Organic Acid-Based Hemicellulose Fractionation and Cellulosic Ethanol Potential of Five Miscanthus Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Conditions and Sample Collection
2.2. Fiber Analysis
2.3. Pretreatment Condition and Sugar and Inhibitor Analysis
2.4. Enzymatic Hydrolysis
2.5. Ethanol Production Potential
2.6. Data Analysis
3. Results
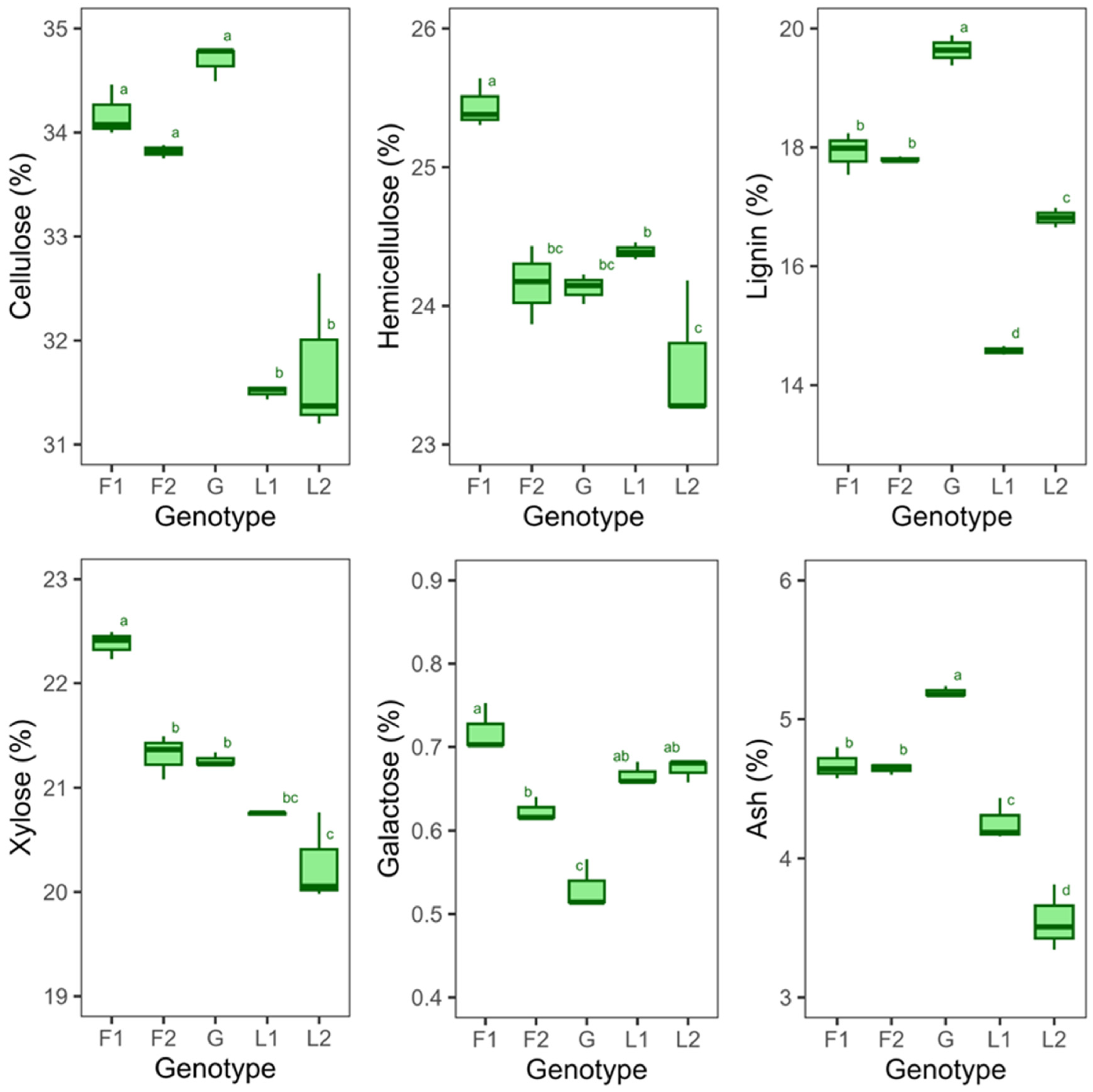
3.1. Characterization of Untreated Feedstock
3.2. Pretreatment of Feedstock
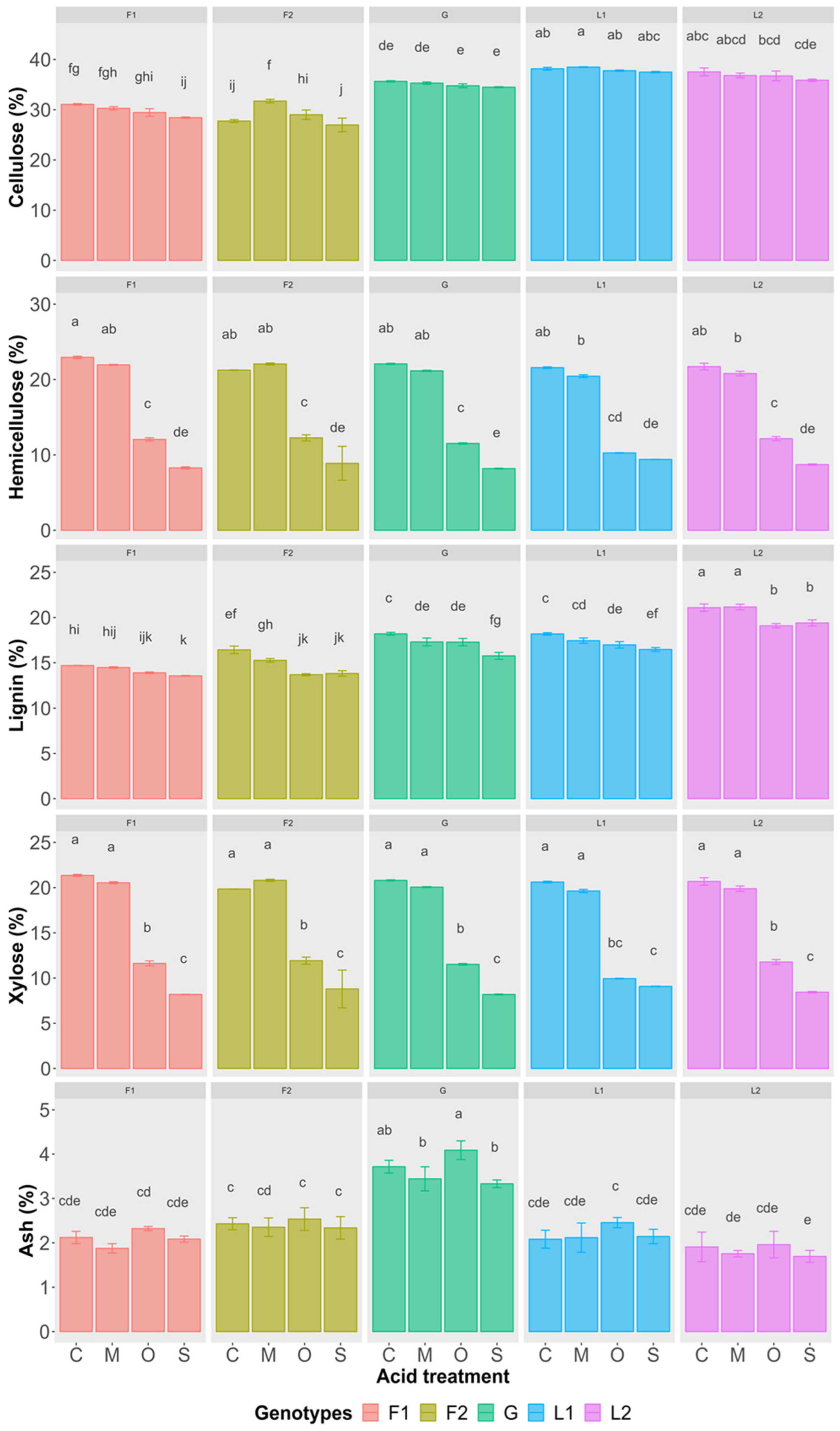
3.2.1. Solid Residue
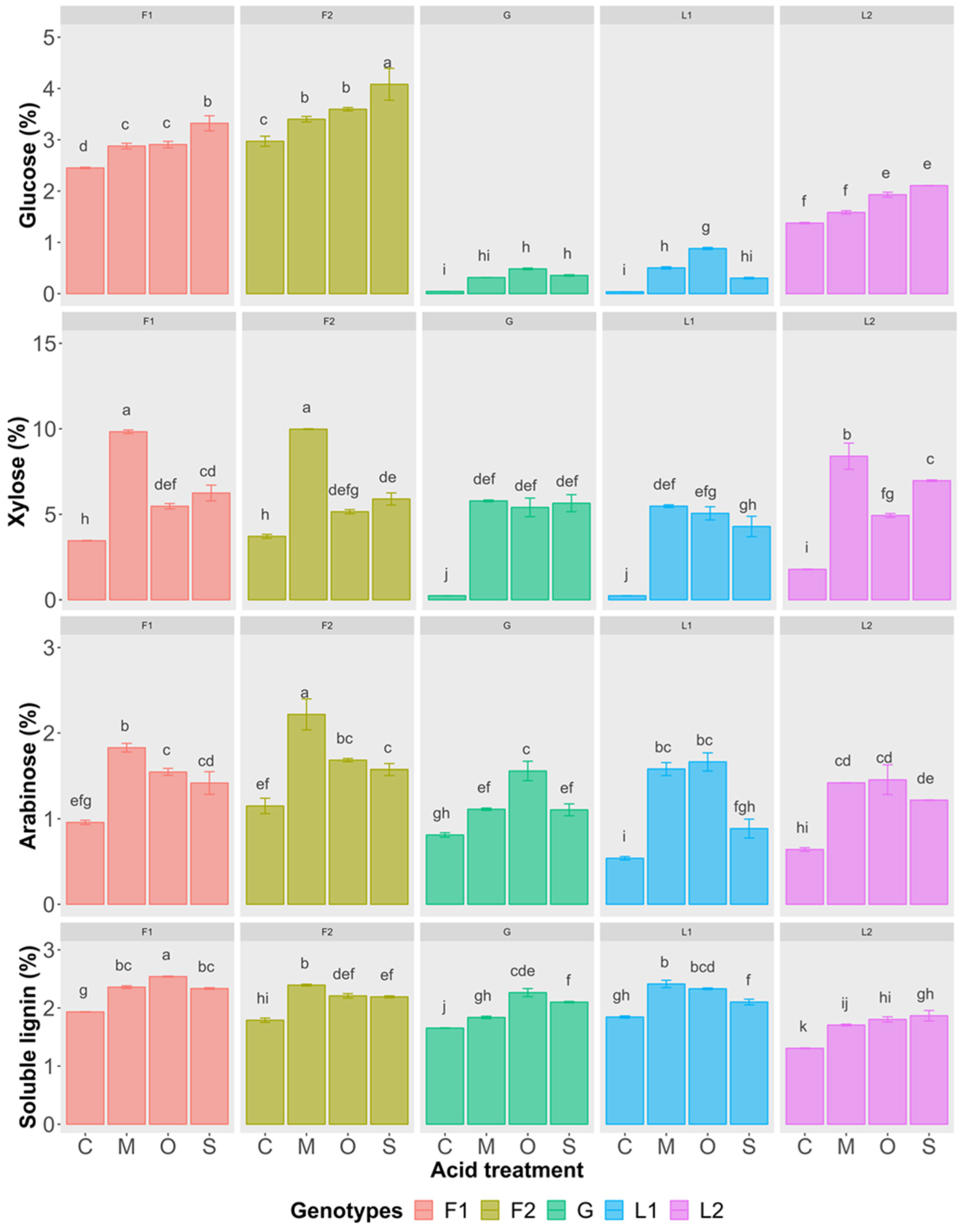
3.2.2. Liquid Hydrolysate
3.2.3. Inhibitor Formation in Hydrolysate
3.3. Enzymatic Hydrolysis
3.4. Relationship between Compositional Traits of Cell Wall
4. Discussion
4.1. Variation in Lignocellulosic Components among Genotypes
4.2. Comparison and Screening of Efficient Acid Pretreatment
4.3. Pretreatment and Response of Genotypes
4.4. Pretreatment and Inhibitor Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Popp, J.; Kovács, S.; Oláh, J.; Divéki, Z.; Balázs, E. Bioeconomy: Biomass and Biomass-Based Energy Supply and Demand. New Biotechnol. 2021, 60, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S. IPCC 2022: Climate Change 2022: Impacts, Adaptation and Vulnerability; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change 2022; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging Renewable and Sustainable Energy Technologies: State of the Art. Renew. Sustain. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-Lignin: Supply, Extraction Processes and Use as Bio-Based Material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Ullah, K.; Kumar Sharma, V.; Dhingra, S.; Braccio, G.; Ahmad, M.; Sofia, S. Assessing the Lignocellulosic Biomass Resources Potential in Developing Countries: A Critical Review. Renew. Sustain. Energy Rev. 2015, 51, 682–698. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal Agricultural Land Low-Input Systems for Biomass Production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef]
- Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Von Cossel, V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; et al. Prospects of Bioenergy Cropping Systems for A More Social-Ecologically Sound Bioeconomy. Agronomy 2019, 9, 605. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in Bioconversion of Lignocellulose: Biofuels, Platform Chemicals & Biorefinery Concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Hastings, A.; von Cossel, M.; Murphy-Bokern, D.; McCalmont, J.; Whitaker, J.; Alexopoulou, E.; Amaducci, S.; Andronic, L.; Ashman, C.; et al. Perennial Biomass Cropping and Use: Shaping the Policy Ecosystem in European Countries. GCB Bioenergy 2023, 15, 538–558. [Google Scholar] [CrossRef] [PubMed]
- Magenau, E.; Clifton–Brown, J.; Awty–Carroll, D.; Ashman, C.; Ferrarini, A.; Kontek, M.; Martani, E.; Roderick, K.; Amaducci, S.; Davey, C.; et al. Site Impacts Nutrient Translocation Efficiency in Intraspecies and Interspecies Miscanthus Hybrids on Marginal Lands. GCB Bioenergy 2022, 14, 1035–1054. [Google Scholar] [CrossRef]
- Stefanoni, W.; Latterini, F.; Pari, L. Perennial Grass Species for Bioenergy Production: The State of the Art in Mechanical Harvesting. Energies 2023, 16, 2303. [Google Scholar] [CrossRef]
