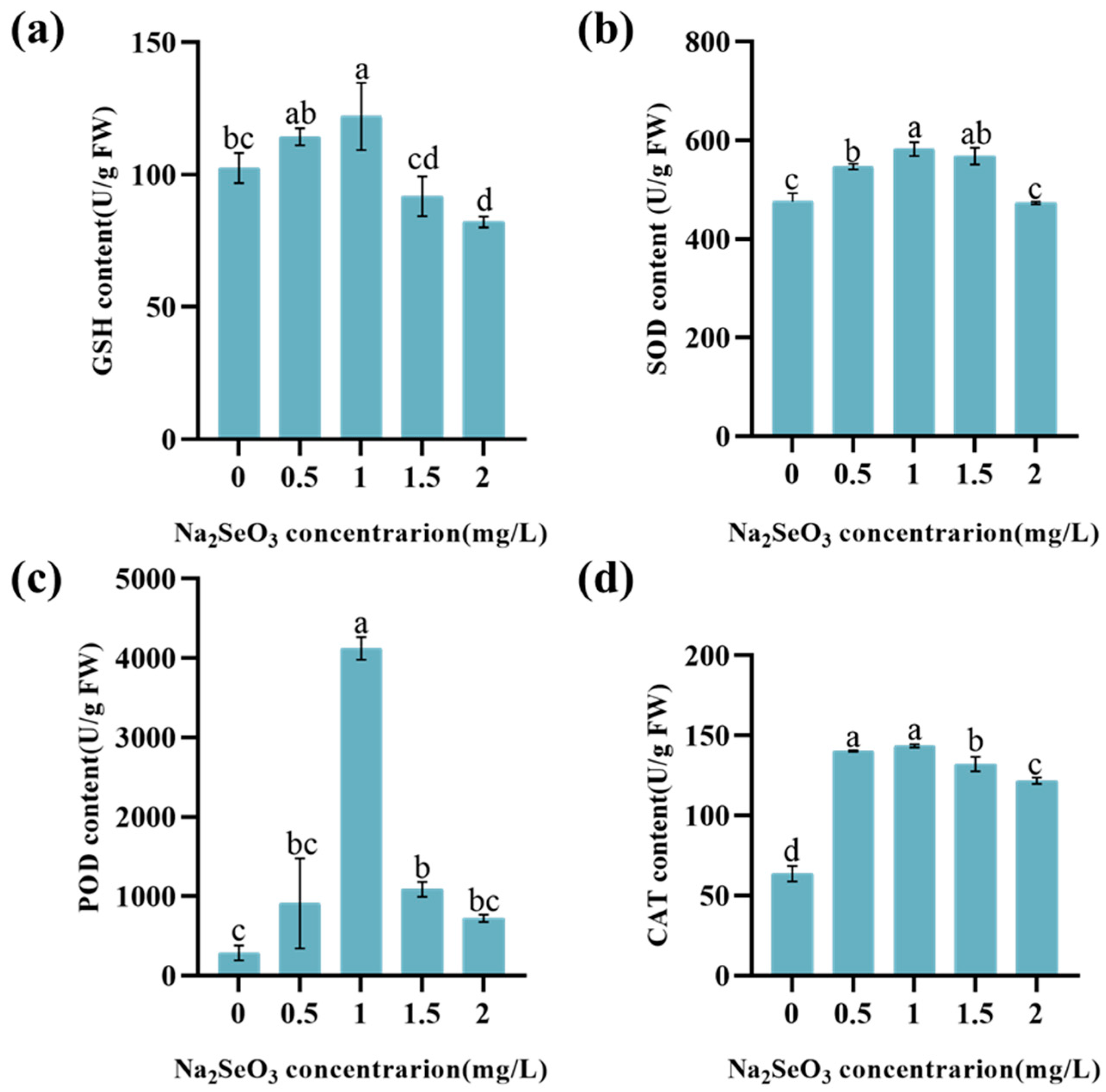
3.4. The Effect of Na2SeO3 on the Antioxidation Performance of Lettuce
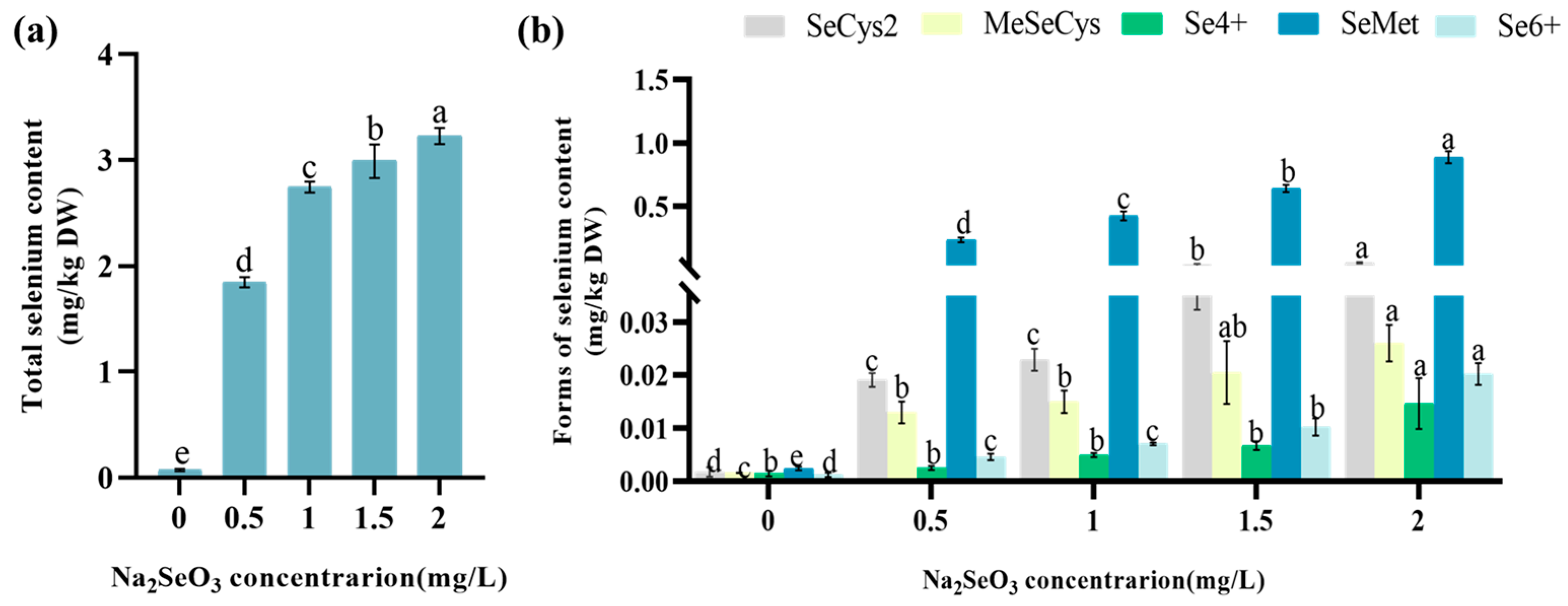
3.5. The Effect of Na2SeO3 Treatment on the Total Se and Se Species Content of Lettuce
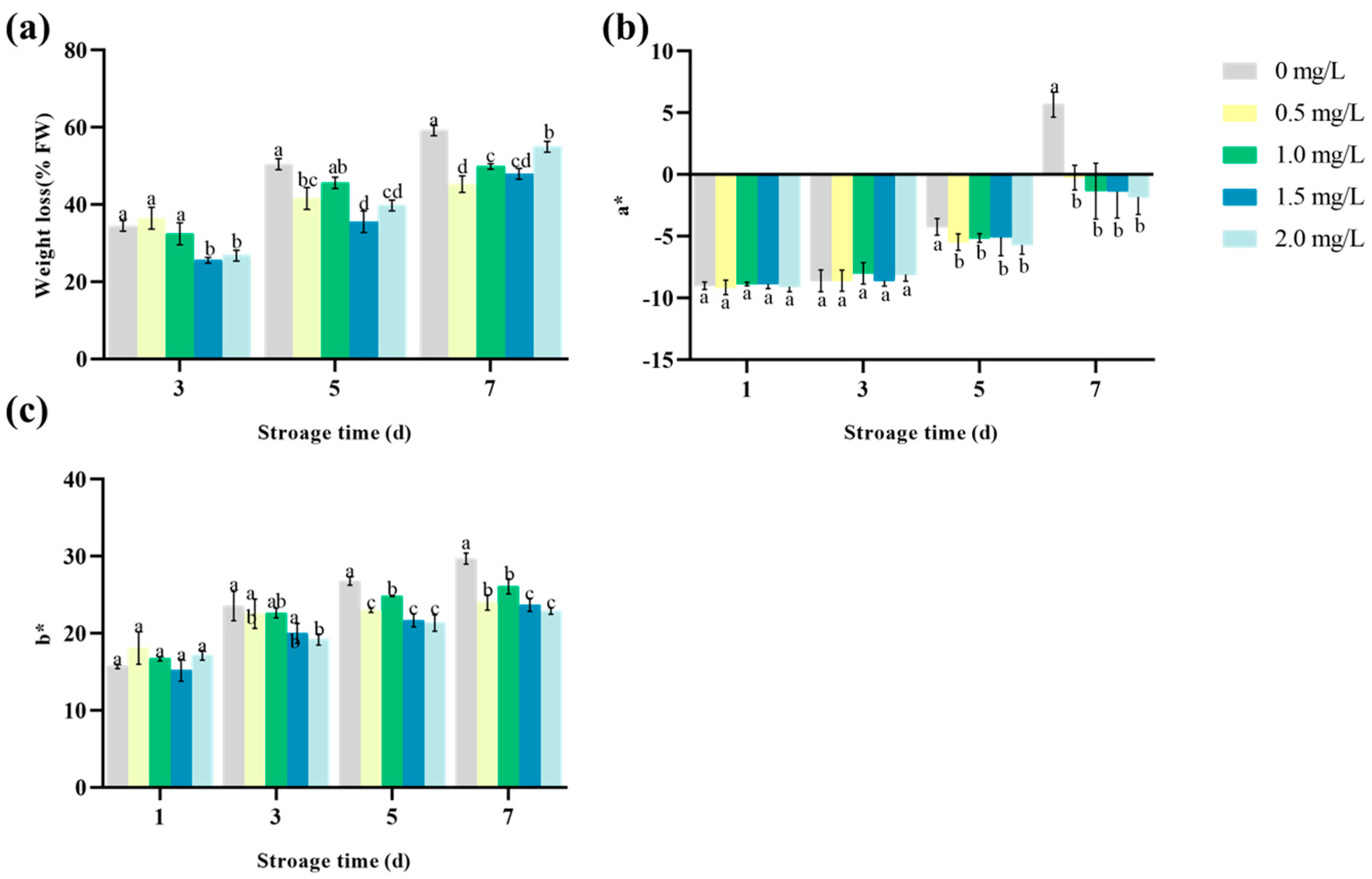
3.6. The Effect of Na2SeO3 on the Weight Loss and Browning of Lettuce
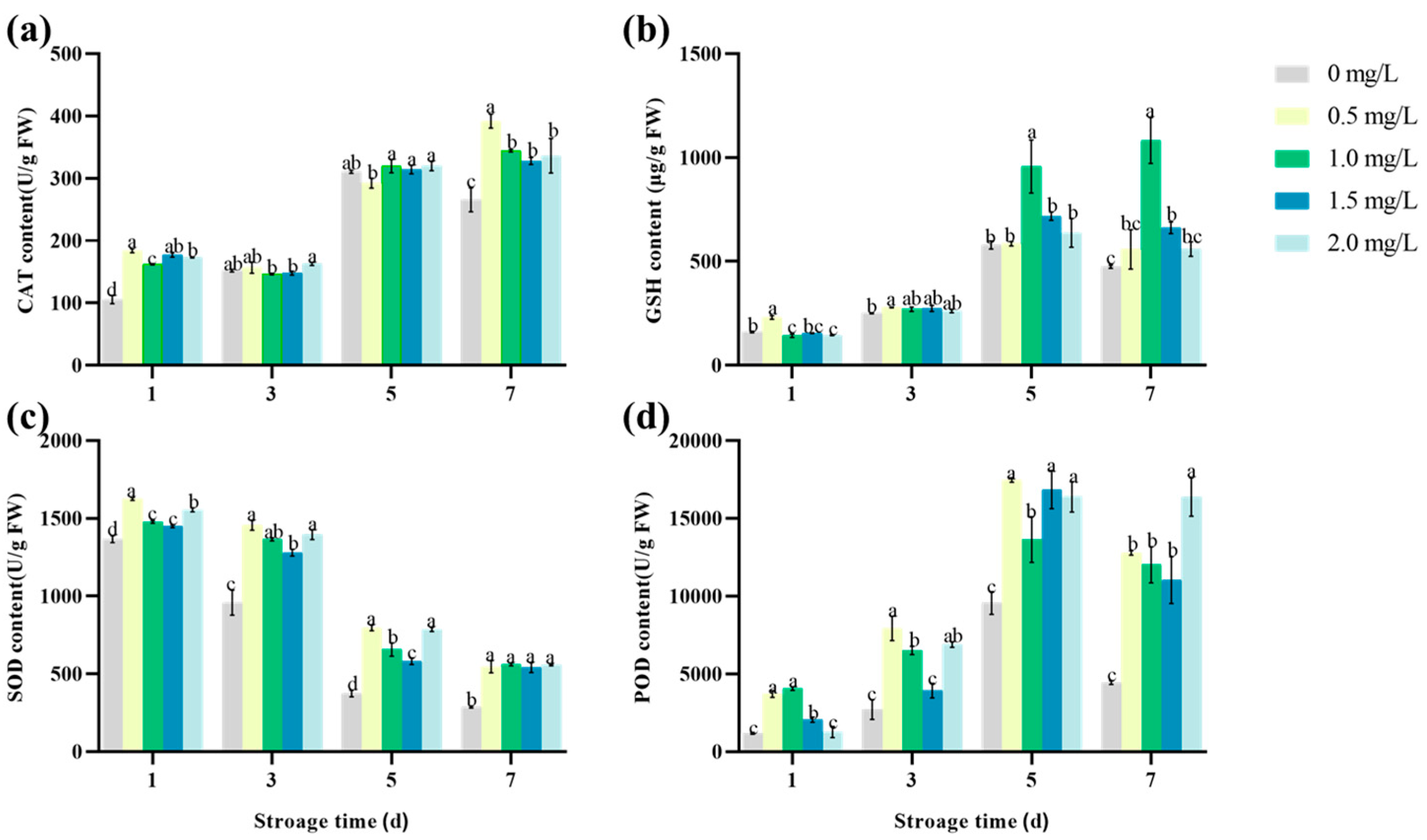
3.7. The Effect of Se on the Antioxidant Properties of Lettuce during Storage
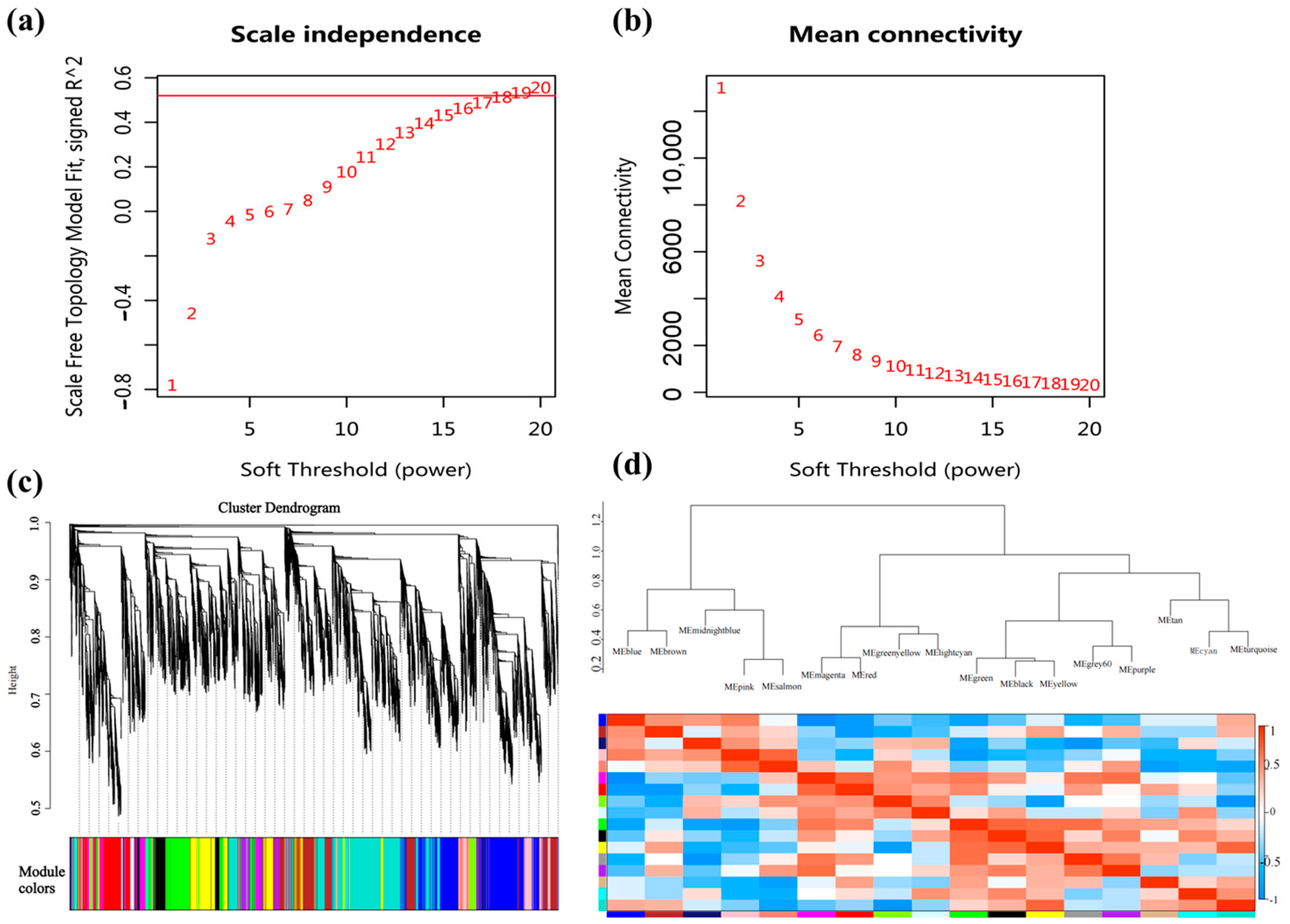
3.8. Transcriptome Data Analysis of Lettuce Treated with Na2SeO3
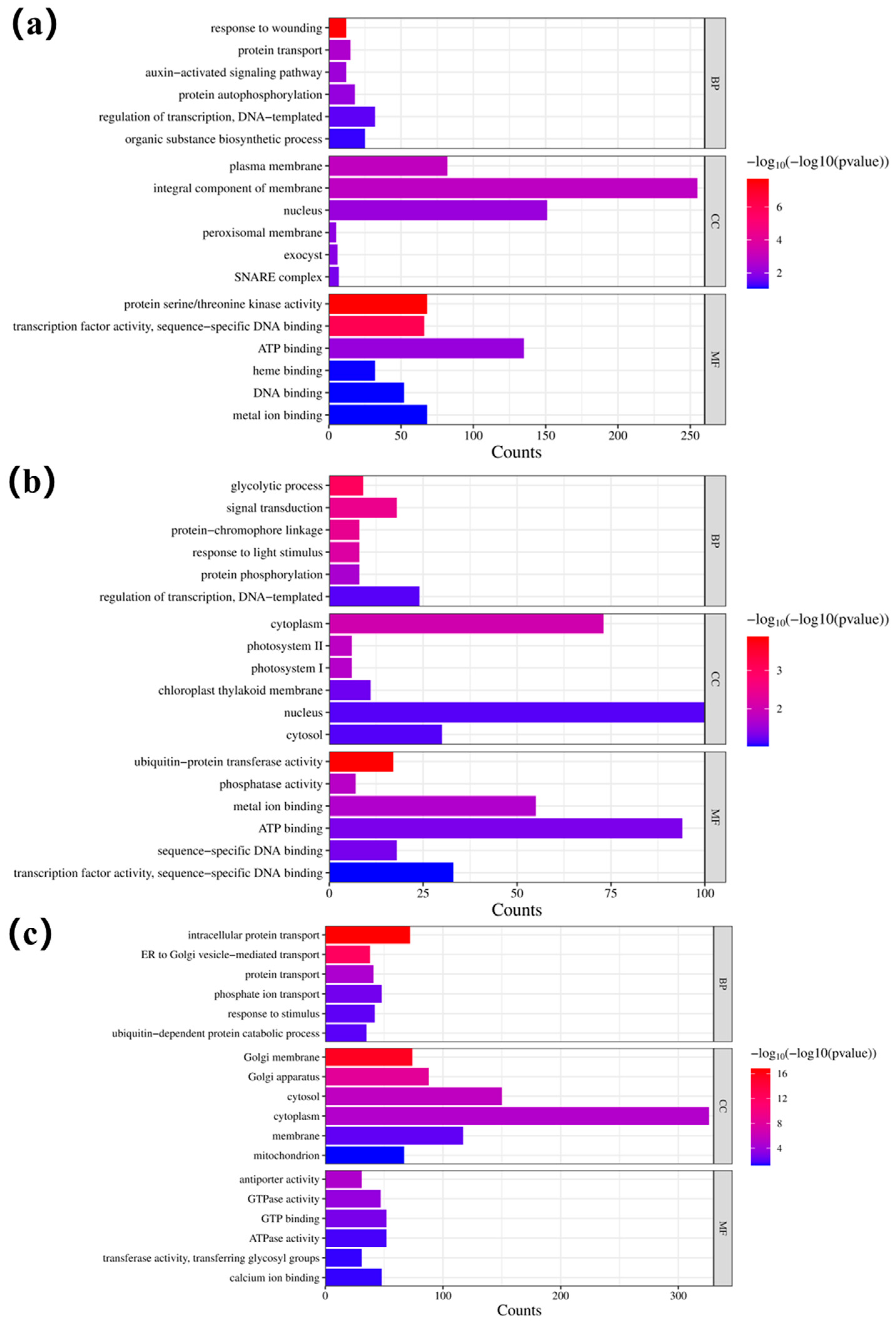
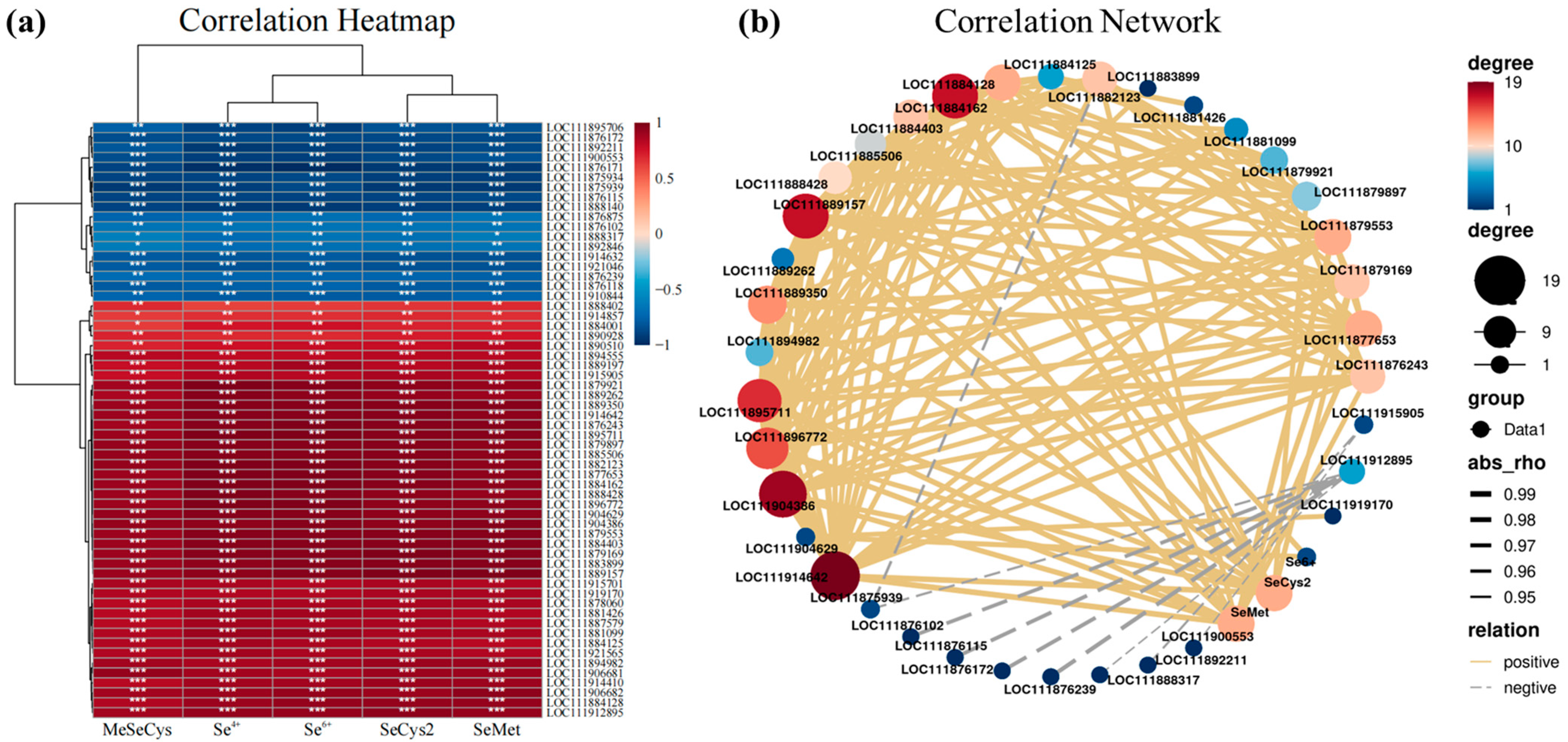
3.9. Gene Function Enrichment and Expression Regulation Analysis of Key Modules
4. Discussion
4.1. The Effect of Na2SeO3 on the Growth and Nutritional Quality of Lettuce
4.2. The Effect of Se on the Antioxidant Capacity of Lettuce
4.3. The Impact of Na2SeO3 on the Changes in Total Se and Se Species Content in Lettuce
4.4. The Effect of Se on the Storage Ability of Lettuce
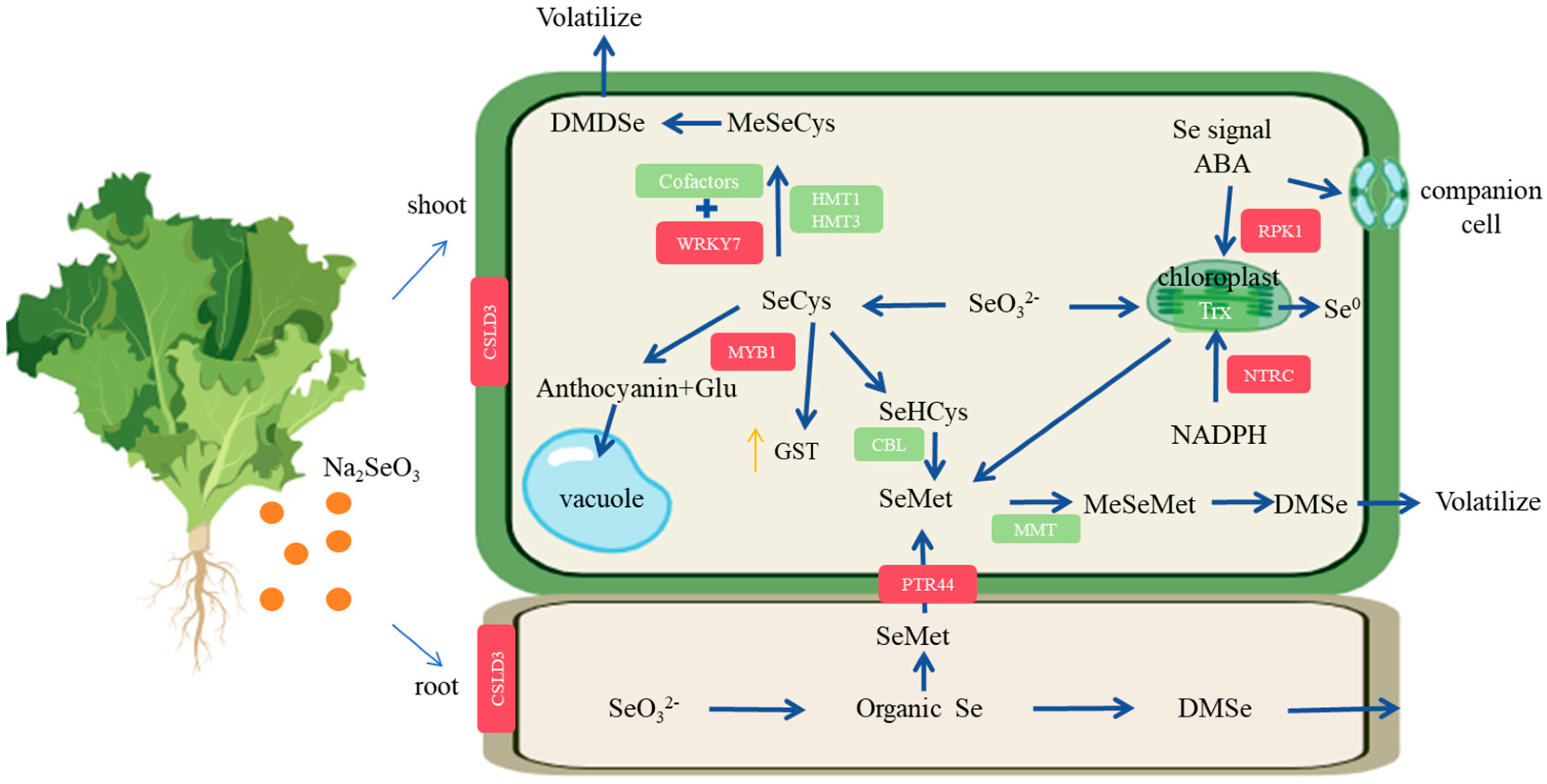
4.5. Molecular Mechanism of Lettuce Absorbing and Transforming Se
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, T.; Boada, R.; Llugany, M.; Valiente, M. Co-application of Se and a biostimulant at different wheat growth stages: Influence on grain development. Plant Physiol. Biochem. 2021, 160, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Rao, S.; Chen, Q.; Zhang, W.; Cheng, S.; Cong, X.; Zhang, Y.; Yang, X.; Xu, F. Research progress on the effects of selenium on the growth and quality of tea plants. Plants 2022, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, H.; Guo, Y.; Fan, B.; Lu, B. Benefit–risk assessment of dietary selenium and its associated metals intake in China (2017–2019): Is current selenium-rich agro-food safe enough? J. Hazard. Mater. 2020, 398, 123224. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Beecher, G.R.; Burk, R.F.; Chan, A.C.; Erdman, J.J.; Jacob, R.A.; Jialal, I.; Kolonel, L.N.; Marshall, J.R.; Taylor Mayne, P.R. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Inst. Med. 2000, 19, 95–185. [Google Scholar] [CrossRef]
