Enhancement of Rhizoma Atractylodis Quality, Soil Nutrients, and Microbial Characters of Vermicompost Preparations from Spent Mushroom and Cow Dung
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant and Soil Sampling
2.3. Plant Analysis
2.4. Soil Physiochemical and Enzymatic Properties Analysis
2.5. DNA Extraction, Quantifications of Gene Abundance, and High-Throughput Sequencing
2.6. Statistical Analyses
3. Results
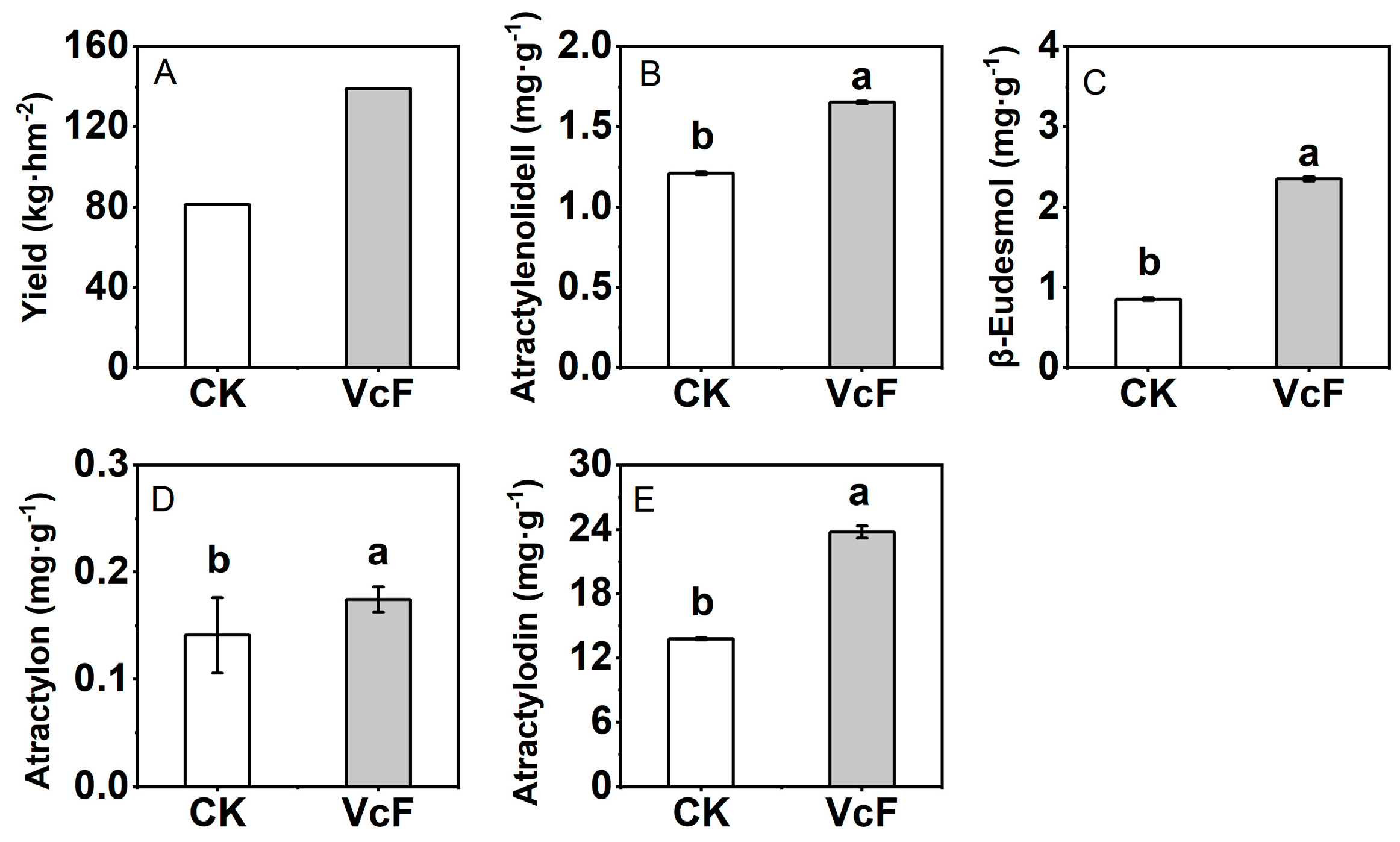
3.1. Effect of Vermicompost on the Yield and Quality of Rhizoma Atractylodis
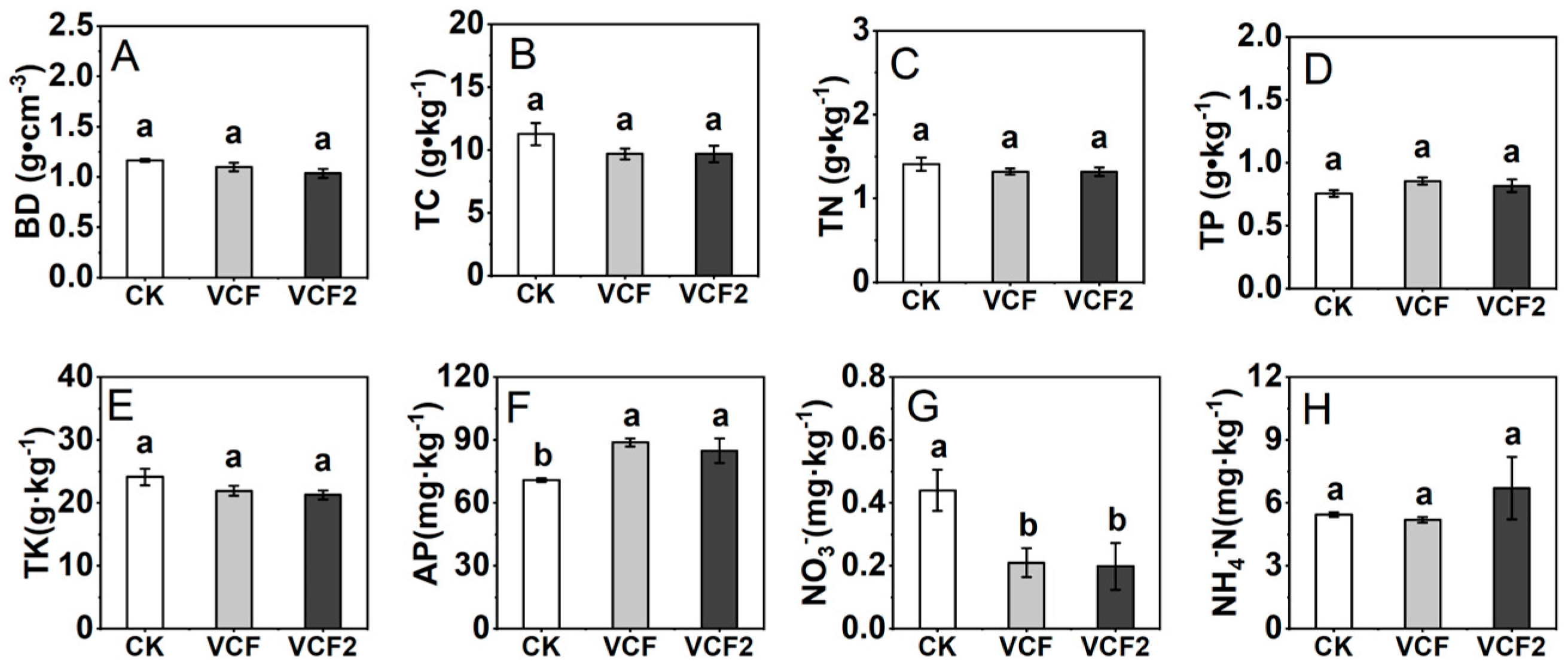
3.2. Effect of Vermicompost Application on Soil Physicochemical Properties and Enzymes Activities
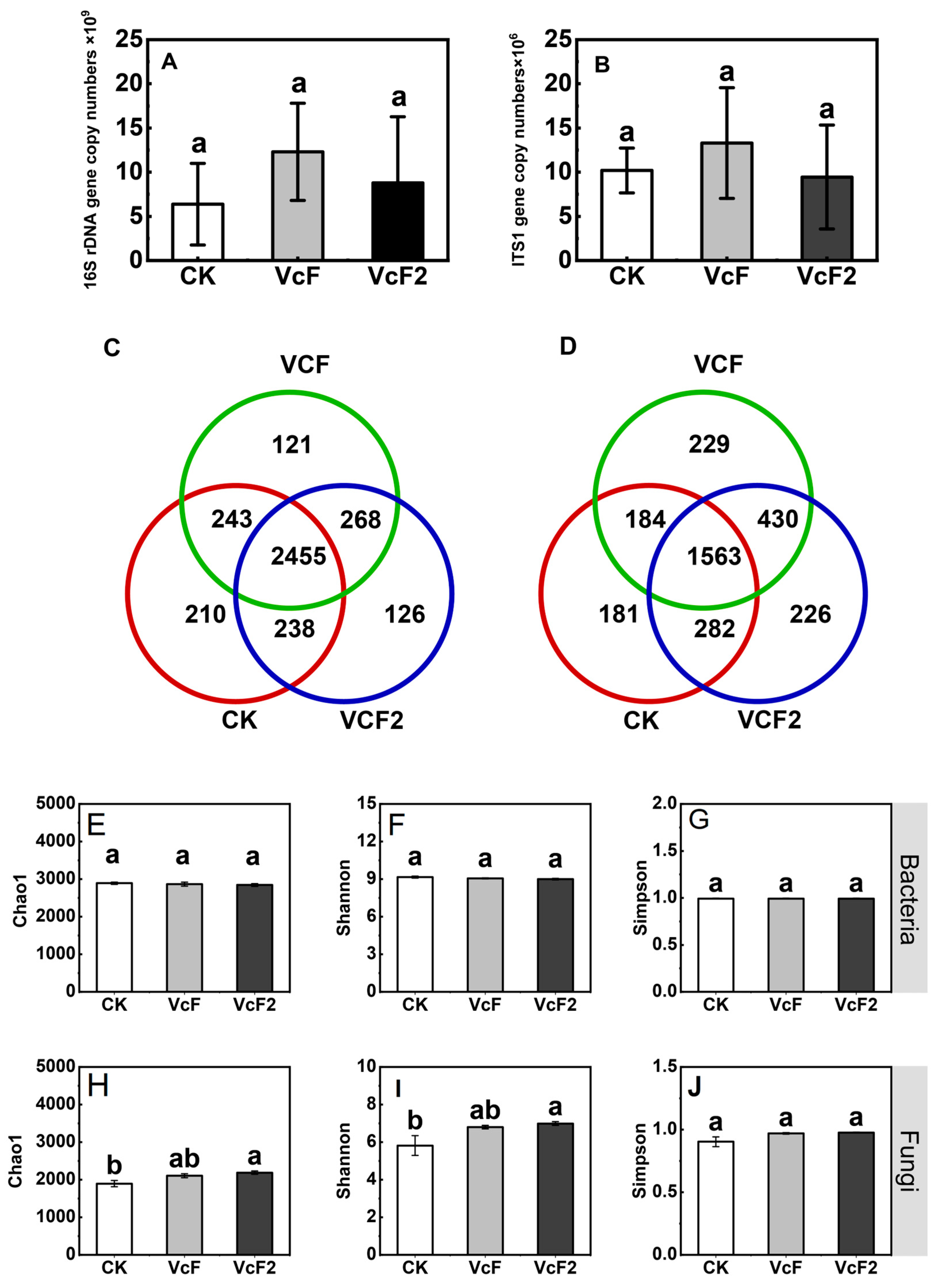
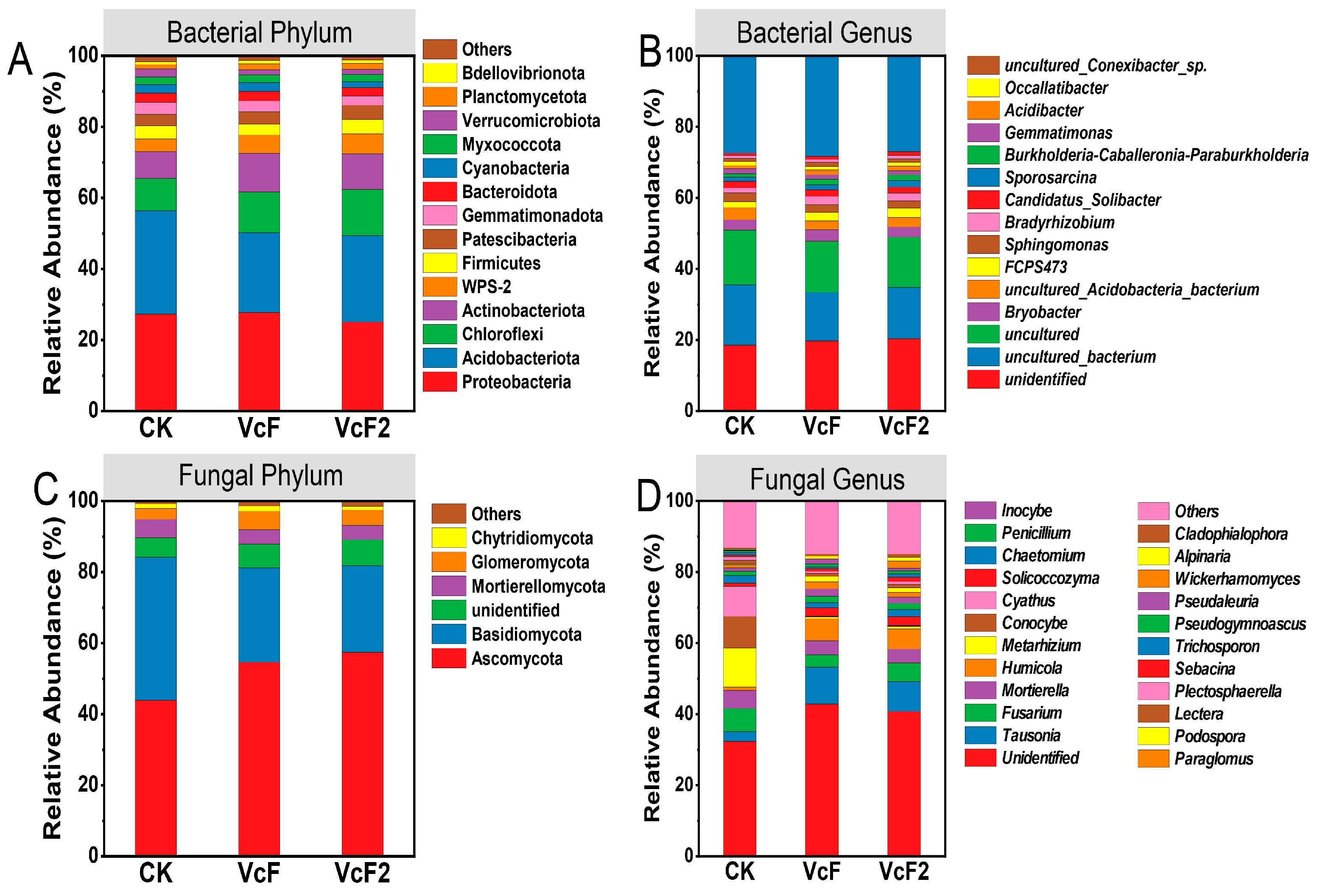
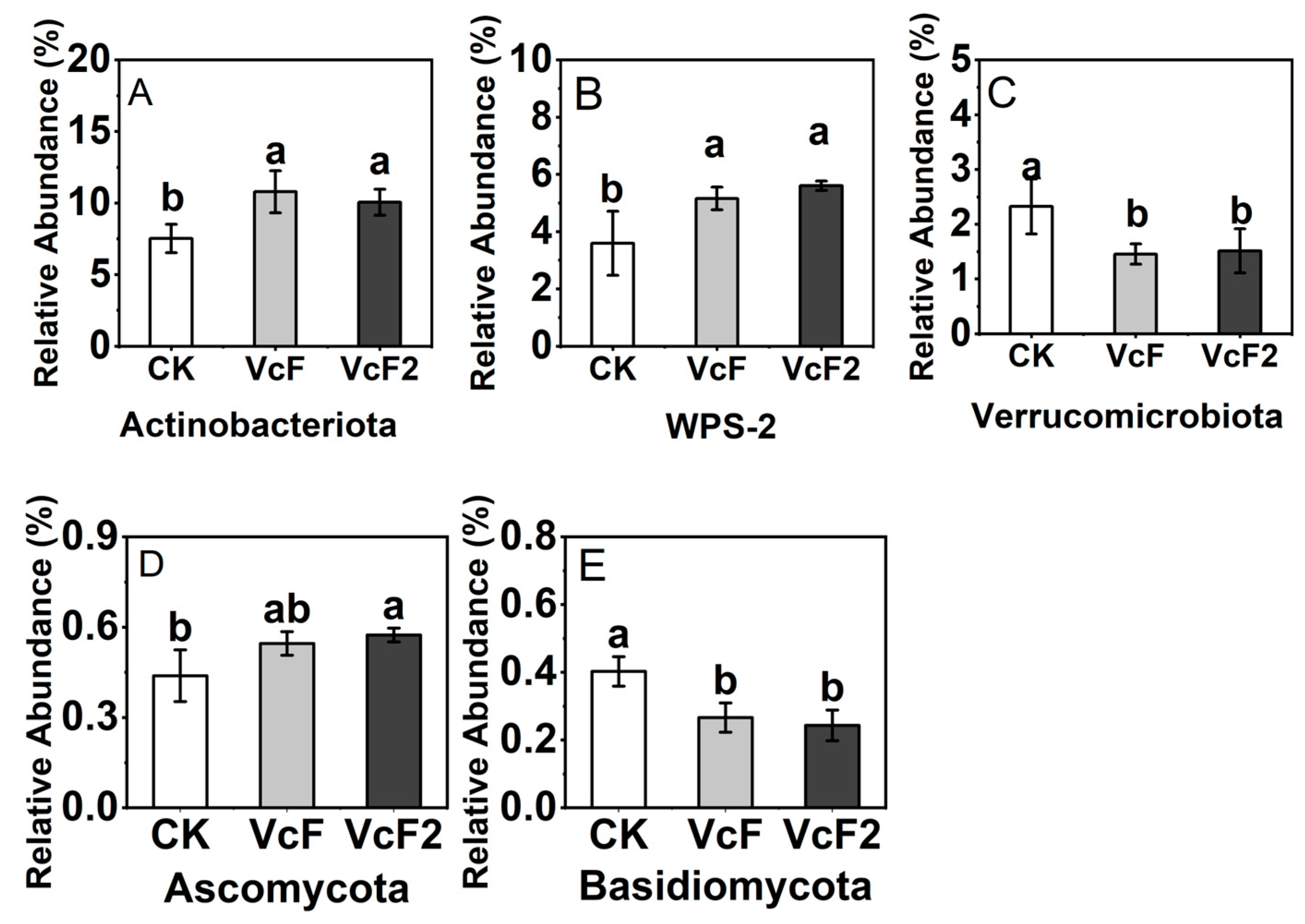
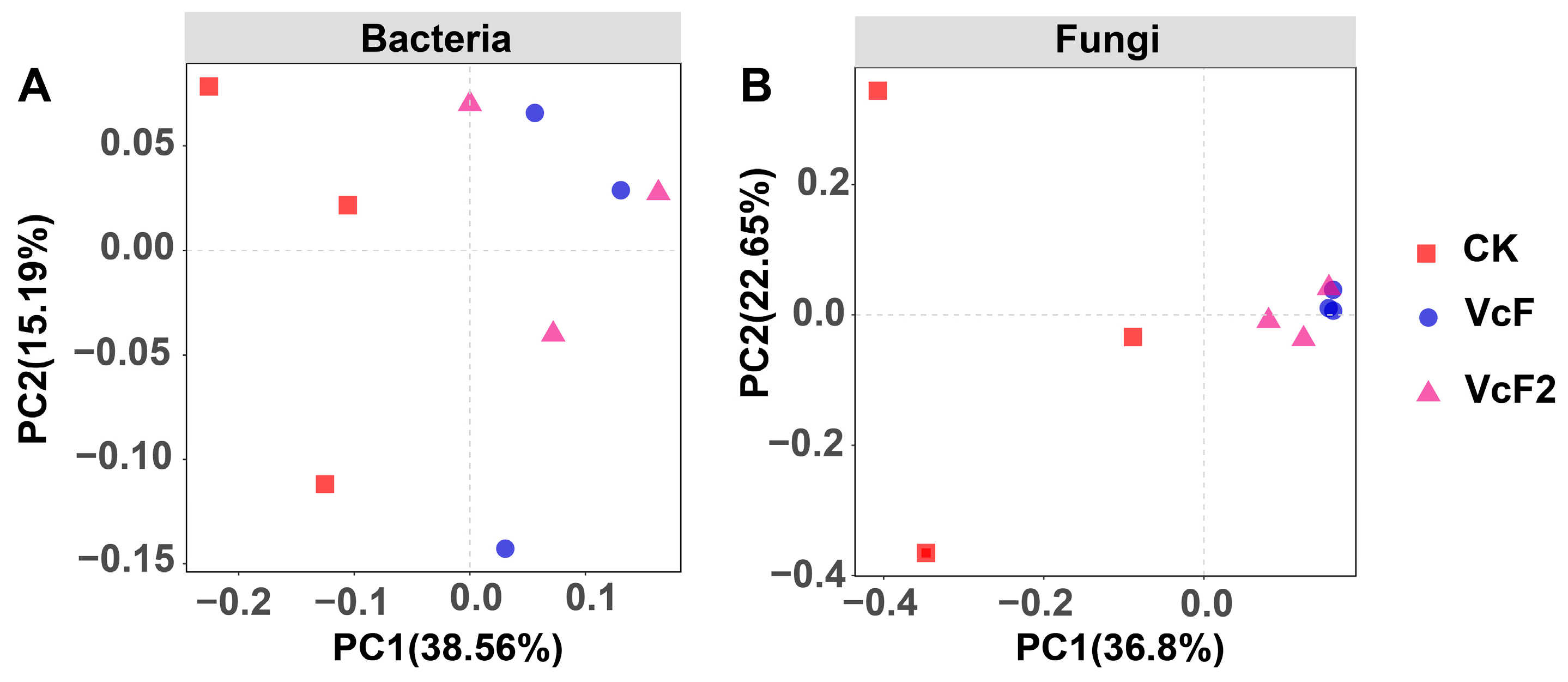
3.3. Effect of Vermicompost on Soil Microbial Quantity and Community Structure
4. Discussion
4.1. Effect of Vermicompost on the Quality of Atractylodes macrocephala
4.2. Effect of Vermicompost on Soil Nutrient Concentration
4.3. Effect of Vermicompost on Soil Microbial Activity, and Community Structure in the Root System of Rhizoma Atractylodis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, D.Q.; Wu, C.R.; Wang, S.Q.; Zhang, Y.L.; Chen, Z.H.; Jiang, N.; Zhang, Y.; Xie, H.T. Vermicompost derived from mushroom residues improves soil C/P cycling, bacterial community, and fungal abundance. Glob. Change Biol. Bioenergy 2023, 15, 1437–1449. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, W.; Luo, L.; Li, Y.; Chen, Z.; Gu, Y.; Chen, Q.; Deng, O.; Xu, X.; Lan, T.; et al. Short-term responses of soil nutrients, heavy metals and microbial community to partial substitution of chemical fertilizer with spent mushroom substrates (SMS). Sci. Total Environ. 2022, 844, 157064. [Google Scholar] [CrossRef] [PubMed]
- Niether, W.; Macholdt, J.; Schulz, F.; Gattinger, A. Yield dynamics of crop rotations respond to farming type and tillage intensity in an organic agricultural long-term experiment over 24 years. Field Crops Res. 2023, 303, 109131. [Google Scholar] [CrossRef]
