Abstract
High light (HL) significantly impacts plant photosynthesis. This study investigated the effects of different magnesium (Mg) levels (0, 1, 2, and 5 mol Mg plant−1; HMg0, HMg1, HMg2, and HMg5) on tobacco (Nicotiana tabacum L. cv. Cuibi No. 1) under HL (1500 μmol m−2 s−1), aiming to understand the role of Mg in mitigating the impact of HL on photosynthesis and carbon–nitrogen metabolism. Plants treated with 1 mol Mg plant−1 under 750 μmol m−2 s−1 light conditions served as the control. HL led to a reduced chlorophyll (Chl) content and inhibited the maximum photosynthetic rate (Pmax). It also decreased energy involved in photosynthetic electron transfer (ET) and electron flux to reduction end-electron acceptors at the photosystems I (PSI) acceptor side (RE) and caused photosynthetic system damage. H2O2 accumulation exacerbated membrane lipid peroxidation damage, disrupting carbon and nitrogen metabolism, and inducing antioxidant enzyme activity. HMg2 increased Chl content, stomatal conductance, intercellular CO2 concentration, and the net photosynthetic rate compared to HMg0. It enhanced ET efficiency, PSI and PSII functionality, reduced dissipated energy flux (DI), and minimized photosynthesis damage. Conversely, excessive Mg application (HMg5) decreased Pmax and PSII activity, increasing DI. Adequate Mg supply alleviated HL’s detrimental effects by enhancing Chl content and ET and RE efficiency.
1. Introduction
Among various environmental factors, high light (HL) intensity has been identified as a primary factor influencing plant growth [1]. HL, characterized by its intensity, duration, and coverage, can severely inhibit plant growth, induce adaptive responses, inflict cellular damage, and ultimately result in plant mortality [2,3]. Numerous studies have illustrated the role of magnesium (Mg2+) in modulating plant responses to environmental stresses, including HL conditions [4,5]. The supplementation of Mg is crucial in augmenting plant biochemical and physiological processes, thereby mitigating the detrimental effects of adverse environmental conditions in crop production [6,7,8]. The investigation of plant exposure to HL reveals significant physiological implications, especially concerning photosynthesis, when augmented with additional Mg.
In the chloroplast, Mg serves as the central atom of the chlorophyll molecule, with its binding to chloroplasts ranging from 6% to 35% depending on the plant’s Mg status [9,10,11]. Mg also influences chlorophyll metabolism by affecting the pheophytin pheophorbide hydrolase (PPH) and chlorophyllide and oxygenase (CAO) pathways [12,13]. The transport and accumulation of carbohydrates are affected in both Mg-sufficient and Mg-deficient plant leaves, resulting in alterations in photosynthetic carbon metabolism and carbon dioxide (CO2) fixation [4,14,15]. Furthermore, the dark reaction of photosynthesis is sensitive to Mg deficiency (MgD), as the activity of photosynthetic enzymes involved in carbon fixation (such as ribulose-1,5-bisphosphate carboxylase/oxygenase) is highly dependent on the concentration of Mg in the leaves [16,17]. In MgD leaves, carbon assimilation is hindered, and the transport of carbon sources to carbon sinks is restricted [17,18]. Mg also plays an essential role in the electron transport chain of chloroplasts [4,19,20], transferring energy from photosystem II (PSII) to nicotinamide adenine dinucleotide phosphate (NADP+), while also mitigating thylakoid membrane damage caused by reactive oxygen species (ROSs) [21,22]. Extra Mg has been found to alleviate the adverse effects on plants under HL stress [23,24,25]. It is worth noting that oxidative stress is one of the components of mineral nutrient deficiency stress [26]. Mg activates various acclimation mechanisms to reduce plants’ susceptibility to HL stress [23,24], likely by improving antioxidant metabolism and reducing oxidative cellular damage caused by ROSs [8,24].
The plant growth depends on the activity of highly efficient photosynthetic complexes and their adaptability to changed environmental condition [27]. Plants have developed many adaptive mechanisms to cope with light intensity [28], and photosynthetic characteristics are one of the most commonly used indicators to evaluate the adaptability of plants to HL stress [29]. Plant photosynthesis is mainly inhibited by the destruction of PSII in the photosynthetic complex when exposed to heat stress. PSII is located in the middle of the thylakoid membrane and is the most sensitive to high temperature [7]. Due to the absorption of excessive light energy, reactive oxygen species are generated in PSI and PSII, causing oxidative damage to cells [8], and the repair of photodamaged PSII is inhibited. This results in photoinactivation of PSII, thermal denouement of oxygen release complex, influences photon energy absorption and electron transfer, and causes irreversible oxidative damage to lipid and pigment molecules [9].
Indeed, there are numerous studies and reviews available on the oxidative stress and antioxidant responses of plants subjected to both Mg toxicity and HL stress [4,30]. It is crucial to ensure an adequate supply of Mg for plants, as it serves as a critical factor in mitigating the impacts of heat stress during plant growth [4,24]. Studies have consistently demonstrated a reduction in photosynthetic efficiency under HL stress conditions [31,32,33]. This decrease is primarily attributed to HL-induced stomatal closure [34], which restricts the entry of CO2 into the leaf. However, it is important to note that other non-stomatal factors can also contribute to this decline in photosynthetic efficiency. These include reduced leaf expansion, leaf senescence, and impaired photosynthetic mechanisms [35].
Research exploring the effects of HL and sufficient Mg on photosynthetic systems, including the photosystem, photosynthetic pigments, and electron transport system, is currently limited. Our specific objectives are to corroborate the hypothesis that additional Mg enhances plants’ resilience to HL exposure. This research seeks to elucidate how Mg alleviates the damage induced by HL stress on multiple facets of the plant’s photosynthetic system, as well as carbon and nitrogen metabolism. This was pivotal in devising strategies to mitigate the adverse effects of HL stress on plant growth processes.
2. Materials and Methods
2.1. Plant Cultivation and Sampling
Tobacco seedlings (Nicotiana tabacum L. cv Cuibi No. 1) were cultivated under controlled environmental conditions in a greenhouse (Temperature test chamber WIPGC-01, Fujian Jiupo Biotechnology Co., Ltd., Fuzhou, China) at the Fujian Institute of Tobacco Sciences during the year 2020. Initially, the seedlings were planted in trays for a period of 5 weeks. Following this, they were transplanted into pots measuring 14.4 cm in diameter and 17.2 cm in height, each filled with 1.4 kg of sand. The exchangeable Mg content in sand was 153.27 mg kg−1 (determined in ammonium acetate extract), indicating a high Mg nutrient level [36]. Sand’s primary nutrient composition is detailed in Table S1. The nutrient content of the sand was determined according to the method described by Lu et al. [37].
Throughout the experimental period, the plants were grown under a photosynthetic photon flux density (PPFD) of approximately 750 μmol m−2 s−1 within a growth chamber. The greenhouse environment was maintained at 75% humidity, and 12 h light, 25 °C, 750 μmol m−2 s−1/12 h dark, 20 °C, 0 μmol m−2 s−1. Temperature conditions within the growth chamber were meticulously regulated. A modified Hoagland nutrient solution, as outlined in Table S2, was employed for the cultivation of tobacco. The experimental design included treatments with different Mg supplementation levels: 0 mol plant−1 (excluding the Mg provided by the sand, thus no additional Mg supply), 1 mol plant−1 (supplemented with 1 mol of MgSO4 7H2O), 2 mol plant−1 (supplemented with 2 mol of MgSO4 7H2O), and 5 mol plant−1 (supplemented with 5 mol of MgSO4 7H2O). After 100 days post-sowing, the seedlings were exposed to high light intensity (HL, 1500 μmol m−2 s−1) for 10 days, and Mg1 treated plants under normal light (NL, 750 μmol m−2 s−1) as a control. Each nutrient solution was prepared by diluting analytical-grade (AR) reagents in water and was administered to the plants every 3 to 5 days. The study investigated the effects of different Mg levels and light intensities on plant growth, with each treatment replicated across 10 pots. According to the preliminary test results, the growth physiology of tobacco treated with Mg1 was the best among different Mg treatments under normal light (Figure S1); thus, Mg1 was used as the control group for high light treatment under natural light conditions (the specific experimental treatment model is shown in Figure S2).