- Schiavon, M.; Nardi, S.; Vecchia, F.D.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Sun, X.; Li, P.; Shen, X.; Fang, Y. Selenium in cereals: Insight into species of the element from total amount. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2914–2940. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H.; Schiavon, M. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyper accumulator Brassicaceae. New Phytol. 2017, 217, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Zhang, S.; Suo, X.; Chen, W.; Shen, Y. Selenite uptake by Medicago sativa L. roots. Grassl. Sci. 2022, 68, 328–335. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium biofortification: Roles, mechanisms, responses and prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, E.; Cao, Q.; Yang, F.; Li, G. Physiological function and absorption enrichment of selenium in staple crops. J. Northeast Agric. Sci. 2020, 45, 115–118. [Google Scholar] [CrossRef]
- Ma, X.; Xu, J.; Zhao, X.; Qu, L.; Gao, Y.; Huang, W.; Han, D.; Dang, B.; Xu, Z.; Jia, W. Selenium improves the control efficacy of Phytophthora nicotianae by damaging the cell membrane system and promoting plant energy metabolism. J. Agric. Food Chem. 2024, 72, 5073–5087. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; Ramezani, M.R.; Rajaii, N. Improving oxidative damage, photosynthesis traits, growth and flower dropping of pepper under high temperature stress by selenium. Mol. Biol. Rep. 2018, 46, 497–503. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium enrichment enhances the quality and shelf life of basil leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef]
- Babalar, M.; Mohebbi, S.; Zamani, Z.; Askari, M.A. Effect of foliar application with sodium selenate on selenium biofortification and fruit quality maintenance of ‘Starking Delicious’ apple during storage. J. Sci. Food Agric. 2019, 99, 5149–5156. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive compounds in lettuce: Highlighting the benefits to human health and impacts of preharvest and postharvest practices. Compr. Rev. Food Sci. Food Saf. 2021, 21, 4–45. [Google Scholar] [CrossRef] [PubMed]
- Sawatdee, S.; Prommuak, C.; Jarunglumlert, T.; Pavasant, P.; Flood, A.E. Combined effects of cations in fertilizer solution on antioxidant content in red lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2021, 101, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.; Alhumaydhi, F.A.; Al-Awthan, Y.S. Phytochemicals, nutrition, metabolism, bioavailability, and health benefits in lettuce-A comprehensive review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef]