- Yu, Y.-C. Research Progress and Comprehensive Utilization of Miscanthus. Chin. Bull. Life Sci. 2014, 26, 474–480. [Google Scholar]
- Alexopoulou, E.; Zanetti, F.; Scordia, D.; Zegada-Lizarazu, W.; Christou, M.; Testa, G.; Cosentino, S.L.; Monti, A. Long-Term Yields of Switchgrass, Giant Reed, and Miscanthus in the Mediterranean Basin. Bioenerg. Res. 2015, 8, 1492–1499. [Google Scholar] [CrossRef]
- Winkler, B.; Mangold, A.; von Cossel, M.; Clifton-Brown, J.; Pogrzeba, M.; Lewandowski, I.; Iqbal, Y.; Kiesel, A. Implementing Miscanthus into Farming Systems: A Review of Agronomic Practices, Capital and Labour Demand. Renew. Sustain. Energy Rev. 2020, 132, 110053. [Google Scholar] [CrossRef]
- Kiesel, A.; Wagner, M.; Lewandowski, I. Environmental Performance of Miscanthus, Switchgrass and Maize: Can C4 Perennials Increase the Sustainability of Biogas Production? Sustainability 2016, 9, 5. [Google Scholar] [CrossRef]
- Martani, E.; Ferrarini, A.; Hastings, A.; Amaducci, S. Soil Organic Carbon Significantly Increases When Perennial Biomass Plantations Are Reverted Back to Annual Arable Crops. Agronomy 2023, 13, 447. [Google Scholar] [CrossRef]
- Grzegórska, A.; Czaplicka, N.; Antonkiewicz, J.; Rybarczyk, P.; Baran, A.; Dobrzyński, K.; Zabrocki, D.; Rogala, A. Remediation of Soils on Municipal Rendering Plant Territories Using Miscanthus × Giganteus. Env. Sci. Pollut. Res. 2023, 30, 22305–22318. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xu, Y.; Xue, S.; Li, J.; Yang, Y.; Yi, Z.; Fu, T. Effects of Soil Physics, Chemistry, and Microbiology on Soil Carbon Sequestration in Infertile Red Soils after Long-Term Cultivation of Perennial Grasses. GCB Bioenergy 2023, 15, 239–253. [Google Scholar] [CrossRef]
