Influencing Factors and Prediction Models of Mercury Phytoavailability and Transference in a Soil–Lettuce System under Chinese Agricultural Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Physicochemical Characteristics of Soils
2.3. Soil Hg Treatments and Experimental Setup
2.4. Soil and Plant Sampling
2.5. Hg Analysis
2.6. Data Analysis
2.7. BCF (Bioconcentration Factor)
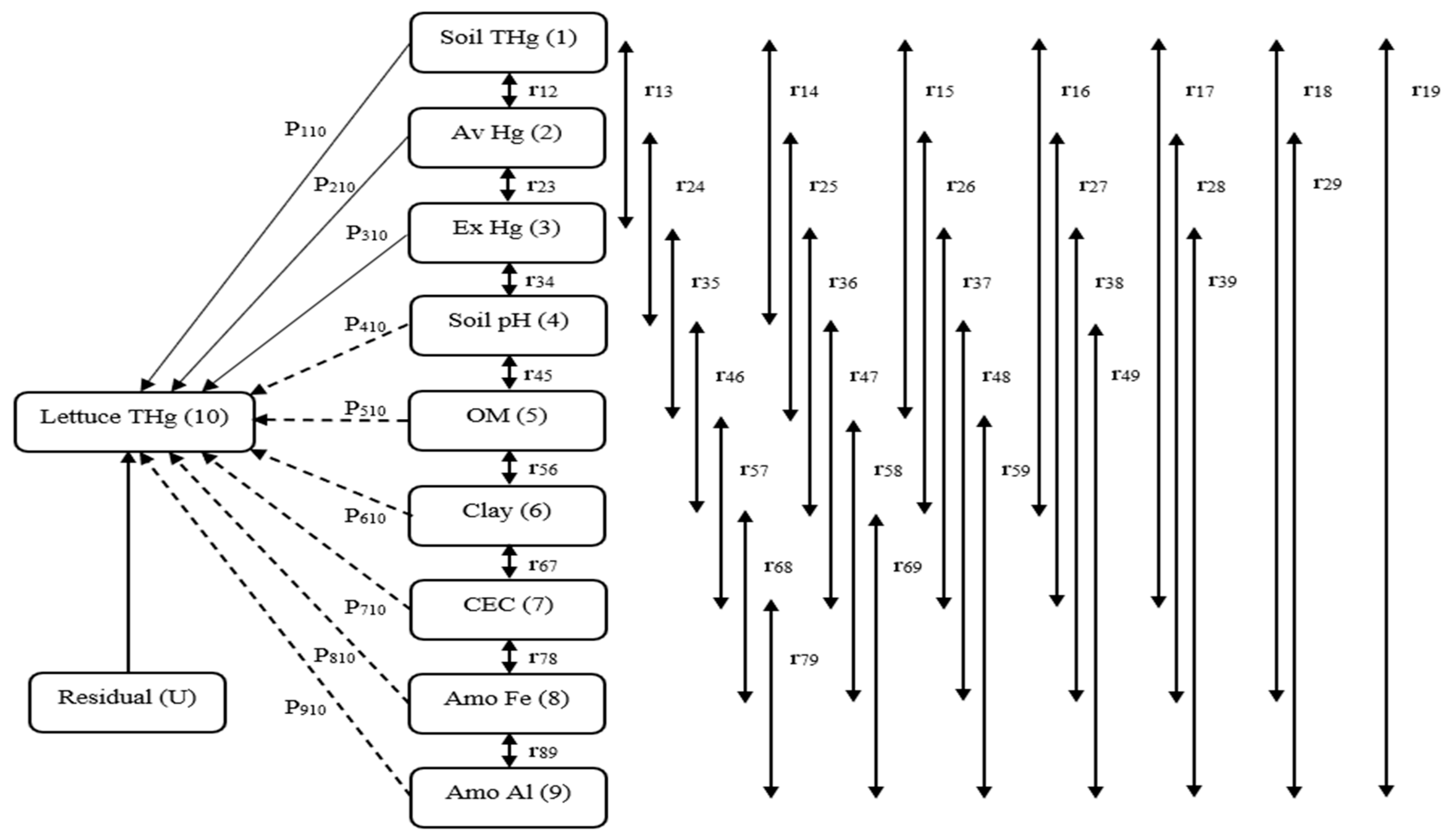
2.8. Path Analysis
2.9. Prediction Model
3. Results
3.1. Soil Total Hg (THg) Concentration (µg kg−1)
3.2. Lettuce Total Hg (THg) Concentrations (µg kg−1)
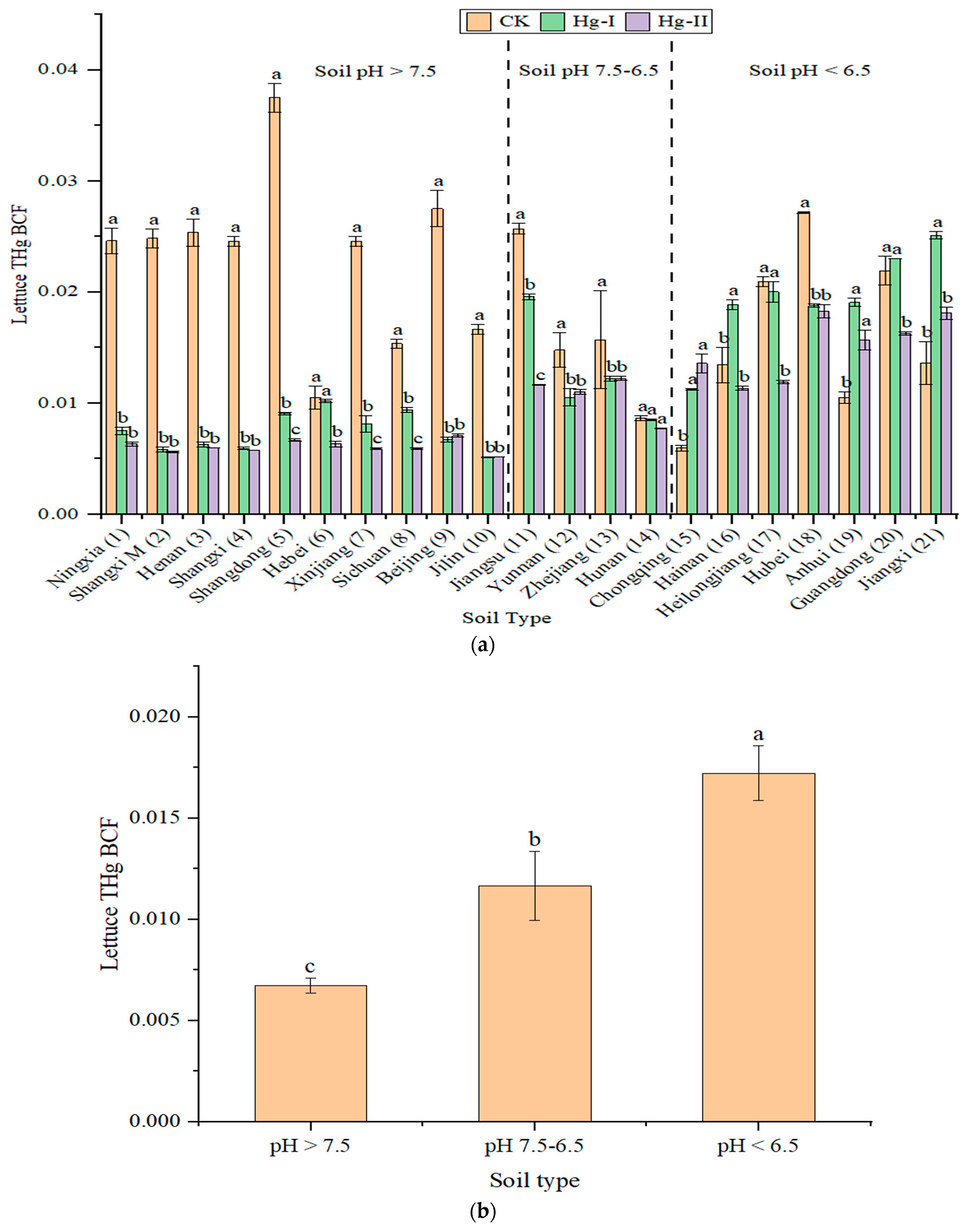
3.3. Uptake and Accumulation of Hg in a Soil–Lettuce System
3.4. Factors Affecting Hg Uptake and Accumulation in a Soil–Lettuce System
3.5. Hg Transfer in a Soil–Lettuce System (Prediction Model)
4. Discussion
4.1. Lettuce Hg Concentration (ug kg−1) in the Investigated Soils
4.2. Uptake and Accumulation of Hg in Soil–Lettuce System
4.3. Factors Affecting Hg Uptake and Accumulation in a Soil–Lettuce System
4.4. Hg Transfer in a Soil–Lettuce System (Prediction Model)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Connor, D.; Hou, D.; Ok, Y.S.; Mulder, J.; Duan, L.; Wu, Q.; Wang, S.; Tack, F.M.; Rinklebe, J. Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef]
- Abdullah, M.; Fasola, M.; Muhammad, A.; Malik, S.A.; Bostan, N.; Bokhari, H.; Kamran, M.A.; Shafqat, M.N.; Alamdar, A.; Khan, M.J.C. Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: A case study from severely contaminated areas. Chemosphere 2015, 119, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, Y.; Ma, J.; Hu, Y.; Su, B.; Fang, G.; Wang, L.; Xiang, B. A review of heavy metal pollution levels and health risk assessment of urban soils in Chinese cities. Environ. Sci. Pollut. Res. 2018, 25, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, X.; Yuan, X.; Chan, H.M.; Qiu, G.; Sun, G.-X.; Zhu, Y.-G. Rice consumption contributes to low level methylmercury exposure in southern China. Environ. Int. 2012, 49, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Wang, Z.; Hu, B.; Wang, Z.; Li, H.; Goodman, R.C. Heavy metals in soil and plants after long-term sewage irrigation at Tianjin China: A case study assessment. Agric. Water Manag. 2016, 171, 153–161. [Google Scholar] [CrossRef]