- Su, S.; Zhou, Y.; Qin, J.G.; Yao, W.; Ma, Z. Optimization of the method for chlorophyll extraction in aquatic plants. J. Freshw. Ecol. 2010, 25, 531–538. [Google Scholar] [CrossRef]
- Deng, X.; Li, R.; Deng, S. Determination of the total content of arsenic, antimony, selenium and mercury in Chinese herbal food by chemical vapor generation-four-channel non-dispersive atomic fluorescence spectrometry. J. Fluoresc. 2020, 30, 949–954. [Google Scholar] [CrossRef]
- Herath, I.; Kumarathilaka, P.; Bundschuh, J.; Marchuk, A.; Rinklebe, J. A fast analytical protocol for simultaneous speciation of arsenic by ultra-high performance liquid chromatography (UHPLC) hyphenated to inductively coupled plasma mass spectrometry (ICP-MS) as a modern advancement in liquid chromatography approaches. Talanta 2020, 208, 120457. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Jia, P.; Luan, H.; Qi, G.; Li, H.; Guo, S. Transcriptomics and metabolomics provide insight into the anti-browning mechanism of selenium in freshly cut apples. Front. Plant Sci. 2023, 14, 1176936. [Google Scholar] [CrossRef] [PubMed]
- Khalofah, A.; Migdadi, H.; El-Harty, E. Antioxidant enzymatic activities and growth response of quinoa (Chenopodium quinoa Willd) to exogenous selenium application. Plants 2021, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, Y.; Zhou, Z.; Li, T.; Wu, F.; Yao, Q. Effect of different concentrations of sodium selenate on the growth and physiological indicators of tea plants. Guihaia 2021, 41, 183–194. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhang, M.; Wei, X.; Zhou, Y. Effects of foliar selenium application on Se accumulation, elements uptake, nutrition quality, sensory quality and antioxidant response in summer-autumn tea. Food Res. Int. 2024, 175, 113618. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.L.O.; de Oliveira, L.C.A.; Mendes, N.A.C.; Silva, V.M.; Vicente, E.F.; dos Reis, A.R. Selenium increases photosynthetic pigments, flavonoid biosynthesis, nodulation, and growth of soybean plants (Glycine max L.). J. Soil Sci. Plant Nutr. 2023, 23, 1397–1407. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, S.; Li, H.; Cong, X. Effects of selenium on physiological characteristics, selenium content and quality of garlic. Plant Nutr. Fertil. Sci. 2012, 18, 733–741. [Google Scholar]