- Zhao, F. The Construction of Efficient Miscanthus Lignocellulose Decomposer Complex and Research of Its Degradation Characteristics; Hunan Agricultural University: Changsha, China, 2013. [Google Scholar]
- Kiesel, A. The Potential of Miscanthus as Biogas Feedstock; University of Hohenheim: Stuttgart, Germany, 2020. [Google Scholar]
- Xu, Q.; Wu, S.; Fu, T.; Xu, Y.; Yang, S.; Li, M.; Yi, Z.; Xue, S. Unlocking the Potential of Dongting Lake-Grown Miscanthus Lutarioriparius Biomass: A Comprehensive Quality Analysis and Bioproduct Application Study. Sci. Total Environ. 2023, 896, 165276. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Lewandowski, I.; Wang, X.; Yi, Z. Assessment of the Production Potentials of Miscanthus on Marginal Land in China. Renew. Sustain. Energy Rev. 2016, 54, 932–943. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of Chemical Pretreatment for Bioconversion of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Yang, H.; Shi, Z.; Xu, G.; Qin, Y.; Deng, J.; Yang, J. Bioethanol Production from Bamboo with Alkali-Catalyzed Liquid Hot Water Pretreatment. Bioresour. Technol. 2019, 274, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wyman, C.E. Pretreatment: The Key to Unlocking Low-Cost Cellulosic Ethanol. Biofuels Bioprod. Biorefining 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, e787532. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, P.F.; Huijgen, W.; Bermudez, L.; Bakker, R. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass; Wageningen UR-Food & Biobased Research: Wageningen, The Netherlands, 2010. [Google Scholar]