- Lou, Z.; Sun, Y.; Zhou, X.; Baig, S.A.; Hu, B.; Xu, X. Composition variability of spent mushroom substrates during continuous cultivation, composting process and their effects on mineral nitrogen transformation in soil. Geoderma 2017, 307, 30–37. [Google Scholar] [CrossRef]
- Hu, W.; Di, Q.; Liang, T.; Liu, J.; Zhang, J. Effects of spent mushroom substrate biochar on growth of oyster mushroom (Pleurotus ostreatus). Environ. Technol. Innov. 2022, 28, 102729. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 24. [Google Scholar] [CrossRef]
- Zhang, T.; Hou, Y.; Meng, T.; Ma, Y.; Tan, M.; Zhang, F.; Oenema, O. Replacing synthetic fertilizer by manure requires adjusted technology and incentives: A farm survey across China. Resour. Conserv. Recycl. 2021, 168, 105301. [Google Scholar] [CrossRef]
- López, R.; Antelo, J.; Silva, A.C.; Bento, F.; Fiol, S. Factors that affect physicochemical and acid-base properties of compost and vermicompost and its potential use as a soil amendment. J. Environ. Manag. 2021, 300, 113702. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Song, N. Biochar and vermicompost improve the soil properties and the yield and quality of cucumber (Cucumis sativus L.) grown in plastic shed soil continuously cropped for different years. Agric. Ecosyst. Environ. 2021, 315, 107425. [Google Scholar] [CrossRef]
- Carnimeo, C.; Gelsomino, A.; Cirrottola, G.; Panuccio, M.R.; Loffredo, E. Compost and vermicompost in cucumber rhizosphere promote plant growth and prevent the entry of anthropogenic organic pollutants. Sci. Hortic. 2022, 303, 111250. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Ren, C.; Wang, J.; Wang, C.; Zou, Y.; Wang, X.; Li, G.; Li, Q. Co-composting with cow dung and subsequent vermicomposting improve compost quality of spent mushroom. Bioresour. Technol. 2022, 358, 127386. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, B.; Lee, S.R.; Chang, S.W.; Nguyen, D.D.; Chung, W.J.; Balasubramanian, B.; Mupambwa, H.A.; Arasu, M.V.; Al-Dhabi, N.A.; Sekaran, G. Positive effects of compost and vermicompost produced from tannery waste-animal fleshing on the growth and yield of commercial crop-tomato (Lycopersicon esculentum L.) plant. J. Environ. Manag. 2019, 234, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Gholami, H.; Raouf Fard, F.; Saharkhiz, M.J.; Ghani, A. Yield and physicochemical properties of inulin obtained from Iranian chicory roots under vermicompost and humic acid treatments. Ind. Crops Prod. 2018, 123, 610–616. [Google Scholar] [CrossRef]
- Jami, N.; Rahimi, A.; Naghizadeh, M.; Sedaghati, E. Investigating the use of different levels of Mycorrhiza and Vermicompost on quantitative and qualitative yield of saffron (Crocus sativus L.). Sci. Hortic. 2020, 262, 109027. [Google Scholar] [CrossRef]
- Argüello, C.R. Sector agrícola y política de competencia x1—Agricultural sector and competition policy. Rev. Econ. Inst. 2006, 8, 227–249. [Google Scholar]
- Shi, Y.; Wang, Z.; Wang, Y. Optimizing the amount of pig manure in the vermicomposting of spent mushroom (Lentinula) substrate. PeerJ 2020, 8, e10584. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.T.; Zhu, B.; Yao, Z.; Wu, J.; Chen, Z.; Ali, Z.; Tang, J.L. Impacts of vermicompost application on crop yield, ammonia volatilization and greenhouse gases emission on upland in Southwest China. Sci. Total Environ. 2023, 860, 160479. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; González, J.L. Application of Two Vermicomposts on a Rice Crop: Effects on Soil Biological Properties and Rice Quality and Yield. Agron. J. 2009, 101, 336–344. [Google Scholar] [CrossRef]
- Song, X.; Liu, M.; Wu, D.; Griffiths, B.S.; Jiao, J.; Li, H.; Hu, F. Interaction matters: Synergy between vermicompost and PGPR agents improves soil quality, crop quality and crop yield in the field. Appl. Soil Ecol. 2015, 89, 25–34. [Google Scholar] [CrossRef]