2.2. Measurements of Plant Biomass, Nitrogen (N) Percentage, Carbon (C) Concentration, Mg Percentage, Starch Percentage, and Sucrose Percentage
For each treatment, three representative tobacco plants were selected, and their aboveground parts were separated from the roots by cutting at the stem base. The upper and lower leaves were halved based on the total number of tobacco leaves. The roots were washed with water to remove any sand. Both roots and aboveground parts were dried in an oven at a constant temperature of 105 °C for 30 min, and then baked to a constant weight at 70 °C. Subsequently, their dry weights were measured. The different plant parts were then ground into a fine powder for various treatments, and the powdered samples were tested for N, C, and Mg percentages. C and N percentages of the plants were determined using a carbon and nitrogen analyzer (SKALAR PrimacsSCN100-1C, Breda, The Netherlands). The magnesium percentage of the plants was extracted using Lu’s method [37] and measured with an ICP-OES (iCAP 7000 series, Thermo Fisher Scientific, Inc., Waltham, MA, USA). Plants’ starch and sucrose percentages were extracted using Sinobestbio test kits (YX-C-C40 and YX-W-B502, Sino Best Biological Technology Co., Ltd., Shanghai, China) and measured with a microplate reader (Varioskan LUX, Thermo Fisher Scientific, Waltham, MA, USA).
From each treatment group, leaves from the 2nd to 3rd position from the top of three fresh specimens were selected, and the main veins were removed. A portion of these leaves was quickly frozen with liquid nitrogen and stored at −80 °C for the determination of physiological indicators. The remaining samples were weighed for in vivo measurement of nitrate reductase (NR) activity. The youngest fully expanded leaves were selected for analyzing the Chl concentration, enzyme activity, light response curves, and photosynthetic fluorescence parameters.
2.3. Assay of Chl Concentration, Hydrogen Peroxide (H2O2), Malondialdehyde (MDA), Superoxide Dismutase (SOD), Catalase (CAT), and Peroxidase Activity (POD) in Leaves
For Chl concentration measurements, the main vein was removed from the leaf, and the remaining tissue was cut into 1 cm pieces. These pieces were mixed and extracted in 80% acetone at room temperature. Pigment absorption was measured spectrophotometrically at 470 nm, 646 nm, and 663 nm using a microplate reader (Varioskan LUX, Thermo Fisher Scientific, Waltham, MA, USA).
The contents of carotene, Chl a, and Chl b were calculated using equations provided by Lichtenthaler [38]. The concentration of H2O2 was measured using the Sinobestbio hydrogen peroxide content assay kit (YX-C-A400, Sino Best Biological Technology Co., Ltd., Shanghai, China) and a UV spectrophotometer (Mapada UV-P5; Shanghai Mapada Instruments Co., Ltd., Shanghai, China). H2O2 was measured in excised leaves after treatment, with the leaves immediately cooled in liquid nitrogen and kept in the dark before measurement. The environmental conditions for H2O2 measurement were as follows: indoor, 450 µmol m−2 s−1, at 24 °C. The content of MDA, an indicator of lipid peroxidation, was determined using the Sinobestbio MDA content assay kit (YX-C-A401, Sino Best Biological Technology Co., Ltd., Shanghai, China). MDA forms a red product with thiobarbituric acid (TBA), with maximum absorption at 532 nm. The absorbance at 532 nm and the correction at 600 nm were used to calculate the MDA content. Leaves’ SOD, CAT, and POD activities were extracted using Sinobestbio test kits (YX-C-A500, YX-C-A501, and YX-C-A502, Sino Best Biological Technology Co., Ltd., Shanghai, China) and measured with a UV spectrophotometer (Mapada UV-P5; Shanghai Mapada Instruments Co., Ltd., Shanghai, China).
2.4. Light Response Curve and Chl a Fluorescence (OJIP) in Leaves
The light response curves of flue-cured tobacco were measured using the CIRAS-3 portable photosynthesis apparatus (USA). The light intensity values used were 0, 250, 500, 750, 1000, and 1500 μmol m−2 s−1. Four plants were selected for each treatment. To determine the initial quantum efficiency (α), maximum net photosynthetic rate (Pmax), light compensation point (Ic), and dark respiration rate (Rd), curve-fitting techniques were employed. The obtained data points from the light response curves were fitted to appropriate mathematical models to derive these physiological parameters [39].
The OJIP transients were measured using a Handy PEA fluorometer (Hansatech Ltd., Norfolk, UK). The analysis of Chl a fluorescence signals was conducted in accordance with the methodologies outlined by Stirbet and Govindjee [40] and De [41]. Various fluorescence parameters were calculated, the specifics of which, including their description, are detailed in Table S3.
2.5. Assay of Sucrose Synthetase (SS), Sucrose Phosphate Synthase (SPS), and Nitrate Reductase (NR) Activities
SS and SPS activities were measured using Sinobestbio assay kits (YX-C-B504 for SS and YX-C-B505 for SPS, Sino Best Biological Technology Co., Ltd., Shanghai, China) and quantified with a UV spectrophotometer (Mapada UV-P5, Sino Best Biological Technology Co., Ltd., Shanghai, China). NR activity was measured in vivo using an ultraviolet spectrophotometer, following the method described by Zou [42].
2.6. Statistical Analysis
Statistical analysis was performed using SPSS 25.0 software. The data from the five treatments were analyzed using a rectangular hyperbola correction model [39] in Photosynthesis Calculation 4.1 software, allowing the determination of α, Pmax, Ic, and Rd values. Duncan’s multiple-range test, at a 0.05 significance level, was employed for statistical comparison. Figures were created using Origin 2024. The JIP-test parameter in the radar chart is controlled by Mg1, and the numerical value represents the ratio of the treatment group to the control group. Each indicator was measured by three biological replicates. Among them, biomass and nutrient indicators were measured using a single plant per replicate, while physiological indicators were measured using a mixed sample of three plants per replicate.
3. Results
3.1. Impacts of Different Mg Supplementation Levels on Plant Phenotypes, Biomass, Mg Concentration, H2O2, MDA, SOD, CAT, and POD Activities
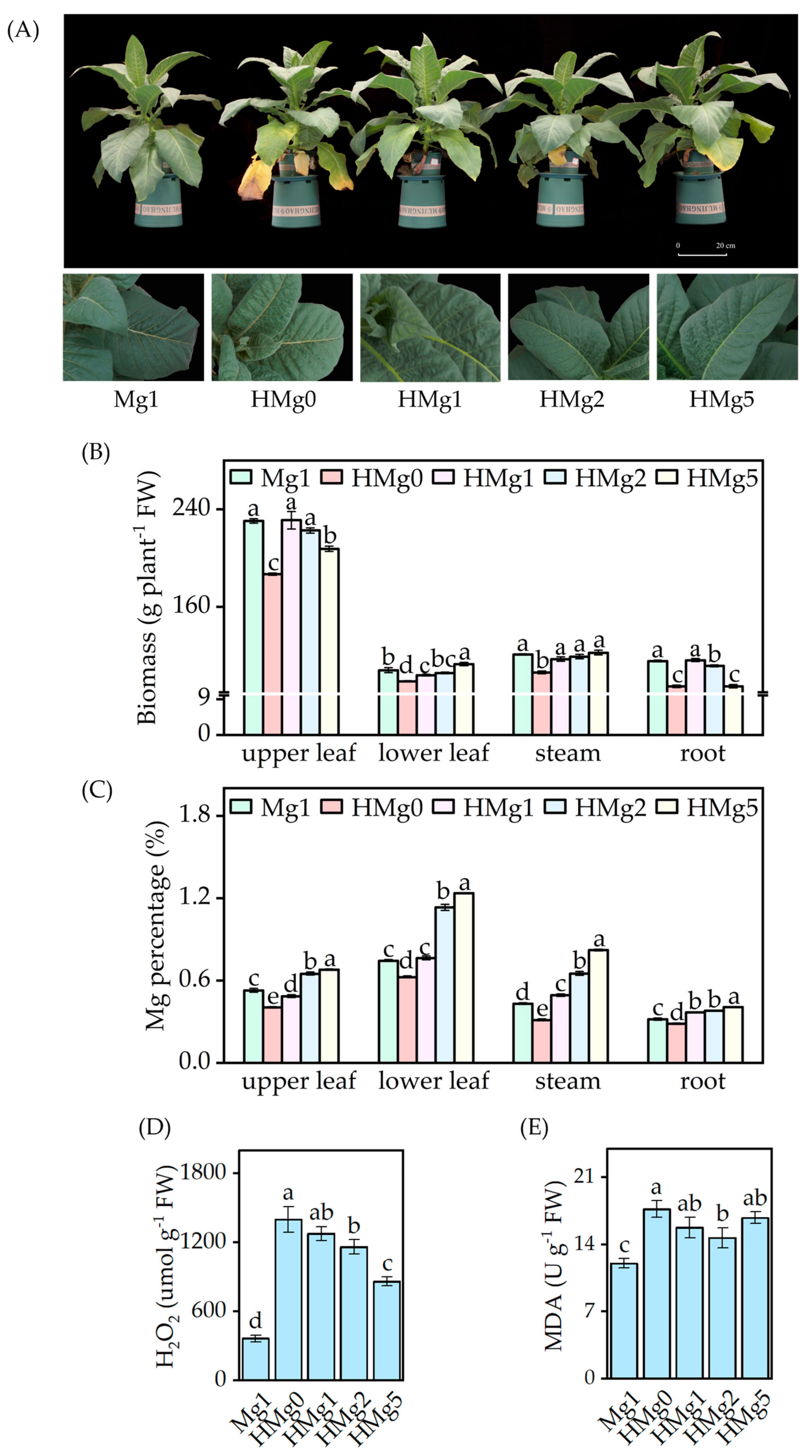
Upon exposure to HL intensity, samples without Mg supplied showed exacerbated visual symptoms, including increased wrinkling and curling at the edges of the upper leaves, browning, and accelerated senescence of the lower leaves (Figure 1A). Plants subjected to HMg0 showed more severe symptoms. Compared to plants treated with 1 mol Mg (Mg1), those treated with 0 mol Mg (HMg0) displayed a reduction in biomass across different components, including upper leaves, lower leaves, stem, and roots, as illustrated in Figure 1B. Similarly, a decreasing trend in the biomass of the upper leaves and roots was observed in plants treated with 5 mol Mg (HMg5). The Mg percentage in different plant parts varied with light intensity and Mg treatments (Figure 1C). Under HL conditions, an increase in Mg supply led to a significant rise in Mg percentage across all plant parts. However, with the same Mg supply, a comparison between Mg1 and HMg1 treatments revealed a decrease in Mg percentage in the upper leaves of HMg1 treated plants, while an increase was noted in the lower leaves, stems, and roots.
