Case Study on the Effects of Sodium Carboxymethyl Cellulose and Biostimulants on Physiological and Photosynthetic Characteristics, Yield, and Quality of Apples
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Area
2.2. Experimental Design
2.3. Measurement of Indicators
- (1)
- Soil bulk density and particle composition
- (2)
- Measurement of leaf SPAD
- (3)
- Determination of leaf nitrogen, phosphorus, and potassium content
- (4)
- Measurement of photosynthetic indexes
- (5)
- Measurement of yield indexes
- (6)
- Measurement of quality indexes
2.4. Data Processing
3. Results and Analysis
3.1. Apple Leaf Nutrients
3.2. Apple Leaf SPAD Values
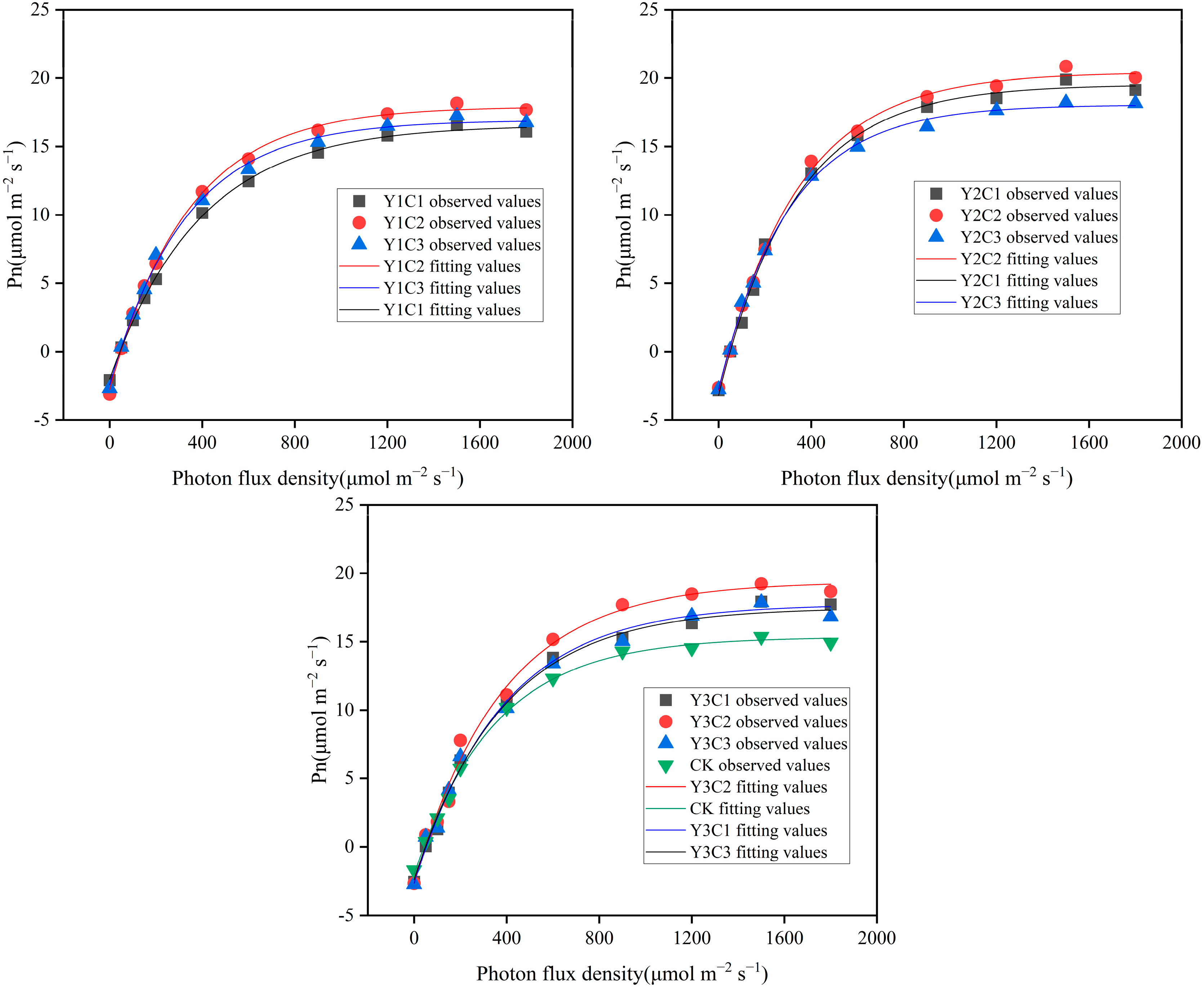
3.3. Apple Light Response Curves
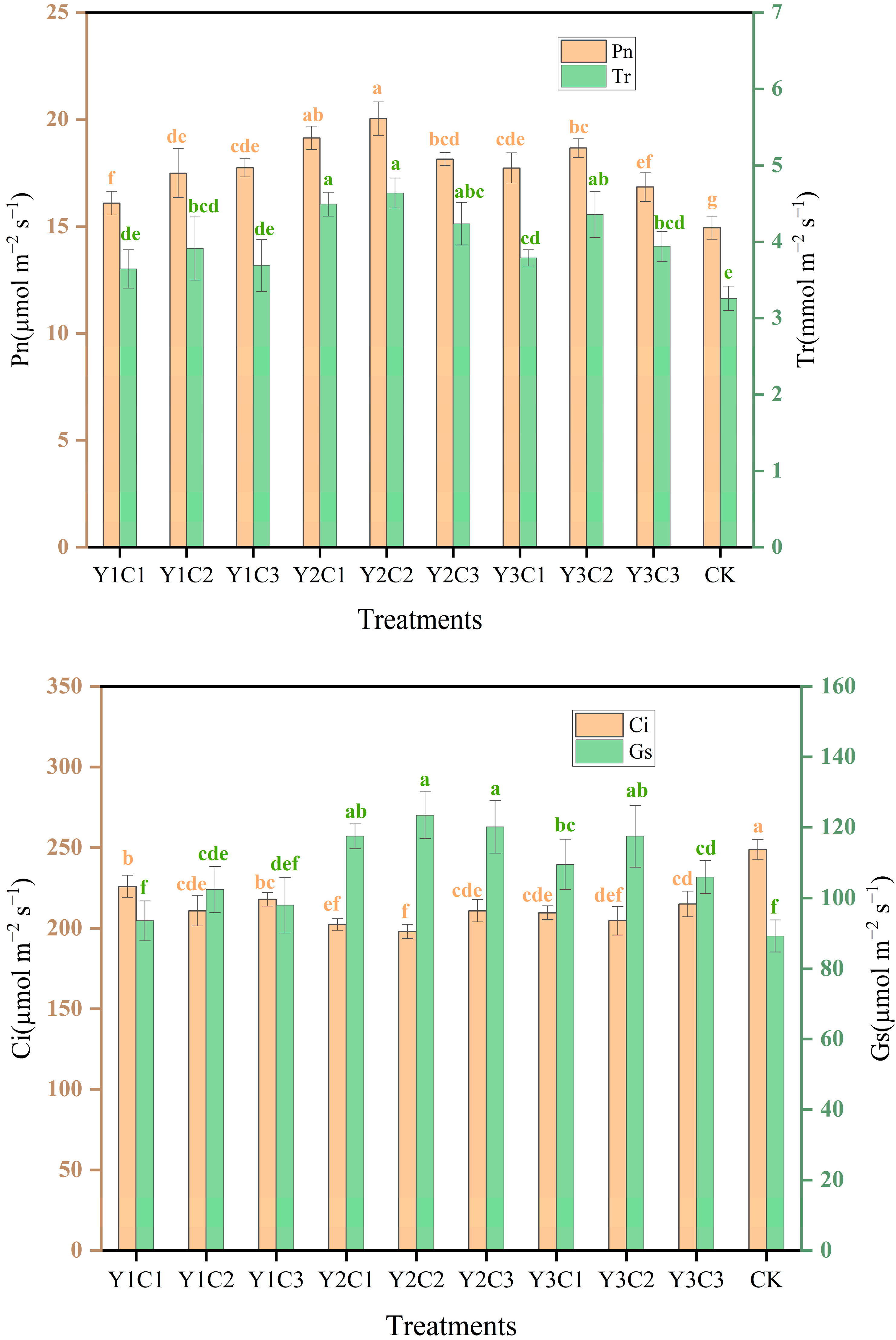
3.4. Photosynthetic Characteristics of Apple Leaves
3.5. Apple Yield and Quality
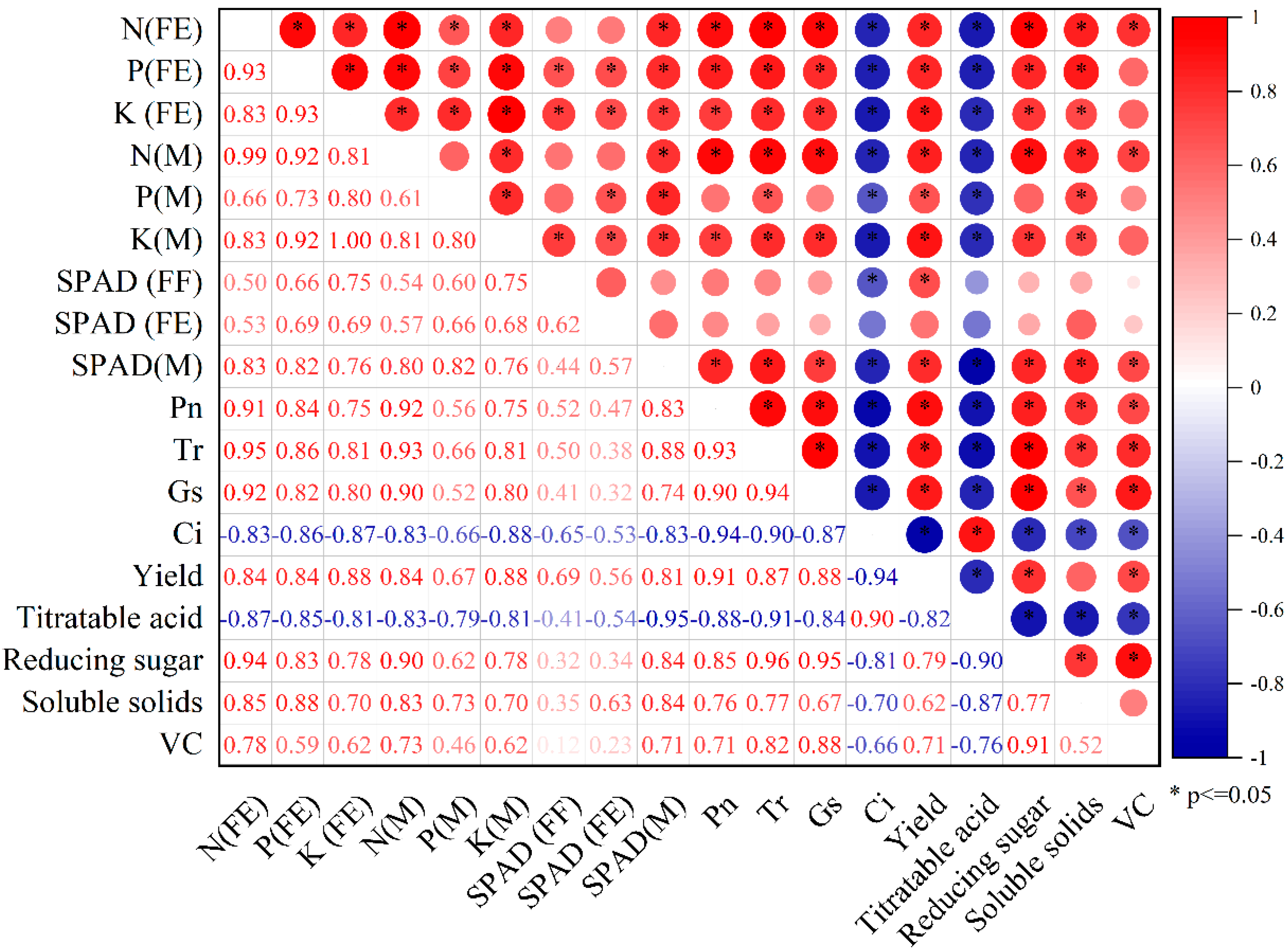
3.6. Correlation Analysis of Apple Yield and Quality with Physiological Indicators
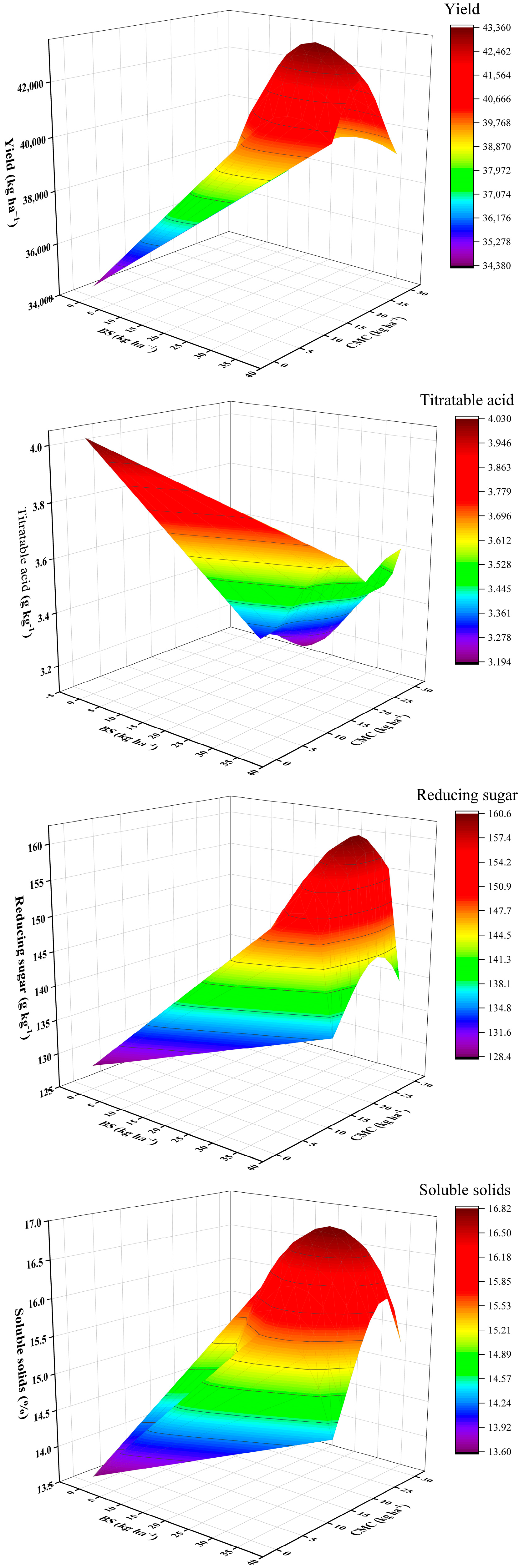
3.7. Functional Relationship between the Optimal Application Rate of BS and CMC and Yield and Quality Indexes
4. Discussion
- (1)
- The mechanism of BS and CMC in improving soil
- (2)
- Effect of BS and CMC on leaf SPAD
- (3)
- Mechanism of the effect of BS and CMC on enhancing the photosynthetic properties of leaves
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shu, H.R.; Chen, X.D. The current task of the development of fruits industry in China. China Fruits 2018, 2, 1–3. [Google Scholar]