- Soobhany, N.; Mohee, R.; Garg, V.K. Inactivation of bacterial pathogenic load in compost against vermicompost of organic solid waste aiming to achieve sanitation goals: A review. Waste Manag. 2017, 64, 51–62. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, J.; Zhao, R.; Dai, H.; Zhang, Z. Application of vermicompost improves strawberry growth and quality through increased photosynthesis rate, free radical scavenging and soil enzymatic activity. Sci. Hortic. 2018, 233, 132–140. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Li, D.; Tang, B.; Man, S.; Jia, Y.; Xu, H. Vermicompost and biochar as bio-conditioners to immobilize heavy metal and improve soil fertility on cadmium contaminated soil under acid rain stress. Sci. Total Environ. 2018, 621, 1057–1065. [Google Scholar] [CrossRef]
- Akinnuoye-Adelabu, D.B.; Steenhuisen, S.; Bredenhand, E. Improving pea quality with vermicompost tea and aqueous biochar: Prospects for sustainable farming in Southern Africa. S. Afr. J. Bot. 2019, 123, 278–285. [Google Scholar] [CrossRef]
- Hřebečková, T.; Wiesnerová, L.; Hanč, A. Change in agrochemical and biochemical parameters during the laboratory vermicomposting of spent mushroom substrate after cultivation of Pleurotus ostreatus. Sci. Total Environ. 2020, 739, 140085. [Google Scholar] [CrossRef]
- Romero, E.; Fernández-Bayo, J.; Díaz, J.M.C.; Nogales, R. Enzyme activities and diuron persistence in soil amended with vermicompost derived from spent grape marc and treated with urea. Appl. Soil Ecol. 2010, 44, 198–204. [Google Scholar] [CrossRef]
- Makkar, C.; Singh, J.; Parkash, C.; Singh, S.; Vig, A.P.; Dhaliwal, S.S. Vermicompost acts as bio-modulator for plants under stress and non-stress conditions. Environ. Dev. Sustain. 2023, 25, 2006–2057. [Google Scholar] [CrossRef]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. SpringerPlus 2012, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Li, X.; Jiao, J.; Ji, G.; Wu, J.; Hu, F.; Li, H. Biocontrol potential of vermicompost through antifungal volatiles produced by indigenous bacteria. Biol. Control 2017, 112, 49–54. [Google Scholar] [CrossRef]
