Geographical Environment and Plant Functional Group Shape the Spatial Variation Pattern of Plant Carbon Density in Subalpine-Alpine Grasslands of the Eastern Loess Plateau, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Measurement of Plant Biomass
2.4. Data Analysis
2.4.1. Estimation of Plant Carbon Density
2.4.2. Spatial Interpolation of Plant Carbon Density
3. Results
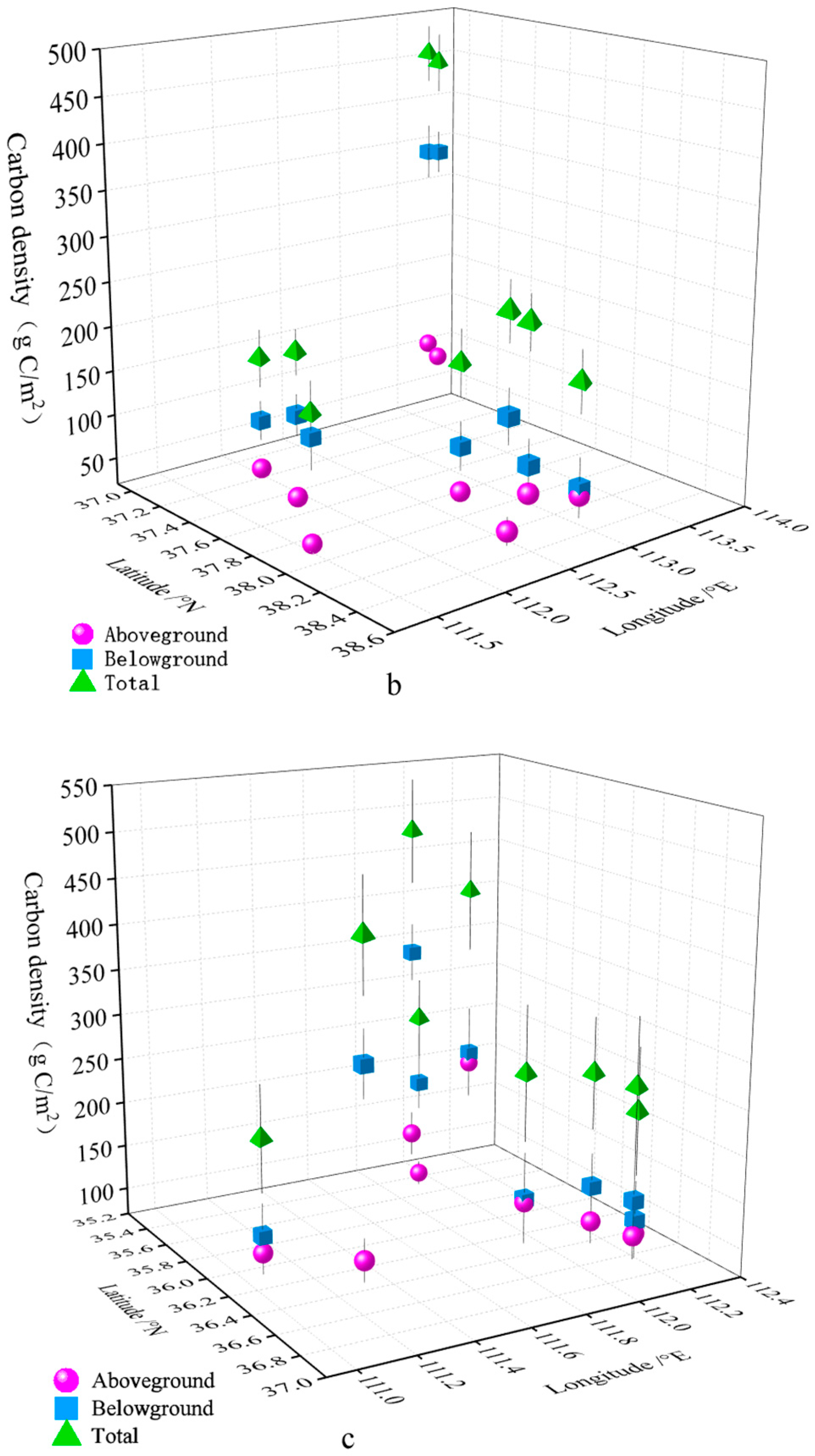
3.1. Estimation of Plant Carbon Density of SGs
3.2. Spatial Distribution Characteristics of Plant Carbon Density in SGs
3.3. Spatial Variations in Plant Carbon Density with Different Functional Groups in SGs
4. Discussion
4.1. Plant Carbon Density and Its Spatial Distribution of SGs
4.2. Spatial Distribution of Plant Carbon Density with Different Functional Groups
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiskopf, S.R.; Isbell, F.; Arce-Plata, M.I.; Marco, M.D.; Harfoot, M.; Johnson, J.; Lerman, S.B.; Miller, B.W.; Morelli, T.L.; Mori, A.S.; et al. Biodiversity loss reduces global terrestrial carbon storage. Nat. Commun. 2024, 15, 4354. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; He, J.L.; Wang, Z.J.; Jiang, Q. Characteristics and composition of vegetation carbon storage in natural grassland in Ningxia, China. J. Appl. Ecol. 2021, 32, 1259–1268. [Google Scholar]
- Zhao, Z.H.; Liu, G.H.; Mou, N.X.; Xie, Y.C.; Xu, Z.R.; Li, Y. Assessment of carbon storage and its influencing factors in Qinghai-Tibet Plateau. Sustainability 2018, 10, 1864. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Wang, S.P. Typical Grassland Ecosystems in China; Science Press: Beijing, China, 2000. [Google Scholar]
- Ashraf, M.; Lone, F.A.; Wani, A.A. Estimating herbaceous plant biomass carbon under different forest strata of Dachigam National Park. Indian For. 2017, 143, 680–684. [Google Scholar]