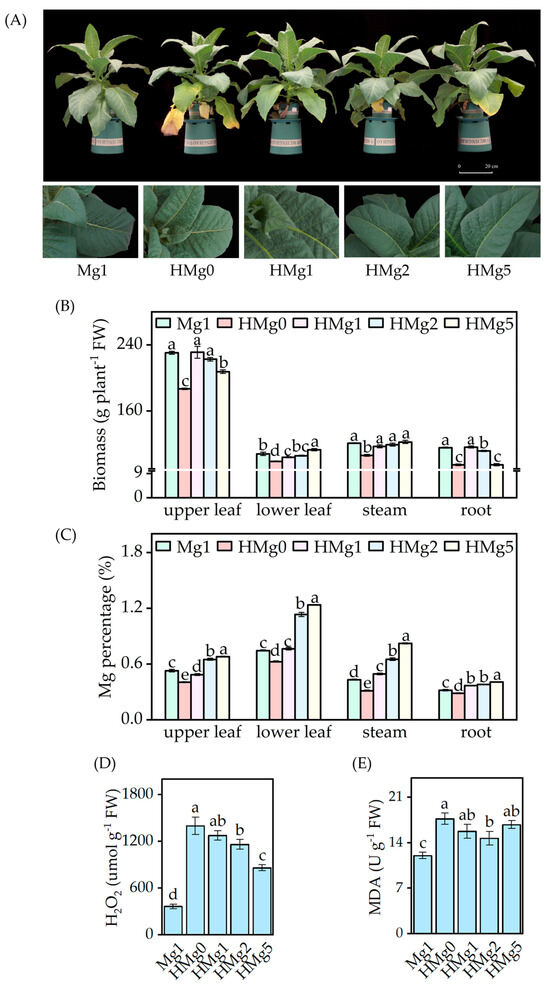
Figure 1.
Effects of different Mg levels supplied to seedings on growth under normal/high light. (A) Phenotype of different Mg levels supplied to tobacco under high light for 10 days, and partial symptom map of tobacco leaves. (B) Biomass of tobacco, including upper leaf, lower leaf, stem, root, and whole plant. (C) Magnesium (Mg) percentage. (D) Hydrogen peroxide (H2O2) concentration. (E) Malondialdehyde (MDA) concentration. Mg1: 1 mol Mg, tobacco seedings grown under 750 μmol m−2 s−1 light intensity. HMg0, HMg1, HMg2, and HMg5 (0, 1, 2, and 5 mol Mg), grown under 1500 μmol m−2 s−1 light intensity for 10 days. The data presented in the bar graphs are expressed as means ± standard errors (n = 3). Means followed by the same small letter are not significantly different (p < 0.05).
Figure 1D,E show the concentrations of H2O2 and MDA in the plants, respectively. Relative to Mg1, H2O2 and MDA levels increased significantly under HL conditions. However, the extent of these changes was dependent on the Mg supply; under HL conditions, H2O2 levels diminished with an increased Mg supply. Regarding MDA levels, HL-treated plants increased compared to Mg1 treatment. The variations in MDA levels under different Mg supplies varied under HL conditions. Specifically, compared to HMg0, MDA levels significantly decreased under the HMg2 treatment, whereas no significant differences were observed under the HMg1 and HMg5 treatments. The increase in MDA content in HL-treated plants was moderated by an appropriate Mg supply, a trend that mirrored changes in H2O2 levels. The MDA and H2O2 content in the HMg1 treated leaves significantly increased compared to Mg1. According to the measurement results of the main antioxidant enzyme activities (Figure S3), the activities of SOD, CAT, and POD in HL-treated leaves were significantly increased compared to Mg1. Under different Mg supplies, the antioxidant enzyme activity in HL-treated leaves showed variations. The SOD and CAT activities in HMg0-treated leaves were significantly higher than those in HMg2 treated leaves. However, POD activity showed an opposite trend, with the POD activity in HL-treated leaves increasing with the increase in Mg supply. The POD activities in HMg2 and HMg5 treated leaves were significantly higher than those in HMg0 and HMg1 treated leaves.
3.2. Impacts of Mg Deficiency on Plant Carbon (C) Percentage and Nitrogen (N) Percentage and Activities of Sucrose Synthetase (SS), Sucrose Phosphate Synthase (SPS), and Nitrate Reductase (NR) in Leaves
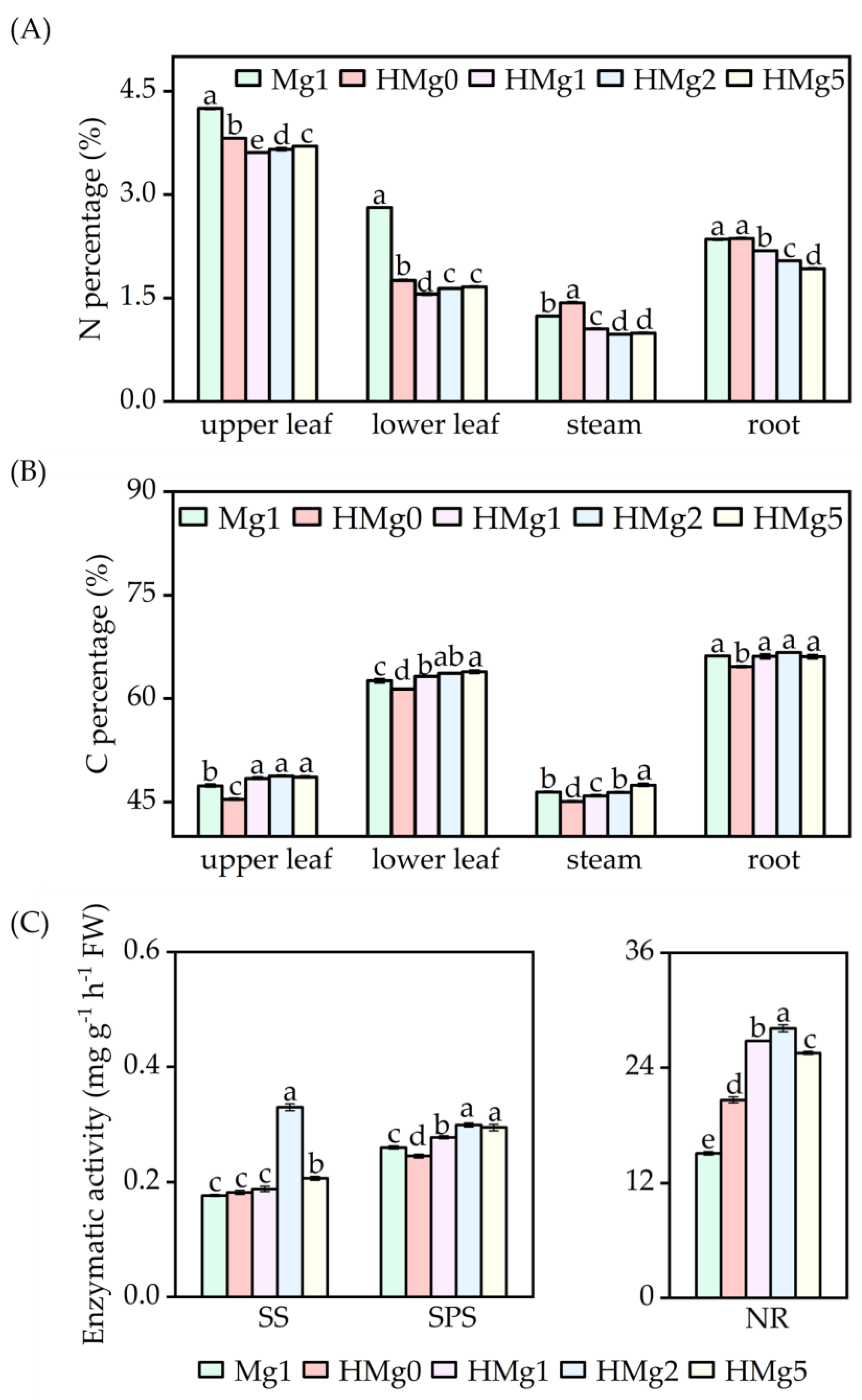
N percentage in different plant tissues, including upper leaves, lower leaves, stems, and roots, as indicated in Figure 2A, showed distinct responses under various treatments. Following HL exposure, the N content in the upper leaves, lower leaves, and roots decreased under the treatments HMg0, HMg1, HMg2, and HMg5, compared to the Mg1 treatment. Furthermore, when compared to HMg0, a significant reduction in N content was observed in the leaves, stems, and roots under HMg1, HMg2, and HMg5 treatments. Conversely, C content varied across different treatments (Figure 2B). Relative to Mg1, a significant increase in C content was noted in the leaves under HMg1, HMg2, and HMg5 treatments, as well as in the stems and roots under HMg2 and HMg5 treatments. The carbon content in different parts of plants treated with different Mg supply levels, compared to HMg0, showed a significant increase under HMg1, HMg2, and HMg5 treatments. According to the analysis results of sucrose and starch contents in different parts (Figure S4), the sucrose content variation trend in plants under different treatments was relatively consistent with the carbon content changes. Compared with Mg1, the sucrose content in various parts of HMg0 plants significantly decreased, and the sucrose content in the upper leaves, stems, and roots of HMg1 treated plants significantly decreased. Compared with HMg0 treatment, the sucrose content in plants treated with HL-Mg supply increased, especially in the upper leaves, stems, and roots. However, the variation trend in starch content was the opposite. Compared with Mg1 treatment, the starch content in the leaves of HL-treated plants significantly increased. Compared with HMg0 treatment, the starch content in the leaves of HL-Mg supply-treated plants decreased, while the starch content in stems and roots increased. With the same Mg supply, the shoot carbon (C) content in HMg1 treated plants significantly increased compared to Mg1, while the nitrogen (N) content decreased. Furthermore, the leaf starch content decreased, whereas the sucrose content increased in HMg1 treated leaves.