- Lin, Y.; Vogt, R.; Larssen, T. Environmental mercury in China: A review. Environ. Toxicol. Chem. 2012, 31, 2431–2444. [Google Scholar] [CrossRef]
- Ha, E.; Basu, N.; Bose-O’Reilly, S.; Dórea, J.G.; McSorley, E.; Sakamoto, M.; Chan, H.M. Current progress on understanding the impact of mercury on human health. Environ. Res. 2017, 152, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Henriques, B.; Reis, A.; Duarte, A.; Pereira, E.; Römkens, P. Hg transfer from contaminated soils to plants and animals. Environ. Chem. Lett. 2012, 10, 61–67. [Google Scholar] [CrossRef]
- Hussain, S.; Yang, J.; Hussain, J.; Zandi, P.; Xia, X.; Zhang, L.; Tian, Y.; Ali, A.; Zhang, K. The rhizospheric transformation and bioavailability of mercury in pepper plants are influenced by selected Chinese soil types. Environ. Geochem. Health 2022, 45, 41–52. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, T.; Li, X.; Wang, X. Major controlling factors and prediction models for mercury transfer from soil to carrot. J. Soils Sediments 2014, 14, 1136–1146. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Nanos, N.; Grau, J.M.; Gil, L.; Lopez-Arias, M. Multiscale analysis of heavy metal contents in Spanish agricultural topsoils. Chemosphere 2008, 70, 1085–1096. [Google Scholar] [CrossRef]
- Hussain, S.; Yang, J.; Hussain, J.; Hussain, I.; Kumar, M.; Ullah, S.; Zhang, L.; Xia, X.; Jia, Y.; Ma, Y. Phytoavailability and transfer of mercury in soil-pepper system: Influencing factors, fate, and predictive approach for effective management of metal-impacted spiked soils. Environ. Res. 2022, 207, 112190. [Google Scholar] [CrossRef]
- Rengel, Z. Role of pH in availability of ions in soil. In Handbook of Plant Growth pH as the Master Variable; CRC Press: Boca Raton, FL, USA, 2002; pp. 317–342. [Google Scholar]