- Keith, H.; Mackey, B.; Berry, S.; Lindenmayer, D.; Gibbons, P. Estimating carbon carrying capacity in natural forest ecosystems across heterogeneous landscapes: Addressing sources of error. Glob. Chang. Biol. 2010, 16, 2971–2989. [Google Scholar] [CrossRef]
- Matsuura, S.; Sasaki, H.; Kohyama, K. Organic carbon stocks in grassland soils and their spatial distribution in Japan. Grassl. Sci. 2012, 58, 79–93. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Hayes, D. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Post, W.M.; Emanuel, W.R.; Zinke, P.J.; Stangenberger, A.G. Soil carbon pools and world life zones. Nature 1982, 298, 156–159. [Google Scholar] [CrossRef]
- Arneth, A.; Kelliher, F.M.; McSeveny, T.M.; Byers, J.N. Net ecosystem productivity, net primary productivity and ecosystem carbon sequestration in a Pinus radiata plantation subject to soil water deficit. Tree Physiol. 1998, 18, 785–793. [Google Scholar] [CrossRef]
- He, H.P.; Wang, W.M.; Zhang, Y.H.; Chen, Q.S. Carbon and nitrogen storage in Inner Mongolian grasslands: Relationships with climate and soil texture. Pedosphere 2014, 3, 391–398. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Li, M.; Yin, P. Effects of Grazing on Soil Organic Carbon in the Rhizosphere of Stipa Grandis in a Typical Steppe of Inner Mongolia, China. Sustainability 2022, 14, 11866. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Li, X.; Wang, S.; Liu, W.; Shi, S.; Cao, W. Nitrogen fertilizer regulates soil respiration by altering the organic carbon storage in root and topsoil in alpine meadow of the north-eastern Qinghai-Tibet Plateau. Sci. Rep. 2019, 9, 13735. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.Z.; Jing, C.Q.; Deng, X.J.; Chen, C.; Zhao, W.K.; Hou, Z.X.; Wang, G.X. Soil respiration characteristics of typical grassland on the northern slopes of the Tianshan Mountains and their response to environmental factors. J. Agric. Sci. Technol. 2022, 24, 189–199. [Google Scholar]