- Cardoso, A.A.d.S.; Gomes, F.T.d.L.; Antonio, J.R.R.; Guilherme, L.R.G.; Liu, J.; Li, L.; Ligia, M.; Silva, S. Sulfate availability and soil selenate adsorption alleviate selenium toxicity in rice plants. Environ. Exp. Bot. 2022, 201, 104971. [Google Scholar] [CrossRef]
- Huang, S.; Yu, K.; Xiao, Q.; Song, B.; Yuan, W.; Long, X.; Cai, D.; Xiong, X.; Zheng, W. Effect of bio-nano-selenium on yield, nutritional quality and selenium content of radish. J. Food Compos. Anal. 2023, 200, 107742. [Google Scholar] [CrossRef]
- Ding, F.; Wei, X.; Dao, Y.; Zhao, F.; Wang, R.; Li, P. Use of fulvic acid-like compounds from pulp-derived black liquor for enhancing the selenium content of peanut buds. BMC Plant Biol. 2022, 22, 1–12. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Liu, J.; Chen, Y.; Zhang, X. Exploring the effects of selenium treatment on the nutritional quality of tomato fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef]
- Groth, S.; Budke, C.; Neugart, S.; Ackermann, S.; Kappenstein, F.S.; Daum, D.; Rohn, S. Influence of a selenium biofortification on antioxidant properties and phenolic compounds of apples (Malus domestica). Antioxidants 2020, 9, 187. [Google Scholar] [CrossRef]
- Woch, W.; Hawrylak-Nowak, B. Selected antioxidant properties of alfalfa, radish, and white mustard sprouts biofortified with selenium. Acta Agrobot. 2019, 72, 1768. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.J.; Kang, H.M.; Lee, Y.T. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Li, T.; Yun, Z.; Zhang, D.; Yang, C.; Zhu, H.; Jiang, Y.; Duan, X. Proteomic analysis of differentially expressed proteins involved in ethylene-induced chilling tolerance in harvested banana fruit. Front. Plant Sci. 2015, 6, 845. [Google Scholar] [CrossRef]
- Walaa, A.; Shatlah, M.; Atteia, M.; Sror, H. Selenium induces antioxidant defensive enzymes and promotes tolerance against salinity stress in cucumber seedlings (Cucumis sativus). Arab Univ. J. Agric. Sci. 2010, 18, 65–76. [Google Scholar]
- Wu, Z.; Yuan, L.; Sun, C.; Xu, X.; Shi, W.; Han, L.; Wu, C. Effects of selenite on the responses of lettuce (Lactuca sativa L.) to polystyrene nano-plastic stress. Ecotoxicol. Environ. Saf. 2023, 262, 115138. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R.; Woch, W.; Hasanuzzaman, M. Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol. Biochem. 2018, 127, 446–456. [Google Scholar] [CrossRef]