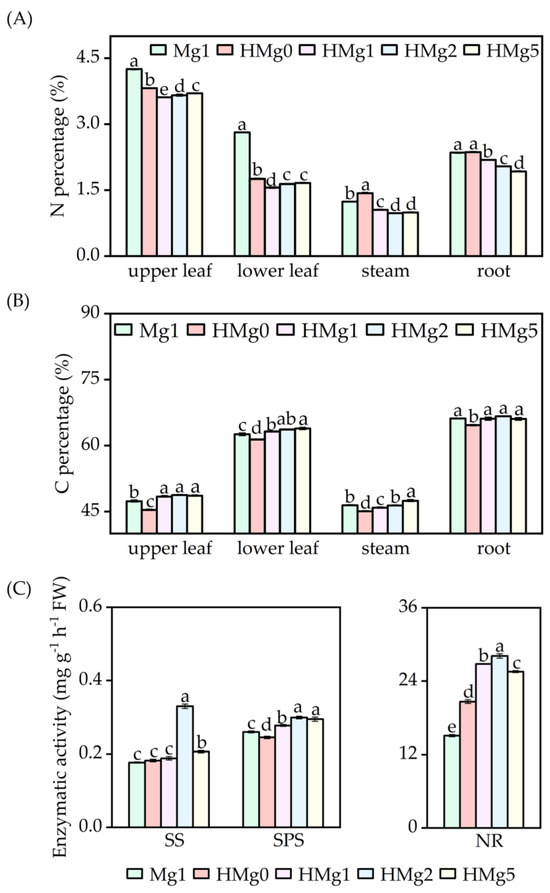
Figure 2.
Effects of Mg application on carbon and nitrogen metabolism under normal/high light. (A) Nitrogen percentage of tobacco. (B) Carbon percentage. (C) Key enzyme activity of carbon and nitrogen metabolism. SS, sucrose synthetase. SPS, sucrose phosphate synthase. NR, nitrate reductase. Mg1: 1 mol Mg, seedings grown under 750 μmol m−2 s−1 light intensity. HMg0, HMg1, HMg2, and HMg5 (0, 1, 2, and 5 mol Mg), grown under 1500 μmol m−2 s−1 light intensity for 10 days. The data presented in the bar graphs are expressed as means ± standard errors (n = 3). Means followed by the same small letter are not significantly different (p < 0.05).
Enzyme activities related to carbohydrate and nitrogen metabolism, including SS, SPS, and NR, are illustrated in Figure 2C. SS activity in the leaves was significantly higher under HMg2 and HMg5 treatments compared to Mg1, while the increases under HMg0 and HMg1 were not significant. SPS activity in the leaves also showed significant increases under HMg1, HMg2, and HMg5 treated leaves compared to Mg1. NR activity was notably higher in all HL-treated plants. As Mg supply levels increased, SS, SPS, and NR activities initially increased and then decreased, reaching a peak in treated leaves.
3.3. Impacts of Different Mg Supplementation Levels on Plant Light Response Curves and Curve-Fitting Parameters
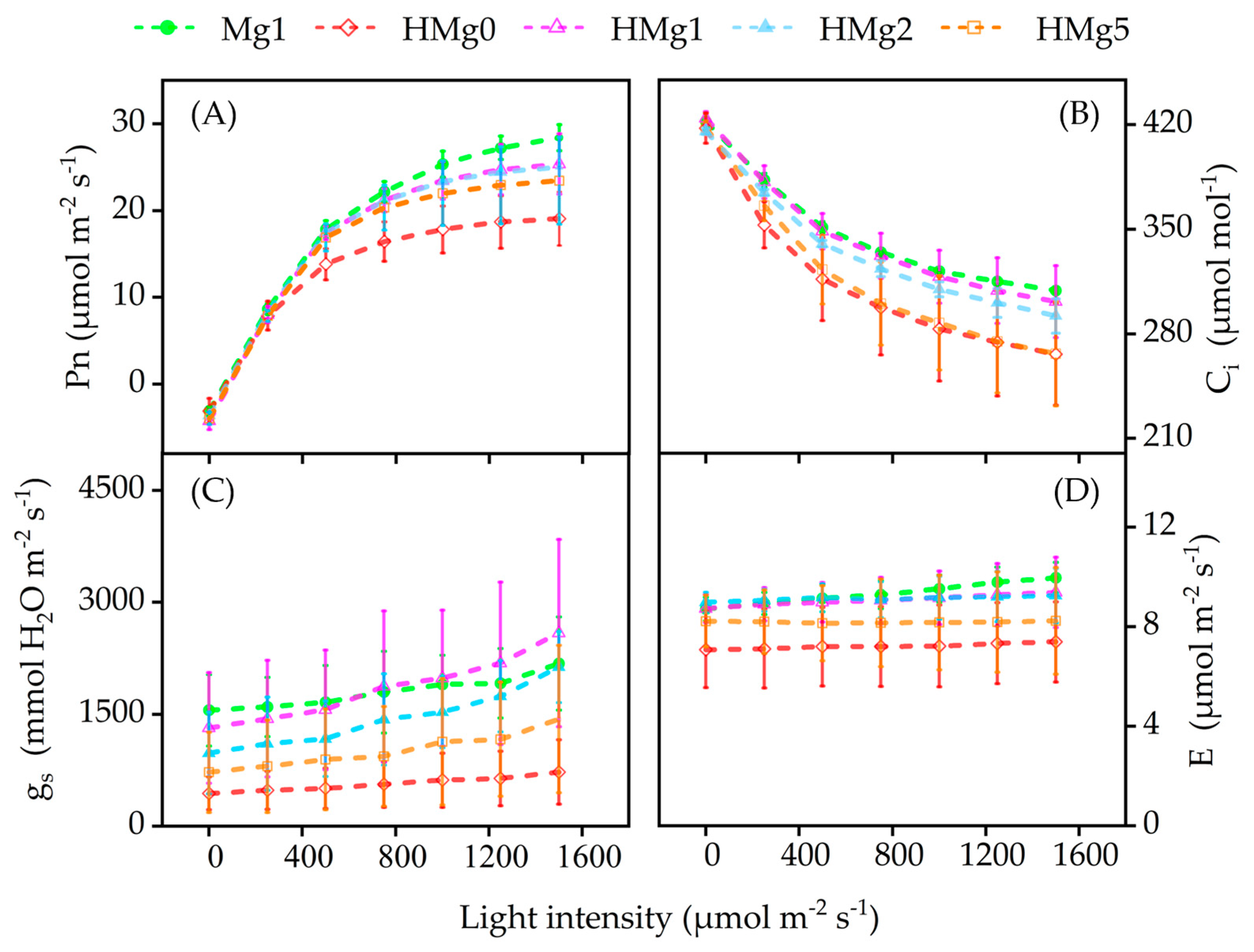
Under varying light intensities, Figure 3 shows a rapid linear increase in the net photosynthetic rate (Pn) from 0 to 500 μmol m−2 s−1, followed by a more gradual rise, as depicted in Figure 3A. Initially, at light intensities up to 250 μmol m−2 s−1, the Pn values across different treatments showed minimal variance. However, discrepancies became more pronounced as light intensity increased. Plants treated with 1 mol Mg (Mg1) showed a significant increase in Pn, peaking at 28.40 μmol CO2 m−2 s−1, exceeding those in HL conditions without Mg. Plants under HL without Mg (HMg0) reached a light saturation point more swiftly than those treated with varying Mg levels (HMg1, HMg2, and HMg5). Mg application significantly increased the Pn, particularly in HMg1 and HMg2 treatments, compared to HMg0. However, the Pn for HMg5 treated plants was lower than that for HMg1 and HMg2.