- Song, B.L.; Yan, M.J.; Hou, H.; Guan, J.H.; Shi, W.Y.; Li, G.Q.; Du, S. Distribution of soil carbon and nitrogen in two typical forests in the semiarid region of the Loess Plateau, China. Catena 2016, 143, 159–166. [Google Scholar] [CrossRef]
- Chen, J.Q. The Biomass and Carbon Storage Distribution of Grassland Ecosystems in Shanxi Province. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2016. [Google Scholar]
- Wang, N.; Fu, F.Z.; Wang, B.T.; Wang, R.J. Carbon, nitrogen and phosphorus stoichiometry in Pinus tabulaeformis forest ecosystems in warm temperate Shanxi Province, north China. J. For. Res. 2018, 29, 1665–1673. [Google Scholar] [CrossRef]
- Qiao, S.W. The Spatial Pattern of Natural Grassland Biomass and Carbon Storage along Geographical Gradients in Shanxi Province. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2014. [Google Scholar]
- Zhang, Z.Y.; Li, G.; Zhang, B.B.; Zhao, X.; Ren, G.H.; Dong, K.H.; Gao, W.J.; Wang, Y.X. Estimation on carbon distribution and storage of typical natural grassland in Shanxi Province. Acta Agrestia Sin. 2017, 25, 69. [Google Scholar]
- Taravat, A.; Wagner, M.P.; Oppelt, N. Automatic grassland cutting status detection in the context of spatiotemporal Sentinel-1 imagery analysis and artificial neural networks. Remote Sens. 2019, 11, 711. [Google Scholar] [CrossRef]
- Xu, M.H.; Li, J.; Liu, Z.Q.; Wang, J.Y.; Wei, K.K.; Yan, J.L.; Cheng, J.W.; Liu, X.J. Eight-year warming might induce a shift toward forbs in an alpine meadow community of the Qinghai-Tibetan Plateau by increasing the soil temperature and nitrogen content. Environ. Exp. Bot. 2024, 219, 105632. [Google Scholar] [CrossRef]
- Negi, G.C.S.; Rikhari, H.C.; Singh, S.P. Phenological features in relation to growth forms and biomass accumulation in an alpine meadow of the Central Himalaya. Vegetatio 1992, 101, 161–170. [Google Scholar] [CrossRef]