- Kootstra, A.M.J.; Beeftink, H.H.; Scott, E.L.; Sanders, J.P.M. Comparison of Dilute Mineral and Organic Acid Pretreatment for Enzymatic Hydrolysis of Wheat Straw. Biochem. Eng. J. 2009, 46, 126–131. [Google Scholar] [CrossRef]
- Kwon, O.-M.; Kim, D.-H.; Kim, S.-K.; Jeong, G.-T. Production of Sugars from Macro-Algae Gracilaria Verrucosa Using Combined Process of Citric Acid-Catalyzed Pretreatment and Enzymatic Hydrolysis. Algal Res. 2016, 13, 293–297. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, E.; Ma, F.; Jiang, K. An Integrated Biorefinery Process for Co-Production of Xylose and Glucose Using Maleic Acid as Efficient Catalyst. Bioresour. Technol. 2021, 325, 124698. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, S.K.; Thimappa, G.S.; Nataraj, L.K.; Dasgupta, P. Optimization of Oxalic Acid Pre-Treatment and Enzymatic Saccharification in Typha Latifolia for Production of Reducing Sugar. J. Genet. Eng. Biotechnol. 2020, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Research on Biomass Pretreatment and Enzymatic Hydrolysis Characteristics Based on Organic Acid System; Qilu University of Technology: Jinan, China, 2021; p. 77. [Google Scholar]
- Energy Industry Standard of the People’s Republic of China, 2017. [WWW Document]. Available online: https://hbba.sacinfo.org.cn/stdDetail/dc2b59364441ef0e1ff7ea0224e079d7 (accessed on 20 February 2022).