- Hussain, S.; Yang, J.; Hussain, J.; Sattar, A.; Ullah, S.; Hussain, I.; Rahman, S.U.; Zandi, P.; Xia, X.; Zhang, L. Mercury fractionation, bioavailability, and the major factors predicting its transfer and accumulation in soil–wheat systems. Sci. Total Environ. 2022, 847, 157432. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Liang, X.; Wang, Q.; Yin, X.; Pierce, E.M.; Gu, B. Competitive exchange between divalent metal ions [Cu (II), Zn (II), Ca (II)] and Hg (II) bound to thiols and natural organic matter. J. Hazard. Mater. 2022, 424, 127388. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qing, C.; Guo, T.; Guo, Y. Effects of humic acid on transport and transformation of mercury in soil-plant systems. Water Air Soil Pollut. 1997, 95, 35–43. [Google Scholar] [CrossRef]
- Montgomery, S.; Lucotte, M.; Rheault, I. Temporal and spatial influences of flooding on dissolved mercury in boreal reservoirs. Sci. Total Environ. 2000, 260, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Dreher, G.; Follmer, L. Mercury content of Illinois soils. Water Air Soil Pollut. 2004, 156, 299–315. [Google Scholar] [CrossRef]
- Gabriel, M.C.; Williamson, D.G. Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environ. Geochem. Health 2004, 26, 421–434. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Xu, X.-H.; Liu, C.-P.; Li, S.-Y.; Liao, X.-R.; Dong, J.; Li, F.-B. Heavy metal accumulation in balsam pear and cowpea related to the geochemical factors of variable-charge soils in the Pearl River Delta, South China. Environ. Sci. Process. Impacts 2014, 16, 1790–1798. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T.; Sun, M.; Bai, Y.; Fu, C.; Zhang, L.; Hu, X.; Hagist, S. Management principles for heavy metal contaminated farmland based on ecological risk—A case study in the pilot area of Hunan province, China. Sci. Total Environ. 2019, 684, 537–547. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Raj, D.; Kumar, A.; Tripti; Maiti, S.K. Health risk assessment of children exposed to the soil containing potentially toxic elements: A case study from coal mining areas. Metals 2022, 12, 1795. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Maiti, S.K.; Raj, D. An approach to quantify heavy metals and their source apportionment in coal mine soil: A study through PMF model. Environ. Monit. Assess. 2023, 195, 306. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, J.; Wang, H.; Han, X.; Ma, J.; Ma, Y.; Luan, H. Distribution and health risk assessment of potentially toxic elements in soils around coal industrial areas: A global meta-analysis. Sci. Total Environ. 