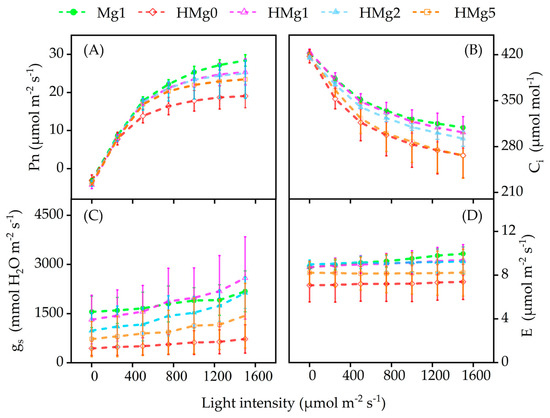
Figure 3.
Effects of different Mg application rates on photosynthesis under normal/high light. (A) Net photosynthetic rate (Pn). (B) Intercellular CO2 concentration (Ci). (C) Stomatal conductance (gs). (D) Evaporation rate (E) of plants under high light. Mg1: 1 mol Mg, seedings grown under 750 μmol m−2 s−1 light intensity. HMg0: 0 mol Mg, grown under 1500 μmol m−2 s−1 light intensity for 10 days. HMg0, HMg1, HMg2, and HMg5 (0, 1, 2, and 5 mol Mg), grown under 1500 μmol m−2 s−1 light intensity for 10 days.
As light intensity increased, the intercellular carbon dioxide concentration (Ci) decreased in all treatments, as shown in Figure 3B. Ci sharply declined up to 750 μmol m−2 s−1 and then gradually reduced. Under HL conditions, Ci values for HMg1 treated plants remained high, similar to those for Mg1 treated plants, with HMg2 being the next closest. Ci levels for HMg0 and HMg5 treatments were similar and consistently lower than those of other treatments. Stomatal conductance (gs) increased linearly with increasing light intensity, as demonstrated in Figure 3C. For Mg1 treated plants, gs increased from 1553.00 to 2179.67 μmol mmol−1. HMg1 treated plants, with gs values similar to Mg1, increased from 1319.00 to 2589.00 μmol mmol−1, and other treatments’ gs values increased as follows: HMg2 treated leaves from 727.33 to 2131.67, HMg5 from 722.67 to 1435.33, and HMg0 from 441.00 to 979.67 μmol mmol−1. The transpiration rate (E) followed a trend similar to gs across different treatments, as shown in Figure 3D, with a decline under HL-treated plants, which was moderated by increasing the Mg supply.
The hyperbolic model fitting of photosynthesis light response curves resulted in parameters (Table 1) with R2 values near 1. Light intensity and Mg application had no significant effect on the initial quantum efficiency (α), light compensation point (Ic), or dark respiration rate (Rd), but significantly influenced the maximum net photosynthetic rate (Pmax). Specifically, HL-treated plants lacking additional Mg (HMg0) showed a notable decrease in Pmax compared to those treated with Mg1.

Table 1.
Parameters of light response characteristic to light intensity of tobacco.
3.4. Alterations in Plant Chl Concentration and OJIP-Test Parameters of Photosynthetic Transient Fluorescence
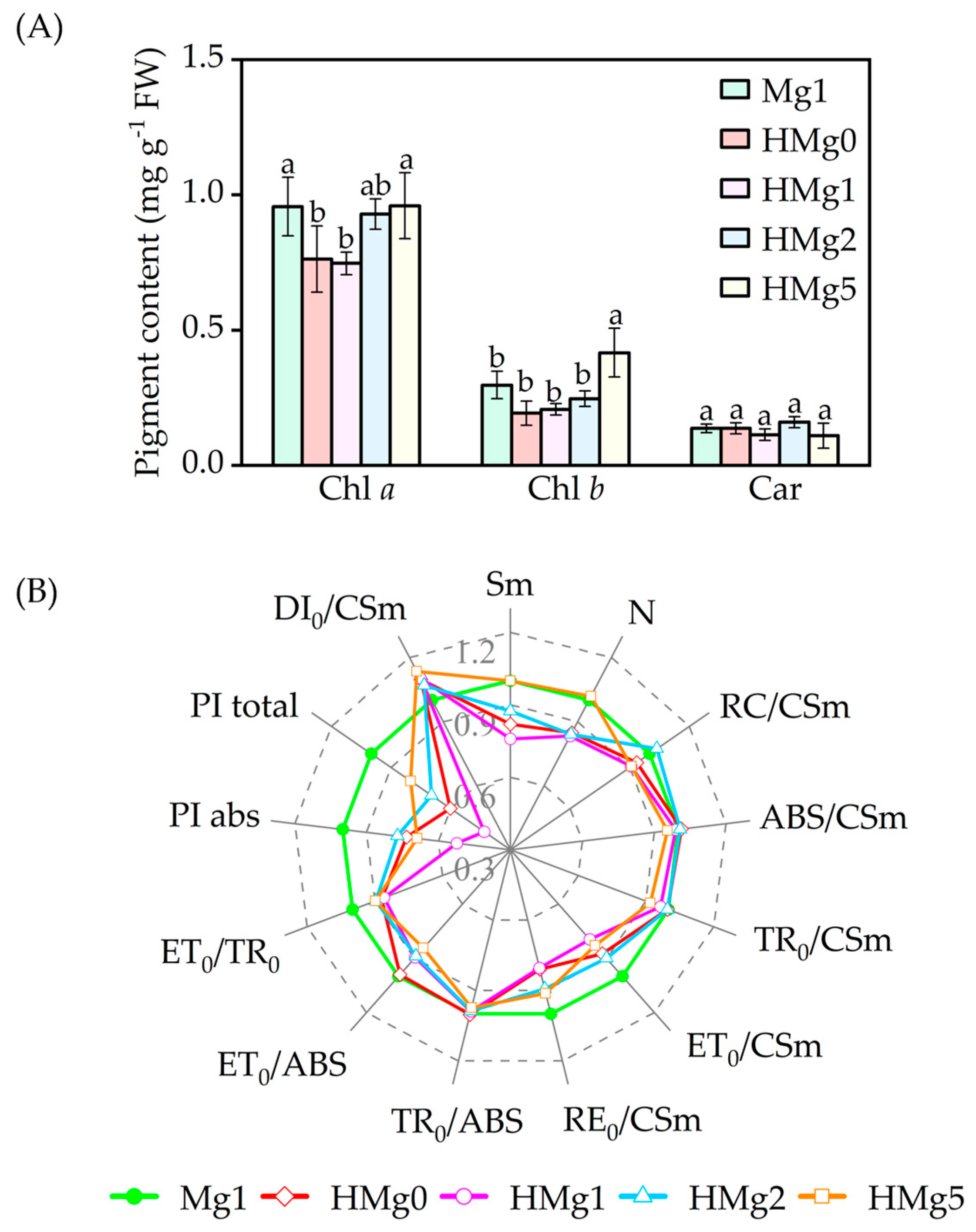
Pigment concentrations significantly declined in HMg0-treated plants, as shown in Figure 4A. Compared to Mg1 treated plants, Chl a and Chl (a + b) contents in HMg0 and HMg1 treatments significantly decreased (p < 0.05). In contrast, chlorophyll b (Chl b) content in HMg5 treated plants increased. No significant differences in pigment content were observed between HMg2 and Mg1. With the same Mg supply, the chlorophyll a (Chla) content in HMg1 treated leaves decreased compared to Mg1.