- Xu, M.H.; Zhang, S.X.; Wen, J.; Yang, X.Y. Multiscale spatial patterns of species diversity and biomass together with their correlations along geographical gradients in subalpine meadows. PLoS ONE 2019, 14, e0211560. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.; Herrero, J.; Polo, M.J. Topographic effects on solar radiation distribution in mountainous watersheds and their influence on reference evapotranspiration estimates at watershed scale. Hydrol. Earth Syst. Sci. 2010, 14, 2479–2494. [Google Scholar] [CrossRef]
- Kimura, R.; Bai, L.; Fan, J.; Takayama, N.; Hinokidani, O. Evapo-transpiration estimation over the river basin of the Loess Plateau of China based on remote sensing. J. Arid Environ. 2007, 68, 53–65. [Google Scholar] [CrossRef]
- De Boeck, H.J.; Lemmens, C.M.H.M.; Zavalloni, C.; Gielen, B.; Malchair, S.; Carnol, M.; Merckx, R.; Van den Berge, J.; Ceulemans, R.; Nijs, I. Biomass production in experimental grasslands of different species richness during three years of climate warming. Biogeosciences 2008, 5, 585–594. [Google Scholar] [CrossRef]
- Hook, P.B.; Lauenroth, W.K.; Burke, I.C. Spatial patterns of roots in a semiarid grassland: Abundance of canopy openins and regeneration gaps. J. Ecol. 1994, 82, 485–494. [Google Scholar] [CrossRef]
- Scurlock, J.M.O.; Hall, D.O. The global carbon sink: A grassland perspective. Glob. Chang. Biol. 1998, 4, 229–233. [Google Scholar] [CrossRef]
- Brown, S.; Pearson, T.; Walker, S.M.; MacDicken, K. Methods Manual for Measuring Terrestrial Carbon; Winrock International: Arlington, VA, USA, 2005. [Google Scholar]
- Kumar, S.; Lal, R.; Liu, D. A geographically weighted regression Kriging approach for mapping soil organic carbon stock. Geoderma 2012, 189, 627–634. [Google Scholar] [CrossRef]
- Bhunia, G.S.; Shit, P.K.; Maiti, R. Comparison of GIS-based interpolation methods for spatial distribution of soil organic carbon (SOC). J. Saudi Soc. Agric. Sci. 2018, 17, 114–126. [Google Scholar] [CrossRef]