2020, 713, 135292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-H.; Cai, L.-M.; Hu, G.-C.; Wen, H.-H.; Luo, J.; Xu, H.-Q.; Chen, L.-G. An integrated exploration on health risk assessment quantification of potentially hazardous elements in soils from the perspective of sources. Ecotoxicol. Environ. Saf. 2021, 208, 111489. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, T.; Wang, X.; Zhou, F.; Yang, Y.; Huang, G. Prediction model for cadmium transfer from soil to carrot (Daucus carota L.) and its application to derive soil thresholds for food safety. J. Agric. Food Chem. 2013, 61, 10273–10282. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J.; Smolders, E.; Degryse, F.; Rietra, R. Uptake of metals from soil into vegetables. In Dealing with Contaminated Sites: From Theory towards Practical Application; Springer: Berlin/Heidelberg, Germany, 2011; pp. 325–367. [Google Scholar]
- Wang, X.; Li, Y.-F.; Li, B.; Dong, Z.; Qu, L.; Gao, Y.; Chai, Z.; Chen, C. Multielemental contents of foodstuffs from the Wanshan (China) mercury mining area and the potential health risks. Appl. Geochem. 2011, 26, 182–187. [Google Scholar] [CrossRef]
- Yang, B.; Gao, Y.; Zhang, C.; Zheng, X.; Li, B. Mercury accumulation and transformation of main leaf vegetable crops in Cambosol and Ferrosol soil in China. Environ. Sci. Pollut. Res. 2020, 27, 391–398. [Google Scholar] [CrossRef]
- Dziubanek, G.; Piekut, A.; Rusin, M.; Baranowska, R.; Hajok, I. Contamination of food crops grown on soils with elevated heavy metals content. Ecotoxicol. Environ. Saf. 2015, 118, 183–189. [Google Scholar] [CrossRef]
- Shatilov, M.; Razin, A.; Ivanova, M. Analysis of the world lettuce market. IOP Conf. Ser. Earth Environ. Sci. 2019, 395, 012053. [Google Scholar] [CrossRef]
- Pelcová, P.; Ridošková, A.; Hrachovinová, J.; Grmela, J. Evaluation of mercury bioavailability to vegetables in the vicinity of cinnabar mine. Environ. Pollut. 2021, 283, 117092. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.; Hutka, J. Particle size analysis. In Soil Physical Measurement and Interpretation for Land Evaluation; Csiro Publishing: Clayton, Australia, 2002; pp. 224–239. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- Mehra, O.; Jackson, M. Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. In Clays and Clay Minerals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 317–327. [Google Scholar]