- Zhang, Q.Q.; Huo, X.X.; Liu, J.D.; Yang, H.L. The analysis and prediction of world apple production pattern and market dynamics. World Agric. 2016, 7, 147–152. [Google Scholar]
- Cheng, C.G.; Zhao, D.Y. Development orientation and trend of the apple industry under the new situation. China Fruits 2019, 1, 1–7. [Google Scholar]
- Wang, J.W.; Ma, Z.W. Research on the Development Path of Apple Industry in Aksu Prefecture, Xinjiang. Xinjiang Farm Res. Sci. Technol. 2022, 45, 25–28. [Google Scholar]
- Deng, Y.; Li, X.P.; Cui, F.R. Taxonomy of saline-alkali soil in Aksu area. J. Northwest A F Univ. 2006, 1, 113–116. [Google Scholar]
- Li, X.H. Effects of drought on apple growth and development and defensive measures. Mod. Agric. Sci. Technol. 2017, 15, 215–219. [Google Scholar]
- Ning, S.R.; Jumai, H.; Wang, Q.J.; Zhou, B.B.; Su, L.J.; Shan, Y.Y.; Zhang, J.H. Comparison of the Effects of Polyacrylamide and Sodium Carboxymethylcellulose Application on Soil Water Infiltration in Sandy Loam Soils. Adv. Polym. Technol. 2019, 2019, 6869454. [Google Scholar] [CrossRef]
- Yang, S.Q.; Xing, L.; Liu, H.Y.; Guo, P. Effects of sodium carboxymethyl cellulose application on soil properties of new cultivating farmland in loess plateau. J. China Agric. Univ. 2021, 26, 185–191. [Google Scholar]
- Xi, Y.Q.; Zhao, Y.; Li, S.Y. Effects of Three Kinds of Soil Amendments on Shear Strength of Aeolian Soil. Acta Pedol. Sin. 2018, 55, 1401–1410. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Gu, D.Y.; Wang, X.F.; Yang, F.J.; Jiao, J.; Wei, M.; Shi, Q.H. Effects of purified humic acid on growth and nutrient uptake of cucumber seedlings under low nitrogen stress. Humic Acid 2018, 4, 52. [Google Scholar]