- Farooq, I.; Bangroo, S.A.; Bashir, O.; Shah, T.I.; Malik, A.A.; Iqbal, A.M.; Biswas, A. Comparison of random forest and Kriging models for soil organic carbon mapping in the Himalayan Region of Kashmir. Land 2022, 11, 2180. [Google Scholar] [CrossRef]
- Von Haden, A.C.; Dornbush, M.E. Depth distributions of belowground production, biomass and decomposition in restored tallgrass prairie. Pedosphere 2019, 29, 457–467. [Google Scholar] [CrossRef]
- Webber, P.J.; May, D.E. The magnitude and distribution of belowground plant structures in the alpine tundra of Niwot Ridge, Colorado. Arct. Alp. Res. 1977, 9, 157–174. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Spiro, B.; Yang, X.M. Oxygen, carbon and strontium isotope study of the carbonatitic dolomite host of the Bayan Obo Fe-Nb-REE deposit, Inner Mongolia, N China. Mineral. Mag. 1997, 61, 531–541. [Google Scholar] [CrossRef]
- Maleki, S.; Khormali, F.; Bodaghabadi, M.B.; Mohammadi, J.; Hoffmeister, D.; Kehl, M. Role of geomorphic surface on the above-ground biomass and soil organic carbon storage in a semi-arid region of Iranian loess plateau. Quat. Int. 2020, 552, 111–121. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate effects on mountain plants. Nature 1994, 369, 448. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Johnson, D.; Grime, J.P.; Booth, R.E. Sustaining ecosystem services in ancient limestone grassland: Importance of major component plants and community composition. J. Ecol. 2008, 96, 894–902. [Google Scholar] [CrossRef]
- Johnson, D.; Phoenix, G.K.; Grime, J.P. Plant community composition, not diversity, regulates soil respiration in grasslands. Biol. Lett. 2008, 4, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Alward, R.D.; Detling, J.K.; Milehunas, D.G. Grassland vegetation changes and nocturnal global warming. Science 1999, 283, 229–231. [Google Scholar] [CrossRef]