- Bailly, C. Atractylenolides, essential components of Atractylodes-based traditional herbal medicines: Antioxidant, anti-inflammatory and anticancer properties. Eur. J. Pharmacol. 2021, 891, 173735. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Q.; Cui, G.; Zhang, X.; Lyu, C.; Sun, J.; Gao, W.; Huang, L.; Guo, L. Recent progress and ongoing challenges in Rhizoma atractylodis research: Biogeography, biosynthesis, quality formation and control. Med. Plant Biol. 2023, 2, 19. [Google Scholar] [CrossRef]
- Zhong, X.-H.; Shi, W.-Y.; Ma, A.-T.; Gong, X.-C.; Zhai, X.-H.; Zhang, T.; Wang, X.-D. Effects of Radix scutellariae and Rhizoma atractylodis on LPS-Induced Abortion and the Uterine IL-10 Contents in Mice. Am. J. Chin. Med. 2008, 36, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, G.; Bhushan, B.; Kumar, S.; Dhurandhar, Y.; Dixit, P. A comprehensive pharmacological review of Atractylodes Macrocephala: Traditional uses, phytochemistry, pharmacokinetics, and therapeutic potential. Pharmacol. Res.—Mod. Chin. Med. 2024, 10, 100394. [Google Scholar] [CrossRef]
- Acharya, B.; Chaijaroenkul, W.; Na-Bangchang, K. Therapeutic potential and pharmacological activities of β-eudesmol. Chem. Biol. Drug Des. 2021, 97, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Chou, J.-Y.; Li, S.-M.; Fu, X.-Q.; Yin, C.; Guo, H.; Amin, A.; Chou, G.-X.; Yu, Z.-L. An ethanol extract of the rhizome of Atractylodes chinensis exerts anti-gastritis activities and inhibits Akt/NF-κB signaling. J. Ethnopharmacol. 2019, 228, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Weng, L.; Xiao, C.; Jiang, Y.; Su, Y.; Liu, Z.; Wu, X. Quality Analysis of Atractylodes chinensis with Different Growth Years by HPLC-QAMS Combined with Color Difference Principle. China Pharm. 2020, 12, 1314–1319. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Wiley: Hoboken, NJ, USA, 1983; Volume 9, pp. 539–579. [Google Scholar]