- Ertani, A.; Pizzeghello, D.; Baglieri, A.; Cadili, V.; Tambone, F.; Gennari, M.; Nardi, S. Humic-like substances from agro-industrial residues affect growth and nitrogen assimilation in maize (Zea mays L.) plantlets. J. Geochem. Explor. 2013, 129, 103–111. [Google Scholar]
- Barrajon-Catalan, E.; Álvarez-Martínez, F.J.; Borras, F.; Perez, D.; Herrero, N.; Ruiz, J.J.; Micol, V. Metabolomic analysis of the effects of a commercial complex biostimulant on pepper crops. Food Chem. 2019, 310, 125818. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.H.; Liu, Z.G.; Zhang, M.; Jia, J.W.; Zheng, L.; Wang, Q.B.; Wang, H.F. Effects of extracts from Paecilomyces variotii on the yield and quality of cherry radish. J. Agric. Resour. Environ. 2019, 36, 176–183. [Google Scholar]
- Feng, J.T.; Xing, Y.; Zhang, X.; Jia, Z.H.; Li, H.F.; Liu, X.Y.; Ge, S.F.; Jiang, Y.M. Effects of salicylic acid, abscisic acid and seaweed extract on drought resistance and nutrient uptake of apple seedlings under drought stress. China Fruits 2019, 06, 42–48. [Google Scholar]
- Panfili, I.; Bartucca, M.L.; Marrollo, G.; Povero, G.; Buono, D.D. Application of a Plant Biostimulant to Improve Maize (Zea mays) Tolerance to Metolachlor. J. Agric. Food Chem. 2019, 67, 12164–12171. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 238366. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Aahraf, M. Induction of Drought Tolerance in Maize (Zea mays L.) due to Exogenous Application of Trehalose: Growth, Photosynthesis, Water Relations and Oxidative Defence Mechanism. J. Agron. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, W.X.; Zhao, X.M.; Yi, H. Effect of chitooligosaccharide on Reducing Cryoinjury and Improving Metabolite of Winter Wheat seedings under Low Temperature Stress. J. Triticeae Crop. 2016, 36, 653–658. [Google Scholar]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal imaging of soybean response to drought stress: The effect of Ascophyllum nodosum seaweed extract. SpringerPlus 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Qu, D.Y.; Gu, W.R.; Li, L.J.; Li, J.; Li, C.F.; Wei, S. Regulation of chitosan on the ascorbate-glutathione cycle in Zea mays seedling leaves under cadmium stress. Plant Sci. J. 2018, 36, 291–299. [Google Scholar]
- Pang, Q.Q.; Sun, G.W.; Cai, X.L.; Zhou, M.; Zhao, J.H.; Li, D.M. Effects of Fulvic Acid on Reactive Oxygen Metabolism and Related Gene Expression in Pakchoi under NO3—Stress. Mol. Plant Breed. 2018, 16, 5812–5820. [Google Scholar]
- Liu, B.Y.; Wang, L.; Yang, L.X.; Tong, X.Y.; Piao, Z.H.; Li, X. Effects of Different Leaf Fertilizers on Chlorophyll, Yield and Fruit Quality of Pingguoli. J. Anhui Agric. Sci. 2021, 49, 156–158. [Google Scholar]
- Li, Z.Q.; Bai, W.B.; Zhang, Y.L.; Wang, W.R.; Yan, H. Effects of Foliar Fertilizers on Fruit Quality of Fuji Line-Jinfu 2. J. Shanxi Agric. Sci. 2012, 40, 41–43. [Google Scholar]
- Erikson, U.G. Muscle Quality of Atlantic Salmon (Salmo salar) as Affected by Handling Stress. Ph.D. Thesis, Trondheim University, Trondheim, Norway, 1997. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=0f6e3924373a83e327fa25232e8ad29b&site=xueshu_se&hitarticle=1 (accessed on 25 June 2024).