- Pereira, M.G.; Hamerski, F.; Andrade, E.F.; Scheer, A.d.P.; Corazza, M.L. Assessment of Subcritical Propane, Ultrasound-Assisted and Soxhlet Extraction of Oil from Sweet Passion Fruit (Passiflora alata Curtis) Seeds. J. Supercrit. Fluids 2017, 128, 338–348. [Google Scholar] [CrossRef]
- Badger, P.C. Ethanol from Cellulose: A General Review. Trends New Crop. New Uses 2002, 14, 17–21. [Google Scholar]
- Zheng, C.; Xiao, L.; Iqbal, Y.; Sun, G.; Feng, H.; Liu, F.; Duan, M.; Yi, Z. Miscanthus Interspecific Hybrids Exceed the Biomass Yield and Quality of Their Parents in the Saline–Alkaline Yellow River Delta. Food Energy Secur. 2022, 11, e347. [Google Scholar] [CrossRef]
- Scordia, D.; Testa, G.; Cosentino, S.L. Perennial grasses as lignocellulosic feedstock for second-generation bioeth-anol production in Mediterranean environment. Ital. J. Agron. 2014, 9, 84–92. [Google Scholar] [CrossRef]
- Xu, P.; Cheng, S.; Han, Y.; Zhao, D.; Li, H.; Wang, Y.; Zhang, G.; Chen, C. Natural Variation of Lignocellulosic Components in Miscanthus Biomass in China. Front. Chem. 2020, 8, 595143. [Google Scholar] [CrossRef] [PubMed]
- Iacono, R.; Slavov, G.T.; Davey, C.L.; Clifton-Brown, J.; Allison, G.; Bosch, M. Variability of Cell Wall Recalcitrance and Composition in Genotypes of Miscanthus from Different Genetic Groups and Geographical Origin. Front. Plant Sci. 2023, 14, 1155188. [Google Scholar] [CrossRef] [PubMed]
- Gismatulina, Y.A.; Budaeva, V.V.; Kortusov, A.N.; Kashcheyeva, E.I.; Gladysheva, E.K.; Mironova, G.F.; Skiba, E.A.; Shavyrkina, N.A.; Korchagina, A.A.; Zolotukhin, V.N. Evaluation of Chemical Composition of Miscanthus × Giganteus Raised in Different Climate Regions in Russia. Plants 2022, 11, 2791. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.; Lewandowski, I.; Möhring, J.; Clifton-Brown, J.; Krzyżak, J.; Mos, M.; Pogrzeba, M.; Kiesel, A. Harvest Date and Leaf: Stem Ratio Determine Methane Hectare Yield of Miscanthus Biomass. GCB Bioenergy 2019, 11, 21–33. [Google Scholar] [CrossRef]
- van der Weijde, T.; Kiesel, A.; Iqbal, Y.; Muylle, H.; Dolstra, O.; Visser, R.G.; Lewandowski, I.; Trindade, L.M. Evaluation of Miscanthus Sinensis Biomass Quality as Feedstock for Conversion into Different Bioenergy Products. GCB Bioenergy 2016, 9, 176–190. [Google Scholar] [CrossRef]
- da Costa, R.M.F.; Pattathil, S.; Avci, U.; Winters, A.; Hahn, M.G.; Bosch, M. Desirable Plant Cell Wall Traits for Higher-Quality Miscanthus Lignocellulosic Biomass. Biotechnol. Biofuels 2019, 12, 85. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, X.; Ling, Z.; Sun, R.-C.; Xu, F. Tissue Specific Response of Miscanthus×giganteus to Dilute Acid Pretreatment for Enhancing Cellulose Digestibility. Carbohydr. Polym. 2016, 154, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijde, T.; Alvim Kamei, C.L.; Torres, A.F.; Vermerris, W.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. The Potential of C4 Grasses for Cellulosic Biofuel Production. Front. Plant Sci. 2013, 4, 107. [Google Scholar] [CrossRef]