- Jiang, S.; Du, B.; Wu, Q.; Zhang, H.; Deng, Y.; Tang, X.; Zhu, J. Selenium decreases the cadmium content in brown rice: Foliar Se application to plants grown in Cd-contaminated soil. J. Soil Sci. Plant Nutr. 2022, 22, 1033–1043. [Google Scholar] [CrossRef]
- Shalaby, T.; Bayoumi, Y.; Alshaal, T.; Elhawat, N.; Sztrik, A.; El-Ramady, H. Selenium fortification induces growth, antioxidant activity, yield and nutritional quality of lettuce in salt-affected soil using foliar and soil applications. Plant Soil 2017, 421, 245–258. [Google Scholar] [CrossRef]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H.Y. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Silva, M.A.; de Sousa, G.F.; Bañuelos, G.; Amaral, D.; Brown, P.H.; Guilherme, L.R.G. Selenium speciation in se-enriched soybean grains from biofortified plants grown under different methods of selenium application. Foods 2023, 12, 1214. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, W.; Wang, M.; Miao, Y.; Cui, Z.; Li, Z.; Liang, D. Effects of selenium application on Se content and speciation in Lentinula edodes. Food Chem. 2018, 265, 182–188. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Chen, F. Effect of selenium enrichment on the quality of germinated brown rice during storage. Food Chem. 2016, 207, 20–26. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Zhang, X.; Zhang, W.; Huang, L.; Zhang, Z.; Yuan, L.; Liu, X. Effects of foliar application of selenate and selenite at different growth stages on Selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Peng, M.; Zhu, S.; Wang, G. Preharvest application of selenite enhances the quality of Chinese flowering cabbage during storage via regulating the ascorbate-glutathione cycle and phenylpropanoid metabolisms. Food Res. Int. 2023, 163, 112229. [Google Scholar] [CrossRef]
- Zhu, Y.; Pilon-Smits, E.A.H.; Zhao, F.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A. Selenium biofortification and phytoremediation phytotechnologies: A review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 107212. [Google Scholar] [CrossRef]
- Van Hoewyk, D.; Takahashi, H.; Inoue, E.; Hess, A.; Tamaoki, M.; Pilon-Smits, E.A.H. Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol. Plant. 2010, 132, 236–253. [Google Scholar] [CrossRef]