- Bao, D.D.; Xing, T.; Xu, Y.; Zhang, Z.J.; Tan, H.L.; Chen, H.Y. Analysis on the Effect of Spraying Different Amino Acid Foliar Fertilizers on the Volatile Substances of Aksu Red Fuji Apple. Mod. Food 2023, 29, 181–184. [Google Scholar]
- Genovese, M.; Viccione, G.; Rossi, F.; Guida, D.; Lenza, T.L.L. Using the Sodium Carboxymethylcellulose (CMC) as viscosity modifier to model the interstitial fluid in laboratory debris flows. In Latest Trends in Engineering Mechanics, Structures, Engineering Geology; Viccione, G., Guarnaccia, C., Eds.; WSEAS Press: Houston, TX, USA, 2014; pp. 179–186. [Google Scholar]
- Ramos, A.C.; Dobbss, L.B.; Santos, L.A. Humic matter elicits proton and calcium fluxes and signaling dependent on Ca2+-dependent protein kinase (CDPK) at early stages of lateral plant root development. Chem. Biol. Technol. Agric. 2015, 2, 1–12. [Google Scholar] [CrossRef]
- Lipson, D.A.; Raab, T.K.; Schmidt, S.K.; Monson, R.K. Variation in competitive abilities of plants and microbes for specific amino acids. Biol. Fertil. Soils 1999, 29, 257–261. [Google Scholar] [CrossRef]
- Zhu, A.M.; Zhang, Y.X.; Wang, X.G.; Tian, Y.L. Effects of Cutting Treaments in Late Autumn on Antioxidant Enzyme Activity and Proline Content in crown of Sandy Land Alfalfa in Wint. Acta Agrestia Sin. 2018, 26, 222–230. [Google Scholar]
- Xu, X.P.; Wang, Y.K.; Feng, H.; Zhao, X.N. Research Summary of the Soil Amendment’s Effect on Improving Soil, Cultivating Fertilizer and Increasing Yield. Chin. Agric. Sci. Bull. 2007, 9, 331–334. [Google Scholar]
- Berbara, R.L.L.; García, A.C. Humic Substances and Plant Defense Metabolism; Springer Science and Business Media: New York, NY, USA, 2014. [Google Scholar]
- Sun, Y.; Wu, J.X.; Qu, Z.; Han, N.; Lu, J.Y.; Ma, Y.Y.; Hu, Z.F. Effects of biochemical fulvic acid on the water-salt transport characteristics of soda saline-alkali soil with different textures. Trans. Chin. Soc. Agric. Eng. 2023, 39, 74–84. [Google Scholar]
- Zhang, Y.H.; He, A.L.; Yang, Z.P.; Zheng, C.F.; Du, J.; Bao, D.J.; Yao, J.; Yang, H.H. Effects of Root-irrigation with Alginate Oligosaccharide Solution on Photosynthetic Characteristics, Dry Matter Accumulation and Yield of Wheat. Acta Agric. Jiangxi 2018, 30, 1–5. [Google Scholar]
- Battacharyya, D.; Babgorahi, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Qiu, Y. Application of Bacillus subtilis in Modern Agriculture. Hortic. Seed 2022, 7, 81–85. [Google Scholar]
- Lu, S.F.; Hu, S.Q.; Yan, C.X.; Shi, C.H. Study on the Preparation of Fulvic Acid from Cotton Stalk in Southern Xinjiang and Its Influence on Soil Properties. Humic Acid 2023, 6, 49–56+67. [Google Scholar]
- Li, Q.X.; Li, M.Y.; Dong, W.H. Research Progress on the Application of Carboxymethyl Cellulose in Soil Improvement. China Resour. Compr. Util. 2024, 42, 102–104. [Google Scholar]
- Halidaimu, J.M.; Ning, S.R.; Wang, Q.J.; Zhang, J.H. Comparative Analysis of the Effects of PAM and CMC on Soil Infiltration and Evaporation. J. Soil Water Conserv. 2020, 1, 121–127. [Google Scholar]
- Feng, J.T. Effects of Seaweed Extract on Drought Resistance and Nutrient Uptake of Apple Seedlings under Drought Stress. Agriculum Res. Areas 2019, 38, 80–85. [Google Scholar]
- Yang, J.; Yin, Y.H.; Shen, H. Effect of seaweed functional substances on drought resistance of flowering cabbage. Phosphate Compd. Fertil. 2019, 34, 34–42. [Google Scholar]
- Horinouchi, H.; Katsuyama, N.; Taguchi, Y.; Hyakumachi, B. Control of Fusarium crown and root rot of tomato in a soil system by combination of a plant growth-promoting fungus, Fusarium equiseti, and biodegradable pots. Crop Prot. 2008, 27, 859–864. [Google Scholar] [CrossRef]
- Li, G.Y.; Ding, H.Q.; Shen, J.L.; Shen, H. Study on preparation of water-soluble chitosan and its effect on drought resistance of Chinese flowering cabbage. Phosphate Compd. Fertil. 2018, 33, 30–34. [Google Scholar]