- GB 15618–1995; Environmental Quality Standard for Soils. Protection Agency of China: Beijing, China, 1995.
- Reis, A.T.; Lopes, C.B.; Davidson, C.M.; Duarte, A.C.; Pereira, E. Extraction of available and labile fractions of mercury from contaminated soils: The role of operational parameters. Geoderma 2015, 259, 213–223. [Google Scholar] [CrossRef]
- Neculita, C.M.; Zagury, G.J.; Deschênes, L. Mercury speciation in highly contaminated soils from chlor-alkali plants using chemical extractions. J. Environ. Qual. 2005, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics, a Biometrical Approach; CABI: Oxfordshire, UK, 1981. [Google Scholar]
- Alloway, B.J.; Jackson, A.P.; Morgan, H. The accumulation of cadmium by vegetables grown on soils contaminated from a variety of sources. Sci. Total Environ. 1990, 91, 223–236. [Google Scholar] [CrossRef]
- Richards, J.R.; Schroder, J.L.; Zhang, H.; Basta, N.T.; Wang, Y.; Payton, M.E. Trace elements in benchmark soils of Oklahoma. Soil Sci. Soc. Am. J. 2012, 76, 2031–2040. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Li, Z.-J.; Yao, F.; Liu, Y.-W.; Xue, J.-M.; Davis, M.; Liang, Y.-C. Prediction model for mercury transfer from soil to corn grain and its cross-species extrapolation. J. Integr. Agric. 2016, 15, 2393–2402. [Google Scholar] [CrossRef]
- Tipping, E.; Lofts, S.; Hooper, H.; Frey, B.; Spurgeon, D.; Svendsen, C. Critical limits for Hg (II) in soils, derived from chronic toxicity data. Environ. Pollut. 2010, 158, 2465–2471. [Google Scholar] [CrossRef]
- Ding, C.; Zhou, F.; Li, X.; Zhang, T.; Wang, X. Modeling the transfer of arsenic from soil to carrot (Daucus carota L.)—A greenhouse and field-based study. Environ. Sci. Pollut. Res. 2015, 22, 10627–10635. [Google Scholar] [CrossRef]