- Barberon, M.; Berthomieu, P.; Clairotte, M.; Shibagaki, N.; Davidian, J.C.; Gosti, F. Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2. New Phytol. 2008, 180, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Shao, H.; Huang, H.; Shen, Y.; Wang, L.; Wu, F.; Han, D.; Song, J.; Jia, H. Overexpression of the phosphate transporter gene OsPT8 improves the Pi and selenium contents in Nicotiana tabacum. Environ. Exp. Bot. 2017, 137, 158–165. [Google Scholar] [CrossRef]
- Zhao, X.; Mitani, N.; Yamaji, N.; Shen, R.; Ma, J. Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiol. 2010, 153, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Xu, J.; Dai, Z.; Elyamine, A.M.; Huang, W.; Han, D.; Dang, B.; Xu, Z.; Jia, W. Selenium in soil-plant system: Transport, detoxification and bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Hayashi, N.; Yamaya, T.; Takahashi, H. Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol. 2004, 136, 4198–4204. [Google Scholar] [CrossRef] [PubMed]
- Trippe, R.C.; Pilon-Smits, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2020, 404, 124178. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, J.; Berkelaar, E. Plant uptake and translocation of inorganic and organic forms of selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hao, R.; Xu, Z.; Yang, W.; Wang, J.; Cheng, T.; Pan, H.; Zhang, Q. Isolation and functional characterization of a R2R3-MYB regulator of Prunus mume anthocyanin biosynthetic pathway. Plant Cell Tissue Organ Cult. 2017, 131, 417–429. [Google Scholar] [CrossRef]
- Schwinn, K.E.; Ngo, H.; Kenel, F.; Brummell, D.A.; Davies, K.M. The onion (Allium cepa L.) R2R3-MYB gene MYB1 regulates anthocyanin biosynthesis. Front. Plant Sci. 2016, 7, e29526. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wu, Q.; Lu, P.; Kang, Q.; Zhao, M.; Wang, A.; Dong, Q.; Sun, M.; Yang, Z.; et al. Selenium application enhances the accumulation of flavones and anthocyanins in bread wheat (Triticum aestivum L.) grains. J. Agric. Food Chem. 2022, 70, 13431–13444. [Google Scholar] [CrossRef]
- Xia, Q.; Shui, Y.; Zhi, H.; Ali, A.; Yang, Z.; Gao, Z. Exogeneous selenium enhances anthocyanin synthesis during grain development of colored-grain wheat. Plant Physiol. Biochem. 2023, 200, 107742. [Google Scholar] [CrossRef]