- Wali, M.; Gunsè, B.; Llugany, M.; Corrales, I.; Abdelly, C.; Poschenrieder, C.; Ghnaya, T. High salinity helps the halophyte Sesuvium portulacastrum in defense against Cd toxicity by maintaining redox balance and photosynthesis. Planta Int. J. Plant Biol. 2016, 244, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.L.; Wu, T.T.; Wang, Y.B.; Zhang, X.Q. The characteristics of cadmiun to lerance and accumulation in three kinds of ornamental plants. Acta Ecol. Sin. 2010, 30, 2491–2498. [Google Scholar]
- Ming, B.Y.; Huang, M.; Fu, X.J.; Yang, Q.S.; Zhou, N.; Guo, D.Q. Correlation analysis of SPAD value and chlorophyll content in leaves of Fritillaria thunbergii. South China Agric. 2023, 17, 34–37. [Google Scholar]
- Pan, J. Correlation analysis of SPAD value and chlorophyll content in apple leaves. Contemp. Hortic. 2017, 21, 39–40. [Google Scholar]
- Lei, Z.X.; AI, T.C.; Li, F.M.; Dong, W. The Relationships between SPAD Readings and the Contents of Chlorophyll and Nitrogen in Strawberry Leaves. J. Hubei Agric. Coll. 2001, 2, 138–140. [Google Scholar]
- Zhao, K.C.; Ye, Y.; Ma, J.; Qian, T.C.; Huang, L.F.; Zhuang, H.Y. Dynamic model and characteristic analysis of SPAD value in rice leaves after flowering. Jiangsu Agric. Sci. 2021, 49, 74–80. [Google Scholar]
- Zhang, Y.J.; Wang, L.; Bai, Y.L.; Lu, Y.L.; Zhang, J.J.; LI, G. Relationship of physiological and biochemical indicators with SPAD values in maize leaves at different layers. J. Plant Nutr. Fertil. 2020, 26, 1805–1817. [Google Scholar]
- Flexas, J.; Carrioui, M. Photosynthesis and photosynthetic efficiencies along the terrestrial plants phylogeny: Lessons for improving crop photosynthesis. Plant J. 2020, 101, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.G.; Keenan, T.F.; Prentice, I.C.; Wang, H.; Wright, I.J.; Niinemets, U.; Crous, K.Y.; Domingues, T.F.; Guerrieri, R.; Ishida, F.K.; et al. Global photosynthetic capacity is optimized to the environment. Ecol. Lett. 2019, 22, 506–517. [Google Scholar] [CrossRef]
- Jones, H.G. Partitioning stomatal and non-stomatal limitations to photosynthesis. Plant Cell Environ. 2010, 8, 95–104. [Google Scholar] [CrossRef]
- Veromann-Jürgenson, L.L.; Brodribb, T.J.; Niinemets, U.; Tosens, T. Variability in the chloroplast area lining the intercellular airspace and cell walls drive mesophyll conductance in gymnosperms. J. Exp. Bot. 2020, 71, 4958–4971. [Google Scholar] [CrossRef] [PubMed]
- Vian, A.; Stankovic, B.; Davies, E. Signalomics: Diversity and Methods of Analysis of Systemic Signals in Plants. In PlantOmics: The Omics of Plant Science; Springer: Berlin/Heidelberg, Germany, 2015; pp. 459–489. [Google Scholar]
- Wang, Y.; Chan, K.X.; Long, S.P. Towards a dynamic photosynthesis model to guide yield improvement. Plant J. 2021, 107, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Improving Photosynthesis. Plant Physiol. 2013, 162, 1780–1793. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.P.; Kong, L.L.; Li, Q.; Yang, J.; Yin, C.X.; Qin, Y.B.; Zhang, L.; Yu, L.; Wang, L.C.; Xie, J.G. Studies on Effect of Different Phosphorus Levels on Yield, Nutrient Absorption of Rice and Soil Phosphorus Balance. J. Northeast Agric. Sci. 2016, 41, 61–66. [Google Scholar]
- Chiou, T.J.; Lin, S.I. Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 2011, 62, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fan, W.H.; Liu, F.W.; Zhang, H.; Wang, G.L.; Yu, M.M.; Tian, L.D. Effects of Phosphorus on Photosynthesis and Antioxidant Enzyme System of Cucumber Seedlings under Cadmium Stress. Chin. J. Soil Sci. 2022, 53, 596–604. [Google Scholar]
- Feng, L.; Liu, S.Q.; Cheng, B.; Wang, Y.; Liu, Q.; Ma, G.Q.; Li, H. Effects of different phosphorus levels on yield, photosynthetic characteristics and quality of garlic under hydroponic culture. Soil Fertil. Sci. China 2014, 03, 38–43. [Google Scholar]
- Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy 2021, 11, 2010. [Google Scholar] [CrossRef]
- Deng, H.Y.; Wang, Y.Y.; Guo, J.H. Uptake and utilization of phosphorus by plants. Rural Sci. Technol. 2022, 13, 71–75. [Google Scholar]
- Dixon, M.; Simonne, E.; Obreza, T.; Liu, G. Crop response to low phosphorus bioavailability with a focus on tomato. Agronomy 2006, 10, 617. [Google Scholar] [CrossRef]