- Peng, F.; Xue, X.; Xu, M.H.; You, Q.G.; Guo, J.; Ma, S.X. Warming-induced shift towards forbs and grasses and its relation to the carbon sequestration in an alpine meadow. Environ. Res. Lett. 2017, 12, 044010. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Kivlin, S.N.; Whitney, K.D.; Price, M.V.; Waser, L.M.; Harte, J. Response of high-altitude graminoids and soil fungi to 20 yr of experimental warming. Ecology 2014, 95, 1918–1928. [Google Scholar] [CrossRef]
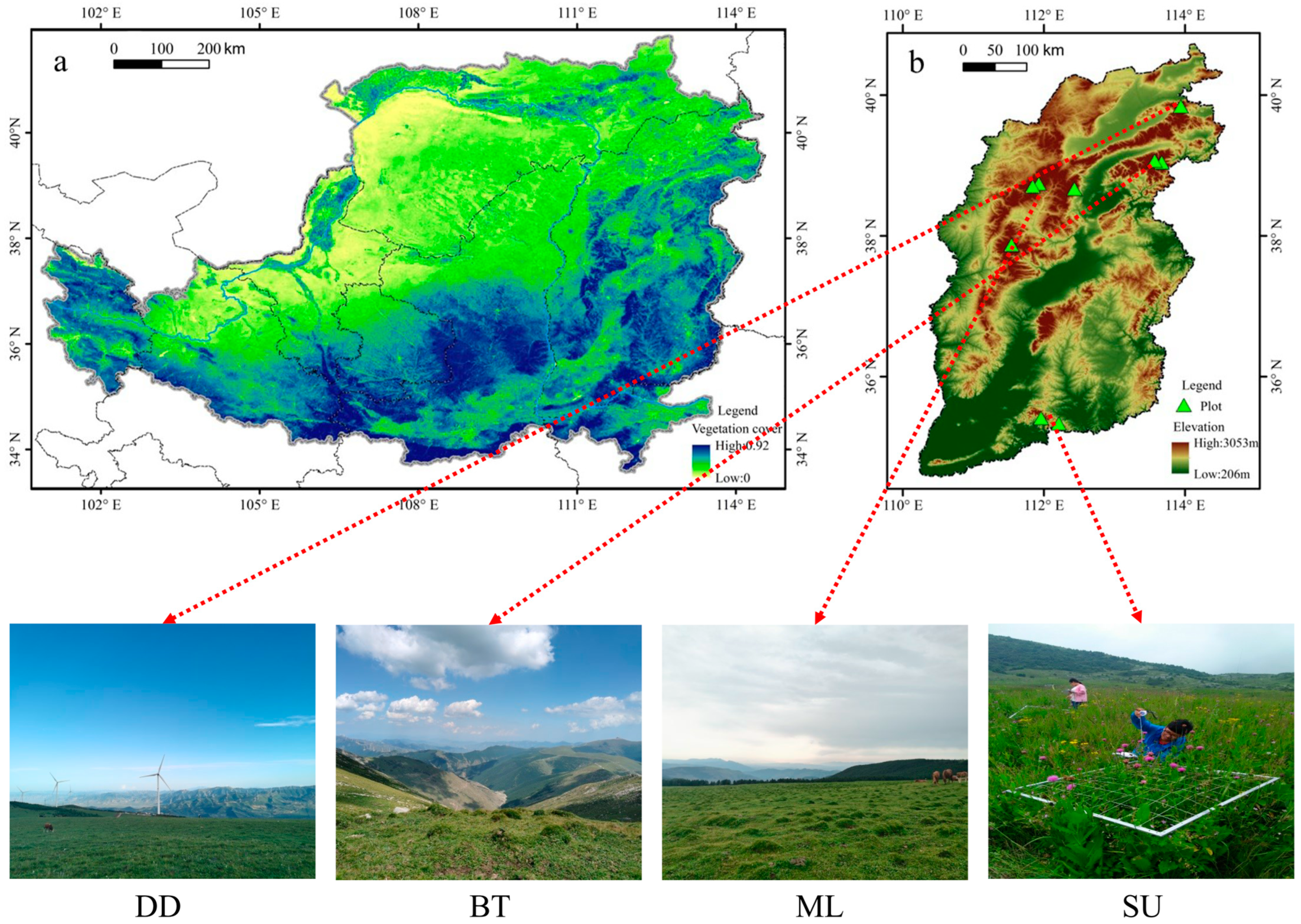
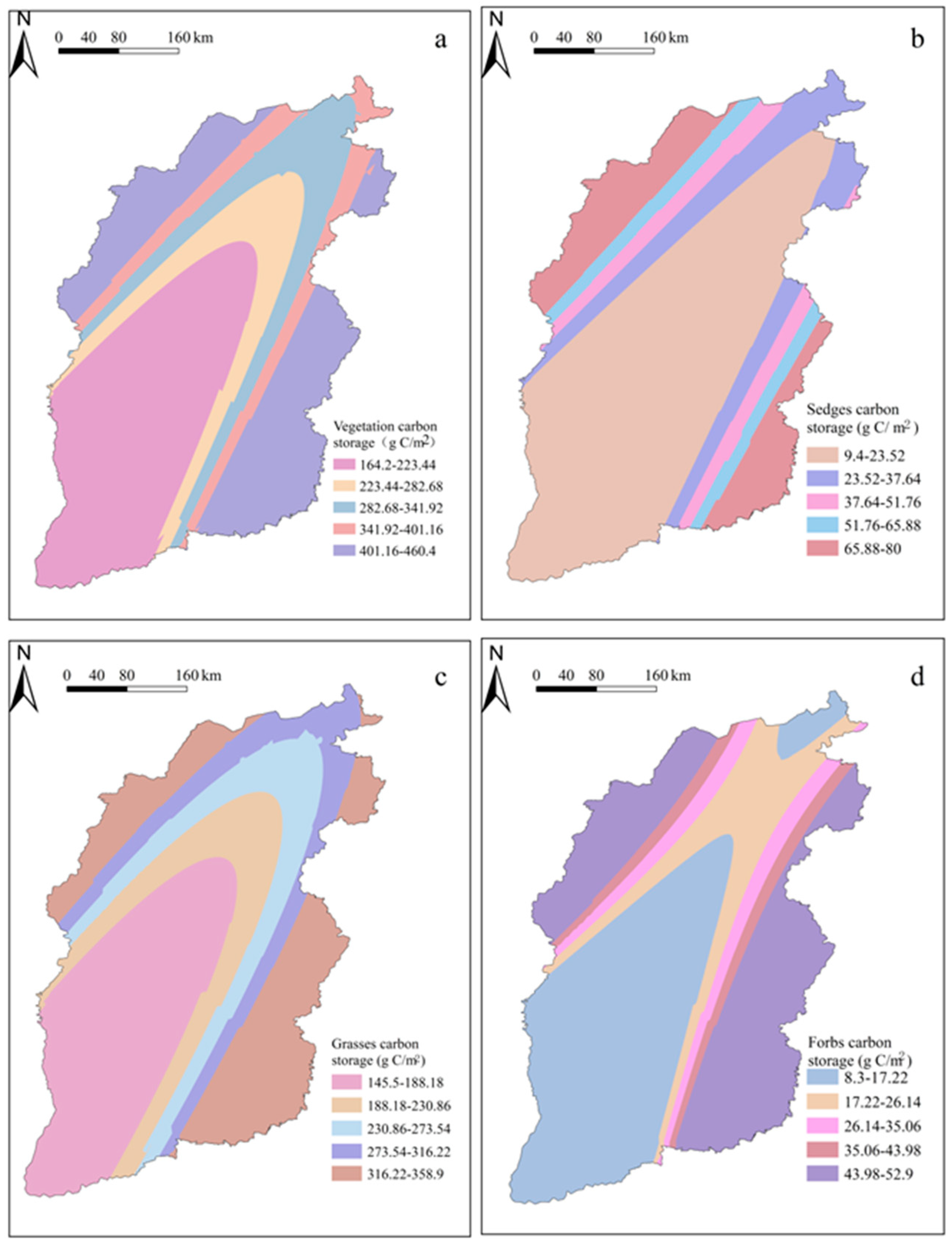



| Location | Mountain Name (Abbreviation) | Latitude (°N) | Longitude (°E) | Elevation (m) | Grassland Type |
|---|---|---|---|---|---|
| North | Dianding (DD) | 39.85 | 113.94 | 2265 | Subalpine grassland |
| Beitai (BT) | 39.08 | 113.57 | 3045 | Alpine grassland | |
| Dongtai (DT) | 39.05 | 113.67 | 2565 | Subalpine grassland | |
| Central | Malun (ML) | 38.75 | 111.93 | 2710 | Subalpine grassland |
| Heyeping (HY) | 38.71 | 111.84 | 2745 | Subalpine grassland | |
| Yunzhong (YZ) | 38.68 | 112.43 | 2260 | Subalpine grassland | |
| Yunding (YD) | 37.88 | 111.54 | 2690 | Subalpine grassland | |
| South | Shunwangping (SU) | 35.42 | 111.96 | 2250 | Subalpine grassland |
| Shengwangping (SE) | 35.34 | 112.21 | 1720 | Subalpine grassland |
| Mountain Name | Aboveground Carbon Density (g C/m2) | Belowground Carbon Density (g C/m2) | Total Carbon Density (g C/m2) |
|---|---|---|---|
| DD | 56.880 ± 8.225 | 248.558 ± 70.221 | 305.438 ± 75.024 |
| BT | 38.498 ± 6.038 | 405.630 ± 52.666 | 444.128 ± 57.372 |
| DT | 56.295 ± 4.338 | 186.345 ± 21.260 | 242.640 ± 19.854 |
| ML | 32.445 ± 2.633 | 166.320 ± 25.920 | 198.765 ± 27.616 |
| HY | 105.863 ± 14.652 | 354.533 ± 28.014 | 460.395 ± 30.230 |
| YZ | 64.530 ± 16.841 | 116.123 ± 31.405 | 180.653 ± 32.708 |
| YD | 24.683 ± 3.500 | 139.523 ± 35.621 | 164.205 ± 34.706 |
| SU | 131.918 ± 25.967 | 187.020 ± 31.056 | 318.938 ± 45.126 |