- Le Ngoc Huyen, T.; Rémond, C.; Dheilly, R.M.; Chabbert, B. Effect of Harvesting Date on the Composition and Saccharification of Miscanthus x Giganteus. Bioresour.Technol. 2010, 101, 8224–8231. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Bergs, M.; Völkering, G.; Kraska, T.; Pude, R.; Do, X.T.; Kusch, P.; Monakhova, Y.; Konow, C.; Schulze, M. Miscanthus x Giganteus Stem Versus Leaf-Derived Lignins Differing in Monolignol Ratio and Linkage. Int. J. Mol. Sci. 2019, 20, 1200. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, J.; Sattler, M.; Iqbal, Y.; Lewandowski, I.; Bunzel, M. Characterization of Miscanthus Cell Wall Polymers. Glob. Chang. Biol. Bioenergy 2019, 11, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose Bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Zhang, X.; Lin, Q.; Xin, F.; Sun, R.; Wang, X.; Ren, J. A New Approach to Recycle Oxalic Acid during Lignocellulose Pretreatment for Xylose Production. Biotechnol. Biofuels 2018, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Gönen, Ç.; Deveci, E.Ü.; Akter Önal, N. Evaluation of Biomass Pretreatment to Optimize Process Factors for Different Organic Acids via Box–Behnken RSM Method. J. Mater. Cycles Waste Manag. 2021, 23, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Rodríguez, Y.F.; Salas-Calderón, K.T.; Orozco-Blanco, D.A.; Gentile, P.; Girón-Hernández, J. Cocoa Pod Husk: A High-Pectin Source with Applications in the Food and Biomedical Fields. ChemBioEng Rev. 2022, 9, 462–474. [Google Scholar] [CrossRef]
- Huang, L.-Z.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9, 690773. [Google Scholar] [CrossRef]
- van der Cruijsen, K.; Al Hassan, M.; van Erven, G.; Dolstra, O.; Trindade, L.M. Breeding Targets to Improve Biomass Quality in Miscanthus. Molecules 2021, 26, 254. [Google Scholar] [CrossRef] [PubMed]
- Valladares-Diestra, K.K.; Porto de Souza Vandenberghe, L.; Zevallos Torres, L.A.; Zandoná Filho, A.; Lorenci Woiciechowski, A.; Ricardo Soccol, C. Citric Acid Assisted Hydrothermal Pretreatment for the Extraction of Pectin and Xylooligosaccharides Production from Cocoa Pod Husks. Bioresour. Technol. 2022, 343, 126074. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.M.; Pattathil, S.; Avci, U.; Lee, S.J.; Hazen, S.P.; Winters, A.; Hahn, M.G.; Bosch, M. A Cell Wall Reference Profile for Miscanthus Bioenergy Crops Highlights Compositional and Structural Variations Associated with Development and Organ Origin. New Phytol. 2017, 213, 1710–1725. [Google Scholar] [CrossRef] [PubMed]
- Thielemans, K.; De Bondt, Y.; Comer, L.; Raes, J.; Everaert, N.; Sels, B.F.; Courtin, C.M. Decreasing the Crystallinity and Degree of Polymerization of Cellulose Increases Its Susceptibility to Enzymatic Hydrolysis and Fermentation by Colon Microbiota. Foods 2023, 12, 1100. [Google Scholar] [CrossRef]