- Mohamed, E.A.H.; Farag, A.G.; Youssef, S.A. Phosphate Solubilization by Bacillus subtilis and Serratia marcescens Isolated from Tomato Plant Rhizosphere. J. Environ. Prot. 2018, 9, 266–277. [Google Scholar] [CrossRef]

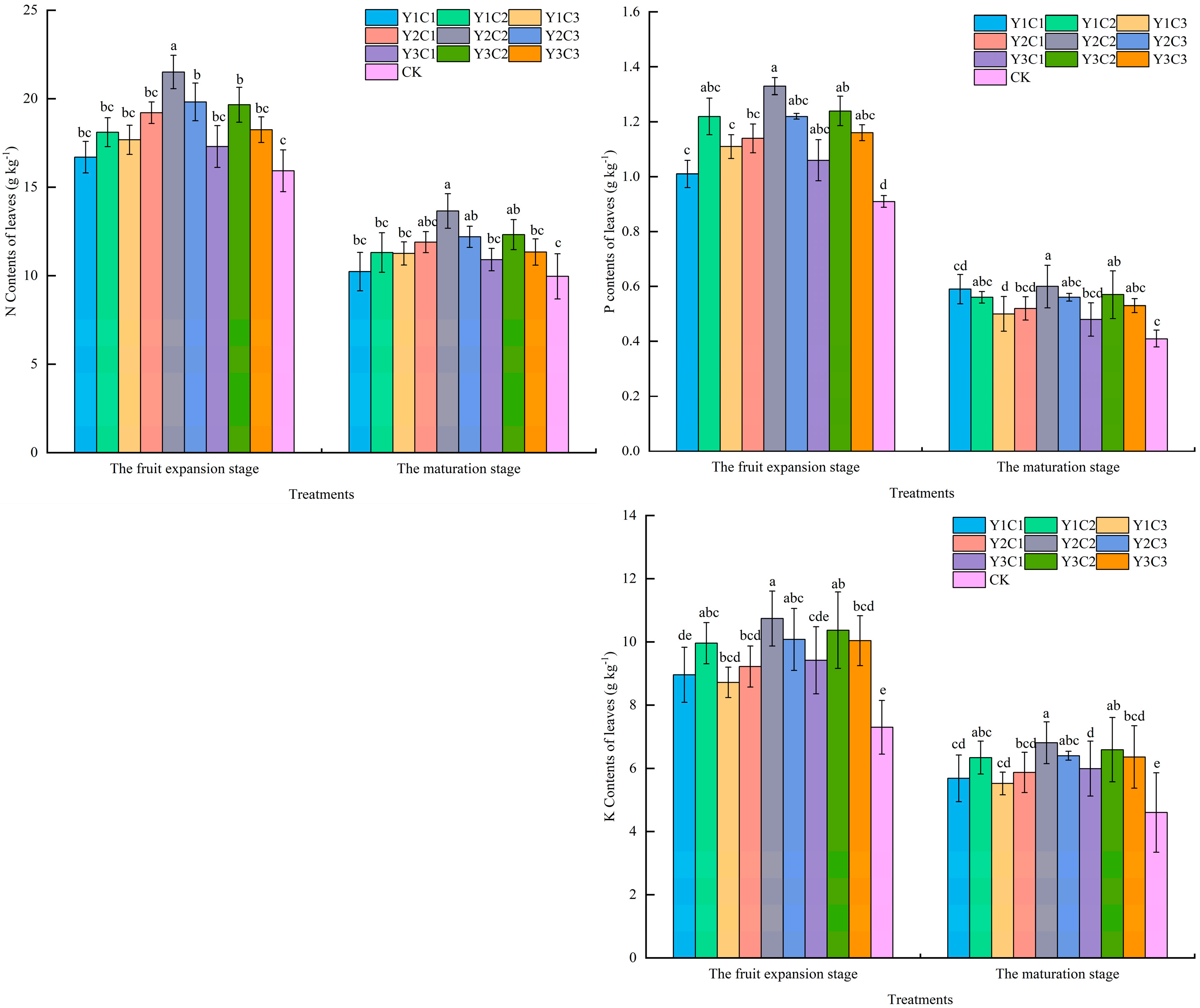


| Soil Depth (cm) | Soil Dry Density (g cm−3) | Sticky Grains (%) | Powder (%) | Sand (%) |
|---|---|---|---|---|
| 0–20 | 1.52 | 0.51 | 3.21 | 96.28 |
| 20–40 | 1.58 | 1.86 | 8.21 | 90.93 |
| 40–60 | 1.51 | 0 | 1.41 | 98.59 |
| 60–80 | 1.53 | 0 | 1.27 | 98.73 |
| 80–100 | 1.48 | 0 | 0.42 | 99.58 |
| 100–120 | 1.51 | 0.42 | 4.43 | 95.15 |
| Ingredient | Physical Property | Chemical Property | Functions |
|---|---|---|---|
| Glycine | White or off-white crystalline powder, odorless, with special sweet taste. Soluble in water, not easy to decompose. | Chemical formula C2H5NO2, the constituent amino acid of the endogenous antioxidant reduced glutathione, is one of the simplest structures in the amino acid family. Glycine is able to undergo a variety of chemical reactions with other substances, such as salt formation, esterification and amidation. | Amino acids that can be directly absorbed and utilized by the roots and leaves of crops are absorbed by plants as raw materials for growth hormones, which are involved in the growth and development of plants, can enhance the plant’s ability to adapt to adversity, and have a growth-promoting effect on the plant. |
| Proline | White crystal or crystalline powder state, soluble in water, not easy to decompose. | Chemical formula C5H9NO2, a cyclic subamino acid whose molecular structure contains a pyrrolidine ring, which can be converted to glutamic acid in living organisms by the action of proline oxidase. | Roots and leaves of crops can absorb amino acids, which are one of the components of plant proteins and can exist widely in the plant body in a free state, which can enhance the crops’ resilience to adversity (drought, salinity, heat, cold and frost). |
| Fulvic acid | Reddish brown or gray-black powdery substance, easily soluble in water. | Humic acid with the molecular weight of a very small organic acid, aqueous solution pH value is usually in the range of 3–5, can reduce water surface tension and reduce contact angle, has certain redox properties. | Can be directly absorbed and utilized by plant roots or leaves, and can adsorb nitrogen in the soil, chelate with phosphorus, transform phosphorus from soil to plant-absorbable form, and transform insoluble potassium to soluble potassium, which can improve plant drought resistance, cold resistance and resistance to pests and diseases, and as a kind of broad-spectrum plant growth regulator, it can effectively promote the growth of plants. |
| Bacillus subtilis | A kind of bacillus genus, no pod membrane, with periplasmic flagellum; can move, reproduce faster, and is a kind of aerobic bacteria. | In the process of metabolism, it produces a variety of enzymes, such as protease, α-amylase, cellulase, etc. It can produce active substances such as chytridiomycin, polymyxin, mycobacteriocin, short mycopeptide, etc., which have obvious inhibitory effects on pathogenic bacteria or endogenous infections of conditionally pathogenic bacteria. | It can decompose organic materials and release nutrients such as nitrogen, phosphorus, and potassium to improve soil fertility. Forms a probiotic environment in the soil, promotes the formation of granular structure, improves the ability of soil to retain fertilizer and water, and increases soil looseness. Secretion of active substances can stimulate crop-growth-type endogenous hormones; for example, indole acetic acid, gibberellin, and other content increased. |