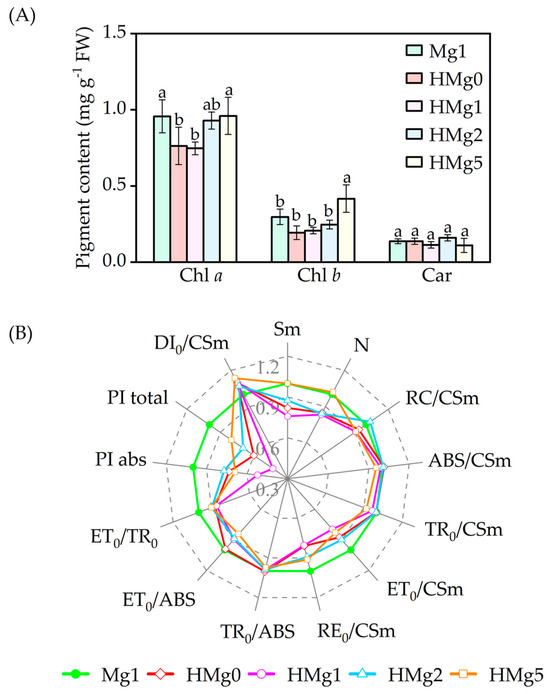
Figure 4.
Effects of magnesium application on pigment content (A) and photosynthetic fluorescence JIP-text parameters (B) under normal/high light. Chlorophyll a, Chl a. Chlorophyll b, Chl b. Carotenoids, Car. The JIP-text parameters were derived from the Chl F transients, normal light intensity (750 μmol m−2 s−1), and magnesium supply with 1 mol (Mg1) as the control, and the changing rate of each treatment relative to Mg1. The radar chart displays data points as means (n = 3) at each point. Means followed by the same small letter are not significantly different (p < 0.05).
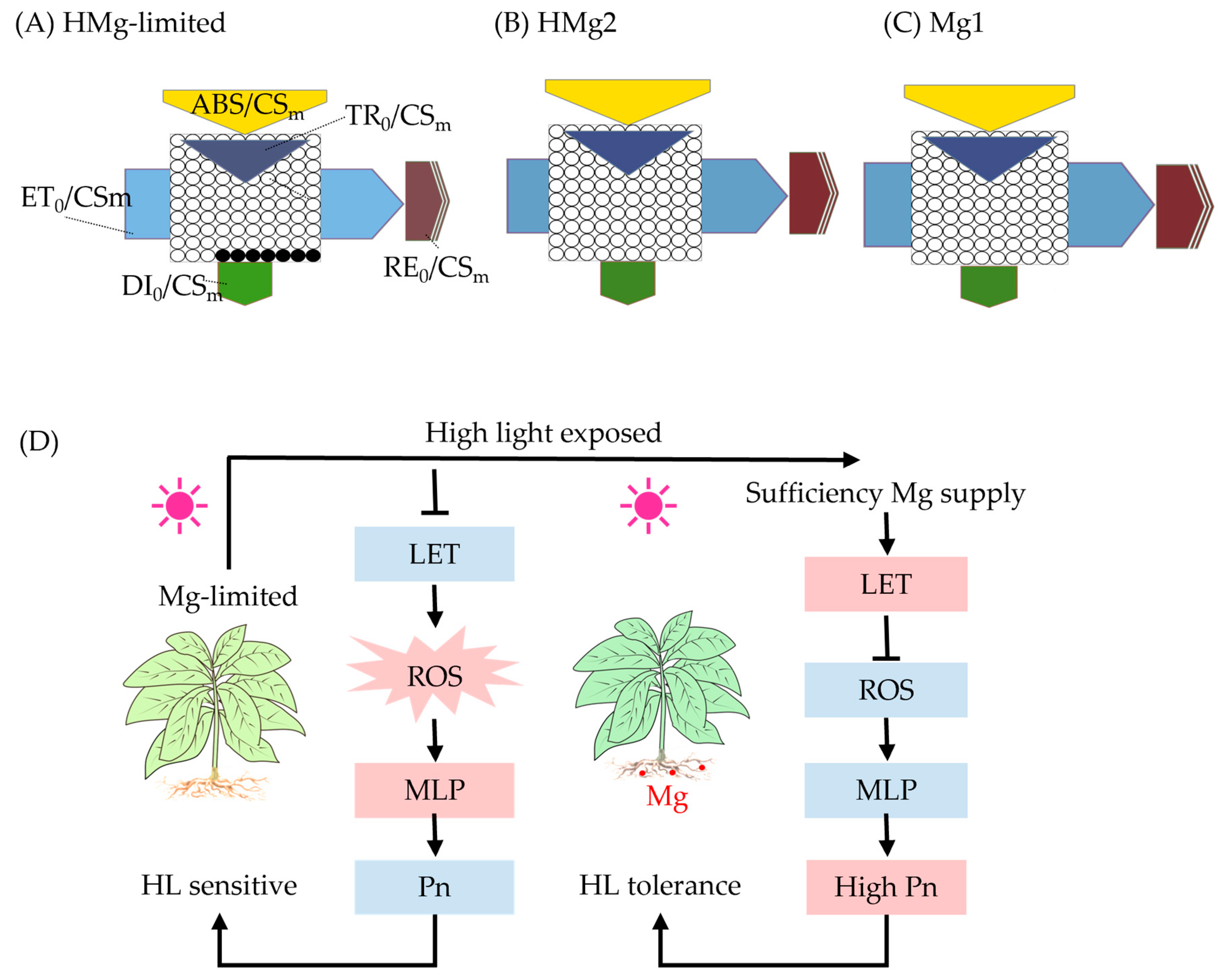
Monitoring Chl a fluorescence is a highly sensitive, reliable, and non-invasive method used extensively to study Photosystem II (PSII) behavior [43,44]. Parameters measured and calculated from OJIP were listed in Table S3. Changes in 12 OJIP transient parameters (Figure 4B) were inconsistent across Mg-supplied treatments. In HL-treated tobacco plants, variations in reaction center density per cross-section (RC/CSm), absorption energy per cross-section (ABS/CSm), energy flux capacity per cross-section (TR0/CSm), and maximum quantum yield of primary photochemistry (TR0/ABS) [45] were not significant compared to Mg1 treated leaves. Decreases in ET0/CSm (electron generation efficiency per cross-section), ET0/ABS (quantum yield for electron transport), and ET0/TR0 (probability of an exciton moving an electron beyond QA− in the electron transport chain) were observed in HL-treated leaves compared to Mg1 treated. As Mg supplies increased in HL-treated plants, ET0/CSm values decreased by 12.5%, 15.9%, 7.1%, and 23.6% in HMg0, HMg1, HMg2, and HMg5 treated leaves, compared with Mg1. Similarly, the trends in ET0/ABS and ET0/TR0 values across leaves supplied with varying Mg levels under HL treatment were consistent with ET0/CSm values.
RE0/CSm values, i.e., electron flux to reduction end electron acceptors at the PSI acceptor side, decreased in HL-treated leaves compared to Mg1, and increased in HMg2 and HMg5 treatments relative to HMg0. In HL-treated plants, the performance index (PI) for electron flux to final PSI electron acceptors and absorption-based PI (PItotal and PIabs) decreased compared to Mg1 and increased in HMg2 and HMg5 relative to HMg0. The pool sizes of electron acceptors (Sm) and the number of quinone protein (QA) reductions (N) on the reducing side of PSII decreased in HMg0, HMg1, and HMg2 treated leaves compared to Mg1. Dissipation rates (DI0/CSm) were increased in HL-treated leaves compared to Mg1.
4. Discussion
4.1. MgD Exacerbated High Light Stress on Plant Growth, and an Imbalance in Nitrogen and Carbohydrate Metabolism Reserves in Leaves
Effective adaptation to constantly changing environmental conditions is a prerequisite for plants to survive and compete in the field [46,47]. Light participates in regulating various biological processes related to plant growth and development [48,49]. Adequate nutrition is crucial for plants to avoid photo-oxidative damage under HL conditions. The results of this study showed that HL conditions promoted leaf wrinkling and premature aging, increased the total C content in plants under HL treatment, and decreased the N content, resulting in a serious imbalance in C and N reserves. Moreover, the activity of key enzymes involved in carbon and nitrogen metabolism increased. HL stress exacerbated the impact on MgD leaves. As the Mg supply increased, the sucrose content and total carbon (TC) content of leaves increased, while the starch content and total nitrogen (TN) content decreased. Through the characterization of tobacco (CoA matrix in Figure S5 and two-factor ANOVA in Table S4), the results showed that Mg positively regulated the carbon and nitrogen metabolism enzymes and their products (including SS, SPS, C, and sucrose percentage) in the HL-treated leaves, and negatively regulated the starch percentage of leaves. Mg influences numerous physiological processes. Previous studies have confirmed that MgD can lead to problems with phloem loading, leaf senescence [50], impaired stomatal opening, and disruption of cellular ionic balance [51]. This has been demonstrated in current research on Mg deficiency. Zhang et al.’s [52] research indicated that decreased light levels led to an increase in total foliar free amino acids and a decrease in soluble carbohydrates. The decrease in net CO2 fixation primarily depends on the extent to which the light and dark reactions are restricted, with MgD leading to significant restrictions on CO2 diffusion and biochemical activities [17,18,53,54]. As elements of tobacco quality, with the increase in leaf maturity or senescence, the contents of total sugar, reducing sugar, and starch in C and N percentage initially rise and then fall, while the percentage of N decreases [55]. This is consistent with the results of our study, where HL or MgD accelerated the senescence of leaves, especially the lower leaves. The C percentage in the upper leaves was lower than that the lower leaves, while the N percentage showed the opposite trend. The accumulation of non-structural carbon occurred in the early stages of tobacco leaf senescence. During leaf senescence, the reduction in photosynthesis occurred in conjunction with the level of nitrogen assimilation. Studies have found that during leaf senescence, leaf nutrient remobilization is manifested as a decrease in free amino acid levels [56].