| SE | 155.228 ± 24.415 | 206.438 ± 53.466 | 361.665 ± 69.013 |
| Carbon Density (g C/m2) | Forbs | Sedges | Grasses | |
|---|---|---|---|---|
| Aboveground | Max. | 16.920 | 16.303 | 129.925 |
| Min. | 1.407 | 1.407 | 21.869 | |
| Mean | 6.122 | 5.333 | 62.386 | |
| Mid. | 4.494 | 3.678 | 49.372 | |
| Belowground | Max. | 46.647 | 54.598 | 327.749 |
| Min. | 4.863 | 6.619 | 100.678 | |
| Mean | 19.950 | 18.138 | 185.034 | |
| Mid. | 17.330 | 9.912 | 172.245 | |
| Total | Max. | 52.945 | 70.901 | 358.855 |
| Min. | 8.292 | 9.360 | 145.486 | |
| Mean | 26.073 | 23.471 | 247.419 | |
| Mid. | 22.566 | 16.188 | 265.120 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Wang, J.; Wei, K.; Li, J.; Yu, X. Geographical Environment and Plant Functional Group Shape the Spatial Variation Pattern of Plant Carbon Density in Subalpine-Alpine Grasslands of the Eastern Loess Plateau, China. Agronomy 2024, 14, 1420. https://doi.org/10.3390/agronomy14071420
Xu M, Wang J, Wei K, Li J, Yu X. Geographical Environment and Plant Functional Group Shape the Spatial Variation Pattern of Plant Carbon Density in Subalpine-Alpine Grasslands of the Eastern Loess Plateau, China. Agronomy. 2024; 14(7):1420. https://doi.org/10.3390/agronomy14071420
Chicago/Turabian StyleXu, Manhou, Jiaying Wang, Kunkun Wei, Jie Li, and Xiuli Yu. 2024. "Geographical Environment and Plant Functional Group Shape the Spatial Variation Pattern of Plant Carbon Density in Subalpine-Alpine Grasslands of the Eastern Loess Plateau, China" Agronomy 14, no. 7: 1420. https://doi.org/10.3390/agronomy14071420
APA StyleXu, M., Wang, J., Wei, K., Li, J., & Yu, X. (2024). Geographical Environment and Plant Functional Group Shape the Spatial Variation Pattern of Plant Carbon Density in Subalpine-Alpine Grasslands of the Eastern Loess Plateau, China. Agronomy, 14(7), 1420. https://doi.org/10.3390/agronomy14071420