- Ding, C.; Li, X.; Zhang, T.; Wang, X. Transfer model of lead in soil–carrot (Daucus carota L.) system and food safety thresholds in soil. Environ. Toxicol. Chem. 2015, 34, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A.; Wenstel, R.; Sappington, K.; Wood, W. Framework for metals risk assessment. Ecotoxicol. Environ. Saf. 2007, 68, 145–227. [Google Scholar] [CrossRef]
- Liang, P.; Li, Y.-C.; Zhang, C.; Wu, S.-C.; Cui, H.-J.; Yu, S.; Wong, M.H. Effects of salinity and humic acid on the sorption of Hg on Fe and Mn hydroxides. J. Hazard. Mater. 2013, 244, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Allen, H.E.; Li, Y.; Huang, C.; Sanders, P.F. Adsorption of Mercury (II) by Soil: Effects of pH, Chloride, and Organic Matter; Wiley Online Library: Hoboken, NJ, USA, 1996. [Google Scholar]
- Naidu, R.; Kookana, R.S.; Sumner, M.E.; Harter, R.D.; Tiller, K. Cadmium sorption and transport in variable charge soils: A review. J. Environ. Qual. 1997, 26, 602–617. [Google Scholar] [CrossRef]
- Rensing, C.; Maier, R.M. Issues underlying use of biosensors to measure metal bioavailability. Ecotoxicol. Environ. Saf. 2003, 56, 140–147. [Google Scholar] [CrossRef]
- Wang, G.; Su, M.-Y.; Chen, Y.-H.; Lin, F.-F.; Luo, D.; Gao, S.-F. Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environ. Pollut. 2006, 144, 127–135. [Google Scholar] [CrossRef]
- Dong, H.; Lin, Z.; Wan, X.; Feng, L. Risk assessment for the mercury polluted site near a pesticide plant in Changsha, Hunan, China. Chemosphere 2017, 169, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Yu, H.; Chen, J.; Li, F.; Zhang, H.; Liu, C. Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environ. Monit. Assess. 2014, 186, 1547–1560. [Google Scholar] [CrossRef]
- Robarge, W.P. Precipitation/dissolution reactions in soils. In Soil Physical Chemistry; CRC Press: Boca Raton, FL, USA, 2018; pp. 193–238. [Google Scholar]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Hung, J.-J.; Lu, C.-C.; Huh, C.-A.; Liu, J. Geochemical controls on distributions and speciation of As and Hg in sediments along the Gaoping (Kaoping) Estuary–Canyon system off southwestern Taiwan. J. Mar. Syst. 2009, 76, 479–495. [Google Scholar] [CrossRef]
- Rodrigues, S.; Pereira, E.; Duarte, A.; Römkens, P. Derivation of soil to plant transfer functions for metals and metalloids: Impact of contaminant’s availability. Plant Soil 2012, 361, 329–341. [Google Scholar] [CrossRef]