4.2. MgD Restricts the Electron Transport Flux (ET) of High Light Plants, Increasing Oxidative Damage
Photosynthesis is crucial for plant growth, and light intensity affects the synthesis of photosynthesis, plant morphology, and primary and secondary metabolites [48]. Plant growth depends on the functionality of efficient photosynthetic complexes and their adaptability to changing environmental conditions [27]. Plant photosynthesis is mainly inhibited by the degradation of photosynthetic complex PSII under heat stress. PSII is located on the thylakoid membrane of chloroplasts and is highly sensitive to high temperatures [7]. Moreover, chloroplasts are most sensitive to MgD, as approximately 15–35% of Mg is bound to Chl molecules in plants [57,58]. Under HL conditions, MgD plants exhibited decreased Chl content, with decreases in ABS, TR, RE, and ET (Figure 5), where the decreases in ET and RE were greater than those in ABS and TR, while leaf H2O2 content and membrane damage product MDA content increased. Meanwhile, the functionality of PSII and the overall activity of the electron transfer chain between PSII and PSI (PIabs, total) were compromised, with a corresponding increase in thermal dissipation. Symptoms were alleviated after Mg supply. In response to fluctuations in light intensity, chloroplasts have multiple mechanisms to regulate electron and proton flux [59]. Absorption of excess light energy by Chl may lead to the production of triplet chlorophyll (3Chl), which subsequently transfers energy to oxygen molecules (O2), resulting in singlet oxygen (1O2) formation [8,60]. The 1O2 is then quenched by carotenoids. Additionally, the superoxide anion (O2−) produced in the Mehler reaction is mitigated through enzymatic action [60]. ROS play crucial roles in plant adaptation to biotic and abiotic stress, serving as signaling molecules in plant stress response. ROS include hydrogen peroxide (H2O2), O2−, and hydroxyl radical (·OH) [9,61,62,63]. Meanwhile, this study found that the activities of SOD, POD, and CAT in MgD leaves were significantly increased compared to leaves under normal growth conditions. Under HL conditions, the functionality of PSII and ETC are affected by HL and Mg [8]. Although chlorophyll fluorescence induction measurements are widely used in plant stress research, their full potential in many cases remains to be fully explored [64]. This study comprehensively examined the impact of MgD in plants exposed to HL on the efficiency of the photosynthetic electron transfer chain. Specifically, the analysis focused on the main energy pathways related to Chl a fluorescence, which are represented by five values: apparent antenna size (ABS), trapped energy in the reaction center (TR), electron transport flux (ET), electron flux of reduction end-electron acceptors at the PSI acceptor side (RE), and dissipated energy flux (DI) [1,60]. Meanwhile, Chl content is the primary factor affecting leaf light absorption, with the absorption rate increasing asymptotically with increasing Chl content per unit area [65,66]. Under HL, MgD plants showed a decrease in Chl content, and ABS, TR, RE, and ET (Figure 5). Particularly, the decrease in ET and RE was greater than that in ABS and TR, while leaf H2O2 content and membrane damage product MDA content increased. Additionally, the functionality of PSII and the overall activity of the electron transfer chain between PSII and PSI (PIabs, total) were compromised, with a corresponding increase in thermal dissipation. Symptoms were alleviated after Mg was supplied. This was consistent with previous research findings that HL inhibits chloroplast formation [12,46], and exposure to HL severely inhibits electron transfer and cyclic electron transfer pathways, leading to PSII damage that is difficult to correct in the short term, resulting in physiological disorders and premature leaf senescence in plants [67].
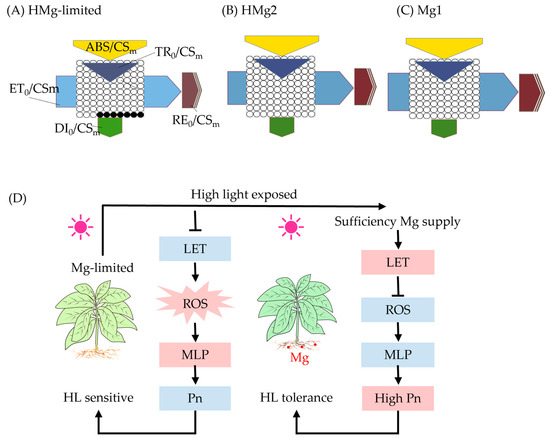
Figure 5.
Overview of Mg-limited HMg0 tobacco leaves or HMg2 response to high light. HMg0 tobacco leaves (A) or HMg2 (B) chlorophyll fluorescence leaf model changes compared to Mg1 treated plants (C). (D) Physiological mechanisms. When Mg0 plants were exposed to high light, the energy absorption (ABS) of PSII, as well as trapping (TR) of PSII photons, showed no significant changes, while the electron transport (ET) and electron flux to reduction end electron acceptors at the PSI acceptor side (RE) decreased. This process is associated with a higher likelihood of energy losses and the formation of highly reactive oxygen species. Consequently, the functionality between the PSII and PSI systems was compromised, leading to an increase in thermal dissipation. HMg2 plants exhibited higher ET and RE efficiency. As shown in (D), when exposed to both excessive light and magnesium deficiency simultaneously, the linear electron flow (LET) in photosynthesis significantly decreased, leading to an increase in dissipation (DI). This process involves energy loss and the formation of highly reactive oxygen species (ROS). The plant’s H2O2 levels increased as a result. The excess ROS interacted with cell membrane lipids (MLP), causing oxidative damage to the cell membrane and exacerbating the damage to PSII. Consequently, the plant became more sensitive to excessive light. Pn, photosynthesis rate; HL, high light. The black ellipses in (A) represent the proportion of inactive reaction centers of Photosystem II (PSII RCs) compared to Mg1 treated leaves in (C). The red fillings in (D) represent increased values or activity, while the blue fillings represent the opposite.
5. Conclusions
Our results demonstrated that exposure to HL inhibited plant growth and photosynthesis while increasing oxidative stress, leading to starch accumulation in leaves and accelerated senescence in lower leaves. However, it also improved the activity of key enzymes involved in carbon and nitrogen metabolism in plants. Mg supplies greatly enhanced the efficiency of the photosynthetic system, boosted the electron transport flux (ET), and reduced oxidative stress. Our study also observed that a high supply of Mg (Mg5) suppressed the risks associated with inhibiting plant photosynthesis and carbon and nitrogen metabolism. These findings provide valuable insights for the development of strategies to help plants cope with the stress caused by exposure to HL.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14071396/s1: Supplementary data available online include five figures and four tables. Figure S1: The impact of magnesium on tobacco physiological metabolism under normal light. Figure S2: The plant treatment model. Figure S3: Activities of superoxide dismutase (SOD), catalase (CAT), and peroxidase activity (POD) in leaves. Figure S4: Effects of different Mg supplies of seedings on starch and sucrose percentage under normal/high light. Figure S5: CoA matrix for traits evaluated in tobacco. Table S1: The physical and chemical properties of cultivated sand and water. Table S2: Improved Hoagland nutrient solution formulate. Table S3: Parameters measured and calculated from the Chl a fluorescence (OJIP) transient [43,44,65,68,69,70]. Table S4: Results of two-way ANOVA on tobacco morphological traits.