- Kuo, S. Chapter 32. Total Organic Phosphorus. In Method of Soil Science Analysis; Wiley: Hoboken, NJ, USA, 1996; Volume 3, pp. 874–876. [Google Scholar]
- Zhao, Z.-Q.; Zhu, Y.-G.; Li, H.-Y.; Smith, S.E.; Smith, F.A. Effects of forms and rates of potassium fertilizers on cadmium uptake by two cultivars of spring wheat (Triticum aestivum, L.). Environ. Int. 2004, 29, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-G.; Zhu, J.-P.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.J.; Mitchell, M.J.; Santos, A.; Destaffen, P. Comparison of two methods for measuring ammonium in solution samples. Commun. Soil Sci. Plant Anal. 1989, 20, 1131–1144. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Antibus, R.K.; Linkins, A.E. An enzymic approach to the analysis of microbial activity during plant litter decomposition. Agric. Ecosyst. Environ. 1991, 34, 43–54. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Bai, X.; Dippold, M.A.; An, S.; Wang, B.; Zhang, H.; Loeppmann, S. Extracellular enzyme activity and stoichiometry: The effect of soil microbial element limitation during leaf litter decomposition. Ecol. Indic. 2021, 121, 107200. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Maiti, S.K. Different Soil Factors Influencing Dehydrogenase Activity in Mine Degraded Lands—State-of-Art Review. Water Air Soil Pollut. 2021, 232, 360. [Google Scholar] [CrossRef]
- Suthar, S. Impact of vermicompost and composted farmyard manure on growth and yield of garlic (Allium stivum L.) field crop. Int. J. Plant Prod. 2009, 3, 1735–6814. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Golovko, T.K. Plant Anthocyanins: Structure, Biosynthesis Regulation, Functions, and Ecology. Russ. J. Plant Physiol. 2023, 70, 161. [Google Scholar] [CrossRef]