- Wang, S.; Nan, Z.; Prete, D.; Ma, J.; Liao, Q.; Zhang, Q. Accumulation, transfer, and potential sources of mercury in the soil-wheat system under field conditions over the Loess Plateau, northwest China. Sci. Total Environ. 2016, 568, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, H.; Du, B.; Shang, L.; Yang, J.; Wang, Y. Influence of soil mercury concentration and fraction on bioaccumulation process of inorganic mercury and methylmercury in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2015, 22, 6144–6154. [Google Scholar] [CrossRef] [PubMed]
- Różański, S.Ł.; Castejón, J.M.P.; Fernández, G.G. Bioavailability and mobility of mercury in selected soil profiles. Environ. Earth Sci. 2016, 75, 1065. [Google Scholar] [CrossRef]
- Boszke, L.; Kowalski, A.; Glosifiska, G.; Szarek, R.; Siepak, J. Environmental factors affecting speciation of mercury in the bottom sediments; an overview. Pol. J. Environ. Stud. 2003, 12, 5–13. [Google Scholar]
- Biester, H.; Müller, G.; Schöler, H. Binding and mobility of mercury in soils contaminated by emissions from chlor-alkali plants. Sci. Total Environ. 2002, 284, 191–203. [Google Scholar] [CrossRef]
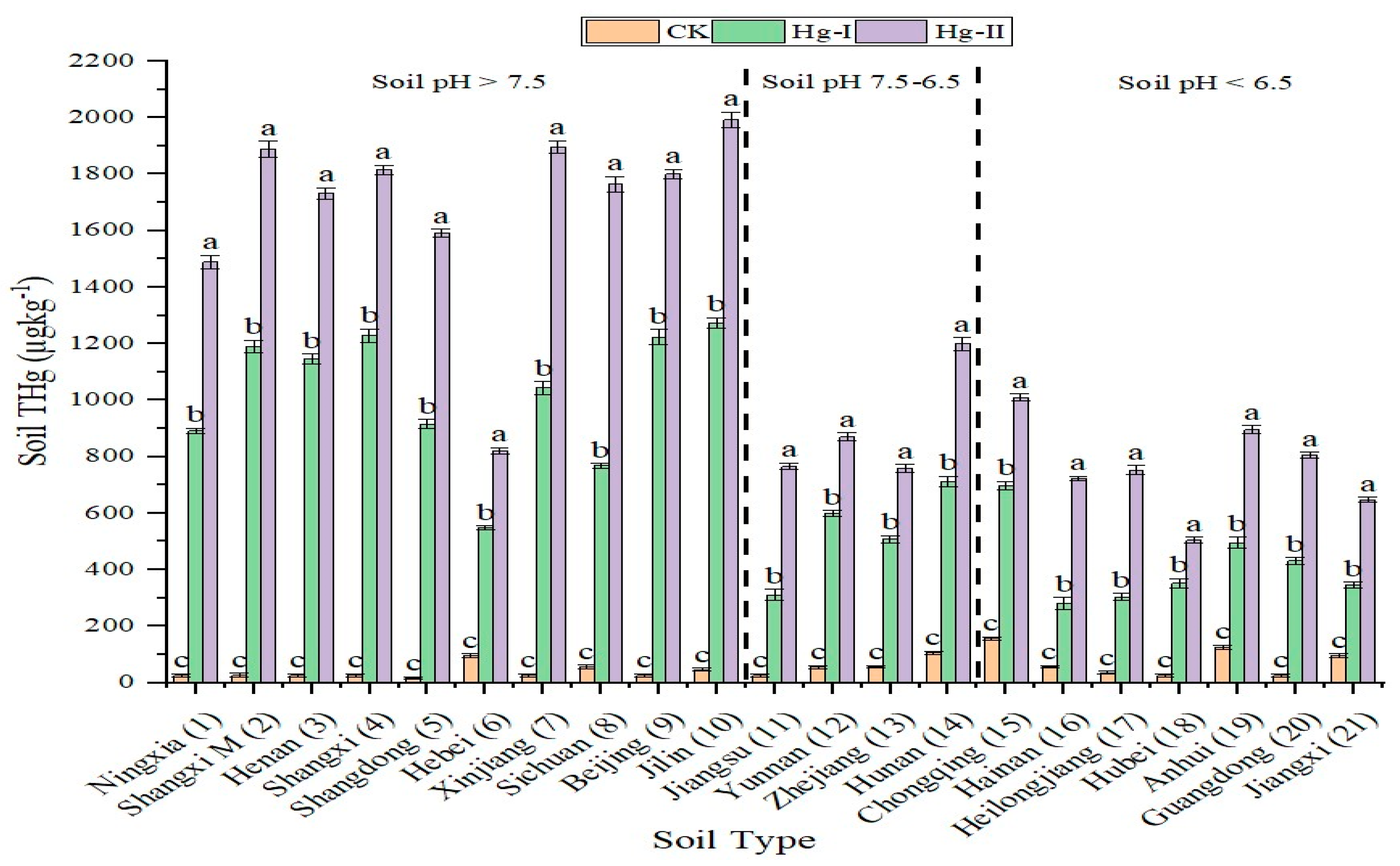

| Hg Treatment | Soil THg Concentration (µg kg−1) | Mean | SD | |
|---|---|---|---|---|
| Min | Max | |||
| CK (Control) | 15.98 | 156.02 | 54.10 c | 27.17 |
| Hg-I (Low-Hg) | 281.04 | 1272.93 | 606.58 b | 248.38 |
| Hg-II (High-Hg) | 503.77 | 1991.90 | 1024.48 a | 314.18 |
| Hg Treatment | Lettuce THg Concentration (µg kg−1) | Mean | SD | |
|---|---|---|---|---|
| Min | Max | |||
| CK (Control) | 0.86 | 1.46 | 1.06 c | 0.15 |
| Hg-I (Low-Hg) | 5.31 | 9.92 | 7.17 b | 1.26 |
| Hg-II (High-Hg) | 5.18 | 14.01 | 10.32 a | 2.01 |
| Hg Treatment | Lettuce Leaf Hg BCF | Mean | SD | |
|---|---|---|---|---|
| Min | Max | |||
| CK (Control) | 0.006 | 0.038 | 0.020 a | 0.008 |
| Hg-I (Low-Hg) | 0.005 | 0.025 | 0.012 b | 0.006 |
| Hg-II (High-Hg) | 0.005 | 0.018 | 0.010 b | 0.004 |
| Property | Soil THg | Av Hg | Ex Hg | pH | OM | Clay | CEC | Amo Fe | Amo Al | r | R2 | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil THg | 0.79 ** | −0.03 | −0.02 | −0.30 | −0.04 | −0.02 | −0.04 | 0.05 | 0.07 | 0.46 * | 0.82 ** | 0.42 |