- Wyman, C.; Decker, S.; Himmel, M.; Brady, J.; Skopec, C.; Viikari, L. Hydrolysis of Cellulose and Hemicellulose; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kärcher, M.A.; Iqbal, Y.; Lewandowski, I.; Senn, T. Comparing the Performance of Miscanthus x Giganteus and Wheat Straw Biomass in Sulfuric Acid Based Pretreatment. Bioresour. Technol. 2015, 180, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Q.; Li, C.-L.; Sang, T.; Xu, J. Pretreatment on Miscanthus Lutarioriparious by Liquid Hot Water for Efficient Ethanol Production. Biotechnol. Biofuels 2013, 6, 76. [Google Scholar] [CrossRef]
- He, O.; Zhang, Y.; Wang, P.; Liu, L.; Wang, Q.; Yang, N.; Li, W.; Champagne, P.; Yu, H. Experimental and Kinetic Study on the Production of Furfural and HMF from Glucose. Catalysts 2021, 11, 11. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.; Wang, X.; Lu, X.; Qi, X.; Yu, H. Recent Progress in Furfural Production from Hemicellulose and Its Derivatives: Conversion Mechanism, Catalytic System, Solvent Selection. Mol. Catal. 2021, 515, 111899. [Google Scholar] [CrossRef]



| Response Variables | Explanatory Variables | ||
|---|---|---|---|
| Genotype | Acid Treatment | Genotype × Acid Treatment | |
| Cellulose | *** | *** | *** |
| Hemicellulose | * | *** | ** |
| Lignin | *** | *** | *** |
| Ash | *** | *** | ns |
| Xylose | ns | *** | ** |
| Arabinose | *** | *** | *** |
| Parameters | Effect | ||
|---|---|---|---|
| Genotype | Acid Treatment | Genotype × Acid Treatment | |
| Glucose | *** | *** | *** |
| Xylose | *** | *** | *** |
| Arabinose | *** | *** | *** |
| Soluble lignin | *** | *** | *** |
| Parameters | Effect | ||
|---|---|---|---|
| Genotype | Acid Treatment | Genotype × Acid Treatment | |
| Acetic acid | *** | *** | *** |
| Formic acid | *** | *** | *** |
| 5-hydroxymethyl furfural | *** | *** | *** |
| Furfural | *** | *** | *** |
| Genotype | Ethanol (in L (Mg DM)−1) |
|---|---|
| M. giganteus (G) | 92 ± 0.4 |
| M. floridulus (F1) | 91 ± 0.7 |
| M. floridulus (F2) | 90 ± 0.2 |
| M. lutarioparius (L1) | 84 ± 0.2 |
| M. lutarioparius (L2) | 84 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Y.; Dai, Y.; Xue, S.; Yi, Z.; Chen, Z.; Li, M.; von Cossel, M. Organic Acid-Based Hemicellulose Fractionation and Cellulosic Ethanol Potential of Five Miscanthus Genotypes. Agronomy 2024, 14, 1389. https://doi.org/10.3390/agronomy14071389
Iqbal Y, Dai Y, Xue S, Yi Z, Chen Z, Li M, von Cossel M. Organic Acid-Based Hemicellulose Fractionation and Cellulosic Ethanol Potential of Five Miscanthus Genotypes. Agronomy. 2024; 14(7):1389. https://doi.org/10.3390/agronomy14071389
Chicago/Turabian StyleIqbal, Yasir, Yu Dai, Shuai Xue, Zili Yi, Zhiyong Chen, Meng Li, and Moritz von Cossel. 2024. "Organic Acid-Based Hemicellulose Fractionation and Cellulosic Ethanol Potential of Five Miscanthus Genotypes" Agronomy 14, no. 7: 1389. https://doi.org/10.3390/agronomy14071389