- Ngo, P.-T.; Rumpel, C.; Doan, T.-T.; Jouquet, P. The effect of earthworms on carbon storage and soil organic matter composition in tropical soil amended with compost and vermicompost. Soil Biol. Biochem. 2012, 50, 214–220. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Fertilizers and fertilization strategies mitigating soil factors constraining efficiency of nitrogen in plant production. Plants 2022, 11, 1855. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef]
- Costa, R.M.; Araujo, E.M.B.; Silva, D.E.O.; Rocha, S.M.B.; Bonifacio, A.; Sousa, R.S.; Pereira, A.P.d.A.; de Medeiros, E.V.; Sagrilo, E.; de Oliveira Junior, J.O.L.; et al. Seasonal responses of soil microbial biomass C and enzymatic activity comparing no-tillage and integrated crop-livestock systems. Eur. J. Soil Biol. 2024, 121, 103628. [Google Scholar] [CrossRef]
- de Medeiros, E.V.; de Oliveira Silva, É.; Duda, G.P.; Andrade Lira Junior, M.; dos Santos, U.J.; Hammecker, C.; da Costa, D.P.; Araujo, F.F.; de Araujo Pereira, A.P.; Mendes, L.W.; et al. Microbial enzymatic stoichiometry and the acquisition of C, N, and P in soils under different land-use types in Brazilian semiarid. Soil Ecol. Lett. 2023, 5, 220159. [Google Scholar] [CrossRef]
- Zareen, S.; Choudhary, M.I.; Akhtar, M.N.; Khan, S.N. α-Glucosidase inhibitory activity of triterpenoids from Cichorium intybus. J. Nat. Prod. 2008, 71, 910–913. [Google Scholar]
- Islas-Espinoza, M.; Solís-Mejía, L.; Esteller, M.V. Phosphorus release kinetics in a soil amended with biosolids and vermicompost. Environ. Earth Sci. 2014, 71, 1441–1451. [Google Scholar] [CrossRef]
- Anastasi, A.; Varese, G.C.; Voyron, S.; Scannerini, S.; Marchisio, V.F. Characterization of Fungal Biodiversity In Compost and Vermicompost. Compos. Sci. Util. 2004, 12, 185–191. [Google Scholar] [CrossRef]
- Frey, S.D. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Myo, E.M.; Boutaj, H.; Sierra, B.E.G.; et al. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere 2022, 32, 149–170. [Google Scholar] [CrossRef]