| Sodium alginate oligosaccharides | White or light yellow powder, easily soluble in water, dissolved in water to form a viscous colloidal solution. | Alginate degradation from an oligosaccharide, containing a large number of carboxyl and hydroxyl groups and other functional groups; these functional groups have good water solubility and bioactivity, and can interact with multivalent ions to form hydrogels. | It can increase the porosity of soil, have a regulating effect on the acidity and alkalinity of soil, stimulate the activity of the defense enzyme system in the plant body, promote the development of the plant root system, and improve the absorption and utilization of soil nutrients and water by the plant. As a new type of plant growth regulator, it can promote the growth of the plant, and improve the efficiency of photosynthesis and the efficiency of water utilization. |
| Deal with | BS (kg ha−1) | CMC (kg ha−1) |
|---|---|---|
| Y1C1 | 12 | 15 |
| Y1C2 | 12 | 22.5 |
| Y1C3 | 12 | 30 |
| Y2C1 | 24 | 15 |
| Y2C2 | 24 | 22.5 |
| Y2C3 | 24 | 30 |
| Y3C1 | 36 | 15 |
| Y3C2 | 36 | 22.5 |
| Y3C3 | 36 | 30 |
| CK | 0 | 0 |
| Experimental Treatments | α | LCP (µmol m−2 s−1) | LSP (µmol m−2 s−1) | Rd (µmol m−2 s−1) | Pnmax (µmol m−2 s−1) | R2 |
|---|---|---|---|---|---|---|
| Y1C1 | 0.049 | 43.92867 | 1620.956 | 2.164 | 16.2871 | 0.9991 |
| Y1C2 | 0.064 | 47.42824 | 1689.886 | 3.047 | 17.92024 | 0.9993 |
| Y1C3 | 0.063 | 43.42894 | 1757.698 | 2.753 | 16.96672 | 0.9982 |
| Y2C1 | 0.067 | 48.52862 | 1469.893 | 3.231 | 19.40255 | 0.9948 |
| Y2C2 | 0.069 | 43.38928 | 1539.73 | 2.973 | 20.28507 | 0.9964 |
| Y2C3 | 0.072 | 41.73269 | 1606.801 | 3.014 | 18.13575 | 0.9971 |
| Y3C1 | 0.056 | 51.7857 | 1874.962 | 2.885 | 17.72405 | 0.9943 |
| Y3C2 | 0.054 | 47.35235 | 1448.919 | 2.559 | 19.02897 | 0.9893 |
| Y3C3 | 0.053 | 48.42088 | 1705.706 | 2.582 | 17.26578 | 0.9922 |
| CK | 0.047 | 41.52662 | 1413.831 | 1.959 | 15.25137 | 0.9966 |
| Treatment | Titratable Acid (g kg−1) | Reducing Sugar (g kg−1) | Soluble Solids (%) | VC (mg 100 g−1) | Yield (kg ha−1) |
|---|---|---|---|---|---|
| Y1C1 | 3.62 ± 0.10 b | 133.83 ± 4.93 cd | 14.57 ± 0.17 bc | 12.16 ± 0.22 cd | 38,070 ± 2792.42 ab |
| Y1C2 | 3.50 ± 0.11 bc | 139.41 ± 4.62 bcd | 15.70 ± 0.08 ab | 11.90 ± 0.36 cde | 40,050 ± 1402.00 ab |
| Y1C3 | 3.65 ± 0.08 b | 132.46 ± 1.59 cd | 15.43 ± 0.50 ab | 10.98 ± 0.55 e | 38,100 ± 2042.20 b |
| Y2C1 | 3.35 ± 0.11 bc | 150.77 ± 4.33 ab | 15.40 ± 0.37 ab | 13.85 ± 0.13 ab | 40,620 ± 750.60 ab |
| Y2C2 | 3.28 ± 0.07 c | 154.72 ± 3.89 a | 16.40 ± 0.29 a | 14.43 ± 0.42 a | 43,890 ± 1571.50 a |
| Y2C3 | 3.37 ± 0.15 bc | 152.35 ± 4.66 ab | 16.13 ± 0.54 a | 14.13 ± 0.20 a | 40,350 ± 2460.73 ab |
| Y3C1 | 3.67 ± 0.08 b | 136.70 ± 2.14 cd | 13.87 ± 0.37 cd | 12.96 ± 0.44 bc | 41,460 ± 2448.63 a |
| Y3C2 | 3.53 ± 0.07 bc | 146.27 ± 6.70 abc | 15.27 ± 0.49 ab | 12.65 ± 0.23 c | 42,480 ± 2682.95 a |
| Y3C3 | 3.64 ± 0.11 b | 140.60 ± 5.93 bcd | 14.73 ± 0.37 bc | 12.44 ± 0.39 cd | 38,730 ± 1837.61 ab |
| CK | 4.03 ± 0.10 a | 128.43 ± 2.37 d | 13.60 ± 0.16 d | 11.51 ± 0.30 de | 34,392 ± 837.38 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Mu, W.; Wang, Q.; Zhang, J.; Bai, Y.; Sun, Y.; Lu, Z.; Wei, X. Case Study on the Effects of Sodium Carboxymethyl Cellulose and Biostimulants on Physiological and Photosynthetic Characteristics, Yield, and Quality of Apples. Agronomy 2024, 14, 1403. https://doi.org/10.3390/agronomy14071403
Zheng M, Mu W, Wang Q, Zhang J, Bai Y, Sun Y, Lu Z, Wei X. Case Study on the Effects of Sodium Carboxymethyl Cellulose and Biostimulants on Physiological and Photosynthetic Characteristics, Yield, and Quality of Apples. Agronomy. 2024; 14(7):1403. https://doi.org/10.3390/agronomy14071403
Chicago/Turabian StyleZheng, Ming, Weiyi Mu, Quanjiu Wang, Jianghui Zhang, Yungang Bai, Yan Sun, Zhenlin Lu, and Xuesong Wei. 2024. "Case Study on the Effects of Sodium Carboxymethyl Cellulose and Biostimulants on Physiological and Photosynthetic Characteristics, Yield, and Quality of Apples" Agronomy 14, no. 7: 1403. https://doi.org/10.3390/agronomy14071403
APA StyleZheng, M., Mu, W., Wang, Q., Zhang, J., Bai, Y., Sun, Y., Lu, Z., & Wei, X. (2024). Case Study on the Effects of Sodium Carboxymethyl Cellulose and Biostimulants on Physiological and Photosynthetic Characteristics, Yield, and Quality of Apples. Agronomy, 14(7), 1403. https://doi.org/10.3390/agronomy14071403