- Pu, Z.; Wei, G.; Liu, Z.; Chen, L.; Zheng, Y. Selenium and anthocyanins share the same transcription factors R2R3MYB and bHLH in wheat. Food Chem. 2021, 356, 129699. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Jon, J.H.; Nam, K.H.G. Identification of a receptor-like protein kinase gene rapidly induced by abscisic acid, dehydration, high salt, and cold treatments in Arabidopsis thaliana. Plant Physiol. 1997, 113, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Yang, D.; Ha, Y.; Nam, K.H. BAK1-induced RPK1 phosphorylation is essential for RPK1-mediated cell death in Arabidopsis. Biochem. Biophys. Res. Commun. 2021, 573, 125–131. [Google Scholar] [CrossRef]
- Wang, X.; Lu, W.; Zhao, Z.; Hao, W.; Du, R.; Li, Z.; Wang, Z.; Lv, X.; Wang, J.; Liang, D.; et al. Abscisic acid promotes selenium absorption, metabolism and toxicity via stress-related phytohormones regulation in Cyphomandra betacea Sendt. (Solanum betaceum Cav.). J. Hazard. Mater. 2023, 461, 132642. [Google Scholar] [CrossRef]
- Wu, J.; Guo, S.; Wang, J.; He, J.; Li, X.; Zhan, Y. The phosphoproteomic response of pepper (Capsicum annuum L.) seedlings to selenium stress. Horticulturae 2023, 9, 935. [Google Scholar] [CrossRef]
- Golz, J.F.; Oh, H.-Y.; Su, V.; Kusaba, M.; Newbigin, E. Genetic analysis of Nicotiana pollen-part mutants is consistent with the presence of an S-ribonuclease inhibitor at the S locus. Proc. Natl. Acad. Sci. USA 2001, 9, 15372–15376. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Bringmann, M.; Combs, J.; Yang, J.; Bergmann, D.; Nielsen, E. The Arabidopsis CSLD5 functions in cell plate formation in a cell cycle dependent manner. Plant Cell 2016, 28, 1722–1737. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Hao, J.; Fan, S.; Dong, R.; Zeng, H.; Liu, C.; Han, Y. Effects of selenate and selenite on selenium accumulation and speciation in lettuce. Plant Physiol. Biochem. 2022, 192, 162–171. [Google Scholar] [CrossRef]
- Chiba, Y.; Shimizu, T.; Miyakawa, S.; Kanno, Y.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of Arabidopsis thaliana NRT1/PTR FAMILY (NPF) proteins capable of transporting plant hormones. J. Plant Res. 2015, 128, 679–686. [Google Scholar] [CrossRef]
- Lei, B.; Bian, Z.; Yang, Q.; Wang, J.; Cheng, R.-f.; Li, K.; Liu, W.-k.; Zhang, Y.; Fang, H.; Tong, Y. The positive function of selenium supplementation on reducing nitrate accumulation in hydroponic lettuce (Lactuca sativa L.). J. Integr. Agric. 2018, 17, 837–846. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Deng, K.; Gao, X.; Sun, G.; Zhang, Z.; Li, P.; Wang, W.; Li, H.; Zhang, Z.; et al. NRT1.1B improves selenium concentrations in rice grains by facilitating selenomethinone translocation. Plant Biotechnol. J. 2018, 17, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, G.; Wu, W.; Lyu, H.; Wang, J.; Liu, C.; Zhong, C.; Shi, S.; He, Z. Expansion and adaptive evolution of the WRKY transcription factor family in Avicennia mangrove trees. Mar. Life Sci. Technol. 2023, 5, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, C.; Sun, L.; Ren, J.; Li, G.; Ding, Z.; Zheng, S. A WRKY transcription factor regulates Fe translocation under Fe deficiency in Arabidopsis. Plant Physiol. 2016, 171, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tao, M.; Meng, Y.; Zhu, X.; Qian, L.; Shah, A.; Wang, W.; Cao, S. The role of WRKY47 gene in regulating selenium tolerance in Arabidopsis thaliana. Springer Singap. 2020, 14, 121–129. [Google Scholar] [CrossRef]
- Hou, L.Y.; Ehrlich, M.; Thormhlen, I.; Lehmann, M.; Geigenberger, P. NTRC plays a crucial role in starch metabolism, redox balance, and tomato fruit growth. Plant Physiol. 2019, 181, 976–992. [Google Scholar] [CrossRef] [PubMed]
- Atsuki, S.; Ryuta, T.; Riku, A.; Masao, I.; Satoru, H.; Kaito, K.; Yosuke, T.; Takuya, O.; Tatsuo, K.; Kei, G.; et al. Initial step of selenite reduction via thioredoxin for bacterial selenoprotein biosynthesis. Int. J. Mol. Sci. 2021, 22, 10965. [Google Scholar] [CrossRef] [PubMed]
- Johansen-Leete, J.; Payne, R.J. Selenium is the chalcogen of choice for selective reporting of thioredoxin reductase activity. Chem 2022, 8, 1175–1177. [Google Scholar] [CrossRef]
- Kim, Y.B.; Garbisu, C.; Pickering, I.J.; Prince, R.C.; Buchanan, B.B. Thioredoxin h overexpressed in barley seeds enhances selenite resistance and uptake during germination and early seedling development. Planta 2004, 218, 186–191. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Shi, X.; Li, L. The Effect of Exogenous Selenium Supplementation on the Nutritional Value and Shelf Life of Lettuce. Agronomy 2024, 14, 1380. https://doi.org/10.3390/agronomy14071380
Cheng H, Shi X, Li L. The Effect of Exogenous Selenium Supplementation on the Nutritional Value and Shelf Life of Lettuce. Agronomy. 2024; 14(7):1380. https://doi.org/10.3390/agronomy14071380
Chicago/Turabian StyleCheng, Hua, Xinyu Shi, and Linling Li. 2024. "The Effect of Exogenous Selenium Supplementation on the Nutritional Value and Shelf Life of Lettuce" Agronomy 14, no. 7: 1380. https://doi.org/10.3390/agronomy14071380