- Ji, H.; Tan, D.; Chen, Y.; Cheng, Z.; Zhao, J.; Lin, M. Effects of different manganese sources on nutrient digestibility, fecal bacterial community, and mineral excretion of weaning dairy calves. Front. Microbiol. 2023, 14, 1163468. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Phour, M.; Choudhary, S.R.; Chaudhary, D. Phosphorus Cycling: Prospects of Using Rhizosphere Microorganisms for Improving Phosphorus Nutrition of Plants. In Geomicrobiology and Biogeochemistry; Parmar, N., Singh, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 199–237. [Google Scholar]
- Bromfield, E.S.; Cloutier, S.; Tambong, J.T.; Thi, T.V.T. Soybeans inoculated with root zone soils of Canadian native legumes harbour diverse and novel Bradyrhizobium spp. that possess agricultural potential. Syst. Appl. Microbiol. 2017, 40, 440–447. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Wang, S.; Zhang, Y.; Chen, B.; Li, P.; Zhang, X.; Wang, Y.; Zhao, M.; Zhang, Y.; Xie, H. Enhancement of Rhizoma Atractylodis Quality, Soil Nutrients, and Microbial Characters of Vermicompost Preparations from Spent Mushroom and Cow Dung. Agronomy 2024, 14, 1384. https://doi.org/10.3390/agronomy14071384
Sun B, Wang S, Zhang Y, Chen B, Li P, Zhang X, Wang Y, Zhao M, Zhang Y, Xie H. Enhancement of Rhizoma Atractylodis Quality, Soil Nutrients, and Microbial Characters of Vermicompost Preparations from Spent Mushroom and Cow Dung. Agronomy. 2024; 14(7):1384. https://doi.org/10.3390/agronomy14071384
Chicago/Turabian StyleSun, Baoyi, Shuqiang Wang, Ying Zhang, Bin Chen, Pengcheng Li, Xianying Zhang, Yonghuan Wang, Mingyi Zhao, Yulan Zhang, and Hongtu Xie. 2024. "Enhancement of Rhizoma Atractylodis Quality, Soil Nutrients, and Microbial Characters of Vermicompost Preparations from Spent Mushroom and Cow Dung" Agronomy 14, no. 7: 1384. https://doi.org/10.3390/agronomy14071384
APA StyleSun, B., Wang, S., Zhang, Y., Chen, B., Li, P., Zhang, X., Wang, Y., Zhao, M., Zhang, Y., & Xie, H. (2024). Enhancement of Rhizoma Atractylodis Quality, Soil Nutrients, and Microbial Characters of Vermicompost Preparations from Spent Mushroom and Cow Dung. Agronomy, 14(7), 1384. https://doi.org/10.3390/agronomy14071384