| AvHg | 0.16 | −0.15 | −0.01 | 0.31 | 0.07 | 0.03 | 0.07 | −0.12 | −0.13 | 0.23 | ||
| ExHg | 0.20 | −0.02 | −0.09 | −0.01 | 0.01 | 0.00 | 0.01 | 0.06 | 0.05 | 0.20 | ||
| pH | 0.28 | 0.05 | 0.00 | −0.86 ** | −0.04 | −0.01 | −0.04 | 0.05 | 0.08 | −0.49 * | ||
| OM | −0.11 | −0.07 | −0.01 | 0.18 | 0.14 | 0.03 | −0.01 | −0.02 | −0.19 | −0.12 | ||
| Clay | −0.21 | −0.09 | 0.00 | 0.19 | 0.09 | 0.06 | 0.08 | −0.10 | −0.14 | −0.15 | ||
| CEC | −0.13 | −0.04 | 0.02 | 0.14 | −0.08 | 0.04 | 0.15 | −0.10 | −0.15 | −0.19 | ||
| Amo Fe | −0.16 | −0.07 | 0.02 | 0.15 | 0.01 | 0.02 | 0.15 | −0.27 * | 0.14 | 0.13 | ||
| Amo Al | −0.17 | −0.04 | 0.03 | 0.16 | 0.09 | 0.06 | 0.12 | 0.12 | −0.47 * | −0.14 |
| Hg Inputs | Prediction Model | R2 | p | SE |
|---|---|---|---|---|
| CK soil | Log (lettuce THg) = 0.16 log (soil THg) − 0.36 | 0.32 | 0.001 | 0.116 |
| Hg-I + Hg-II | Log (lettuce THg) = −1.55 log (soil pH) + 0.48 log (soil THg) − 0.1 log (Amo Al) − 0.07 log (Amo Fe) + 1.91 | 0.82 | 0.000 | 0.085 |
| CK + Hg-I + Hg-II | Log (lettuce THg) = −1.44 log (soil pH) + 0.45 log (soil THg) − 0.1 log (Amo Al) − 0.07 log (Amo Fe) + 1.66 | 0.81 | 0.000 | 0.082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Hussain, S.; Noor, Y.; Khanam, T.; Xia, X.; Darma, A.I.; Feng, Y.; Yang, J. Influencing Factors and Prediction Models of Mercury Phytoavailability and Transference in a Soil–Lettuce System under Chinese Agricultural Soils. Agronomy 2024, 14, 1394. https://doi.org/10.3390/agronomy14071394
Ullah S, Hussain S, Noor Y, Khanam T, Xia X, Darma AI, Feng Y, Yang J. Influencing Factors and Prediction Models of Mercury Phytoavailability and Transference in a Soil–Lettuce System under Chinese Agricultural Soils. Agronomy. 2024; 14(7):1394. https://doi.org/10.3390/agronomy14071394
Chicago/Turabian StyleUllah, Subhan, Sajjad Hussain, Yousaf Noor, Tasawar Khanam, Xing Xia, Aminu Inuwa Darma, Ya Feng, and Jianjun Yang. 2024. "Influencing Factors and Prediction Models of Mercury Phytoavailability and Transference in a Soil–Lettuce System under Chinese Agricultural Soils" Agronomy 14, no. 7: 1394. https://doi.org/10.3390/agronomy14071394