Author Contributions
Conceptualization, R.X., Y.K., C.L., C.Z. and W.L.; data curation, J.G., Z.Y., Y.W. and L.T.; methodology, Y.K., C.L., C.Z. and W.L.; software, R.X., J.G., Z.Y. and Y.W.; visualization, R.X.; writing—original draft, R.X.; writing—review and editing, Y.K., C.L., C.Z. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Science and Technology Innovation Foundation of Fujian Agriculture and Forestry University (CXZX2020076A) and the Open Research Foundation of the International Magnesium Institute (IMI2018-09).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, R.; Li, L.; Huang, J. The magnesium transporter MGT10 is essential for chloroplast development and photosynthesis in Arabidopsis thaliana. Mol. Plant 2017, 10, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Mengutay, M.; Ceylan, Y.; Kutman, U.B.; Cakmak, I. Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant Soil 2013, 368, 57–72. [Google Scholar] [CrossRef]
- Boaretto, R.M.; Hippler FW, R.; Ferreira, G.A.; Azevedo, R.A.; Quaggio, J.A.; Mattos, D. The possible role of extra magnesium and nitrogen supply to alleviate stress caused by high irradiation and temperature in lemon trees. Plant Soil 2020, 457, 57–70. [Google Scholar] [CrossRef]
- Rodrigues, V.A.; Crusciol, C.A.; Bossolani, J.W.; Moretti, L.G.; Portugal, J.R.; Mundt, T.T.; de Oliveira, S.L.; Garcia, A.; Calonego, J.C.; Lollato, R.P. Magnesium foliar supplementation increases grain yield of soybean and maize by improving photosynthetic carbon metabolism and antioxidant metabolism. Plants 2021, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Hoober, J.K.; Eggink, L.L.; Chen, M. Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth. Res. 2007, 94, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, Y.; Chen, L.-S. Magnesium deficiency–induced impairment of photosynthesis in leaves of fruiting Citrus reticulata trees accompanied by up-regulation of antioxidant metabolism to avoid photo-oxidative damage. J. Plant Nutr. Soil Sci. 2012, 175, 784–793. [Google Scholar] [CrossRef]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.K.; Pattanayak, G.K.; Pandey, S.S.; Leelavathi, S.; Reddy, V.S.; Govindjee Tripathy, B.C. Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in tobacco. Plant Physiol. 2012, 159, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, Q.; Yang, C.; Rao, X.; Wu, Z.; Wu, Z.; Fan, J.; Yu, Z. Effects of Mg on chlorophyll degradation and leaf chroma during the airing of cigar tobacco leaves. Acta Soc. Bot. Pol. 2023, 92, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef]
- Hauer-Jákli, M.; Tränkner, M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: A systematic review and meta-analysis from 70 years of research. Front. Plant Sci. 2019, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Tun Xiao, H.; Zhu, L.; Zhu, Y.; Wang, Z. Effects of magnesium on tobacco growth and chlorophyll fluorescence parameters of tobacco leaves. J. Plant Nutr. Fertil. 2008, 14, 151–155. [Google Scholar] [CrossRef]
- Li, J.; Yokosho, K.; Liu, S.; Cao, H.R.; Yamaji, N.; Zhu, X.G.; Liao, H.; Ma, J.F.; Chen, Z.C. Diel magnesium fluctuations in chloroplasts contribute to photosynthesis in rice. Nat. Plants 2020, 6, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Muneer, M.A.; Sun, A.; Guo, Q.; Wang, Y.; Huang, Z.; Li, W.; Zheng, C. Magnesium application improves the morphology, nutrients uptake, photosynthetic traits, and quality of tobacco (Nicotiana tabacum L.) under cold stress. Front. Plant Sci. 2023, 14, 1078128. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, W.; Xu, G. Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Hald, S.; Nandha, B.; Gallois, P.; Johnson, G.N. Feedback regulation of photosynthetic electron transport by NADP(H) redox poise. Biochim. Biophys. Acta (BBA) Bioenerg. 2008, 1777, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Alamri, S.A.; Al-Khaishany MY, Y.; Al-Qutami, M.A.; Ali, H.M.; Al-Whaibi, M.H.; Al-Wahibi, M.S.; Alharby, H.F. Mitigation of adverse effects of heat stress on Vicia faba by exogenous application of magnesium. Saudi J. Biol. Sci. 2018, 25, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Mengütay, M. Adequate Magnesium Nutrition Mitigates Adverse Effects of Heat and High Light Stress on Maize and Wheat. Ph.D. Thesis, Sabanci University, Istanbul, Turkey, 2014. [Google Scholar]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant-soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, P. Oxidative stress under macronutrient deficiency in plants. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Cao, L.; Chen, L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotoxicol. Environ. Saf. 2020, 191, 110206. [Google Scholar] [CrossRef] [PubMed]
- Guo, W. Magnesium homeostasis mechanisms and magnesium use efficiency in plants. In Plant Macronutrient Use Efficiency; Academic Press: London, UK, 2017; pp. 197–213. [Google Scholar]
- Thussagunpanit, J.; Jutamanee, K.; Sonjaroon, W.; Kaveeta, L.; Chai-Arree, W.; Pankean, P.; Suksamrarn, A. Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica 2015, 53, 312–320. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Sofo, A.; Dippolito, I.; Khan, N.A. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 2021, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed MH, M.; Ibrahim MF, M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Li, J. Nutrients and Fertilization of Tobacco Soils in China; China Agricultural Press: Beijing, China, 2001. [Google Scholar]
- Lu, R.K. Analysis Method of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Lightenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–383. [Google Scholar] [CrossRef]
- Ye, Z.P. A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica 2007, 45, 637–640. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, J.A.; Cress, W.A.; Krüger GH, J.; Strasser, R.J.; Van Staden, J. Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J. Plant Physiol. 2004, 161, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q. Plant Physiology Experiments Guide; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Strasserf, R.J.; Srivastava, A.; Govindjee. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Jiang, H.X.; Chen, L.S.; Zheng, J.G.; Han, S.; Tang, N.; Smith, B.R. Aluminum-induced effects on Photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008, 28, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Cheng, L. Photosystem 2 is more tolerant to high temperature in apple (Malus domestica Borkh.) leaves than in fruit peel. Photosynthetica 2009, 47, 112–120. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Schumann, T.; Paul, S.; Melzer, M.; Dörmann, P.; Jahns, P. Plant growth under natural light conditions provides highly flexible short-term acclimation properties toward high light stress. Front. Plant Sci. 2017, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Chen, Y.; Zhang, H.; Xia, C.; Zhang, Q.; Li, W. The effect of colored plastic films on the photosynthetic characteristics and content of active ingredients of Dysosma versipellis. Hortic. Environ. Biotechnol. 2018, 59, 519–528. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Tanoi, K.; Kobayashi, N.I. Leaf senescence by magnesium deficiency. Plants 2015, 4, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Kleczkowski, L.A.; Igamberdiev, A.U. Magnesium signaling in plants. Int. J. Mol. Sci. 2021, 22, 1159. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, T.; Ni, L.; Xie, P.; Li, Z. Carbon, nitrogen and antioxidant enzyme responses of Potamogeton crispus to both low light and high nutrient stresses. Environ. Exp. Bot. 2010, 68, 44–50. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Ye, X.; Gao, Z.; Xu, K.; Li, B.; Ren, T.; Li, X.; Cong, R.; Lu, Z.; Cakmak, I.; Lu, J. Photosynthetic plasticity aggravates the susceptibility of magnesium—Deficient leaf to high light in rapeseed plants: The importance of Rubisco and mesophyll conductance. Plant J. 2024, 117, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, J.; Gu, G.; Jin, L.; Chen, C.; Lin, Z.; Song, J.; Xie, X. Integrative analyses of biochemical properties and transcriptome reveal the dynamic changes in leaf senescence of tobacco (Nicotiana tabacum L.). Front. Genet. 2021, 12, 790167. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, J.; Zhao, Y.; Lu, X.; Zhou, Z.; Zhao, C.; Xu, G. Comprehensive investigation of tobacco leaves during natural early senescence via multi-platform metabolomics analyses. Sci. Rep. 2016, 6, 37976. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition. Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Tränkner, M.; Jamali Jaghdani, S. Minimum magnesium concentrations for photosynthetic efficiency in wheat and sunflower seedlings. Plant Physiol. Biochem. 2019, 144, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Avenson, T.J.; Cruz, J.A.; Kanazawa, A.; Kramer, D.M. Regulating the proton budget of higher plant photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9709–9713. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Weronika, C.; Stanisław, K. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Irato, P.; Santovito, G. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Strasser, R.J.; Qiang, S. In vivo assessment of effect of phytotoxin tenuazonic acid on PSII reaction centers. Plant Physiol. Biochem. 2014, 84, 10–21. [Google Scholar] [CrossRef]
- Evans, J.R. Developmental constraints on photosynthesis: Effects of light and nutrition. In Photosynthesis and the Environment; Springer: Dordrecht, The Netherlands, 1996; pp. 281–304. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.; Hannaway, D.; Lu, H. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Wang, F.; Sun, H.; Rong, L.; Li, Z.; An, T.; Hu, W.; Ye, Z. Genotypic-dependent alternation in D1 protein turnover and PSII repair cycle in psf mutant rice (Oryza sativa L.), as well as its relation to light-induced leaf senescence. Plant Growth Regul. 2021, 95, 121–136. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).