Insights for Soil Improvements: Unraveling Distinct Mechanisms of Microbial Residue Carbon Accumulation under Chemical and Anaerobic Soil Disinfestation
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Preparation
2.2. Microcosm Design
2.3. Sample Collection and Analysis
2.4. Statistical Analysis
3. Results and Discussion
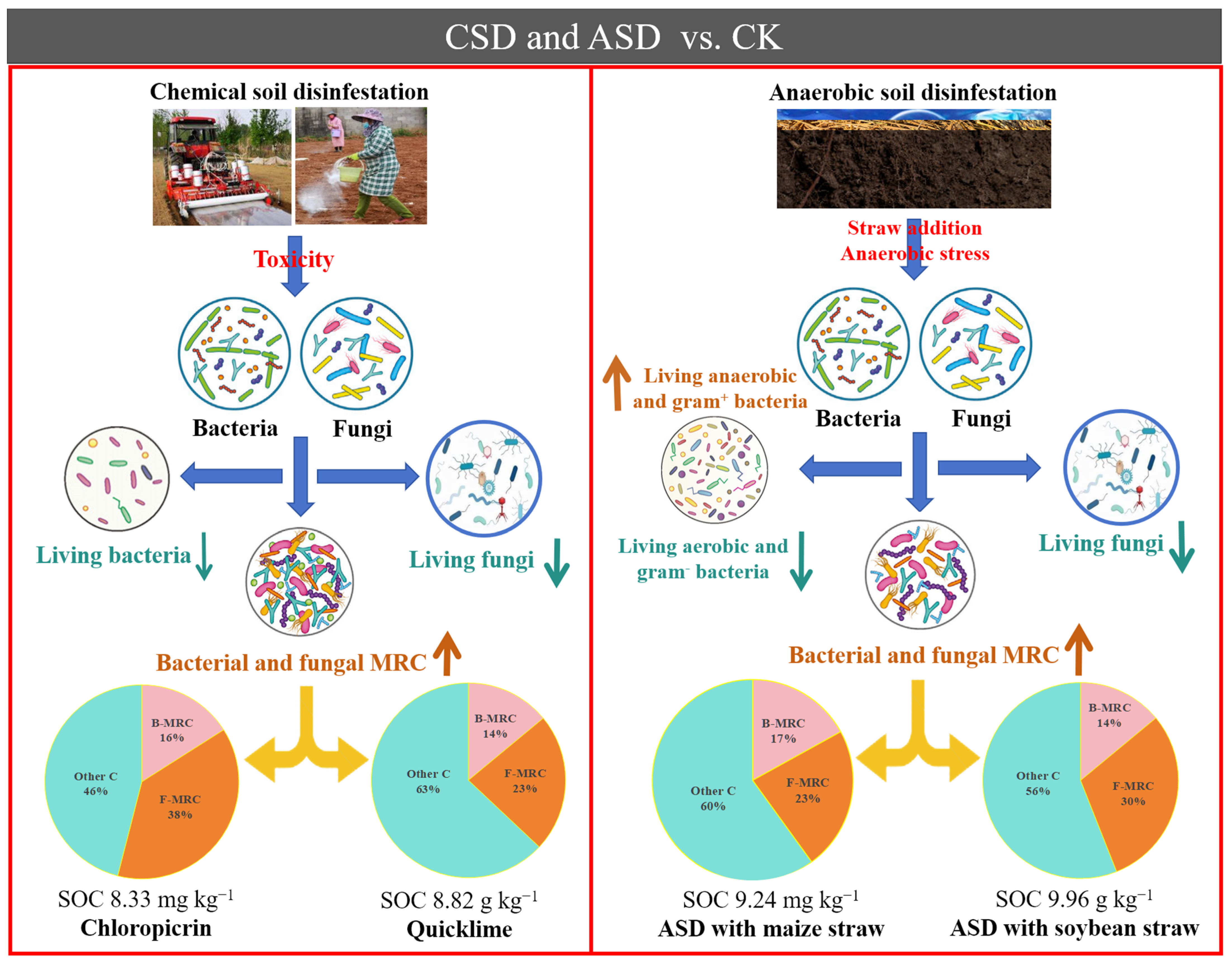
3.1. Soil Organic C Fractions and Concentration after ASD and CSD
3.2. The Effects of ASD and CSD on Soil Components and Concentrations of Amino Sugars
3.3. Changes in Phospholipid Fatty Acids (PLFAs) and Their Relationship with Soil Organic C after ASD and CSD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Zhang, Y.; Chen, D.; White, R.E.; Li, Y. A quantitative evaluation system of soil productivity for intensive agriculture in China. Geoderma 2004, 123, 319–331. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, X.; Jiang, A.Q.; Fan, J.Z.; Lan, T.; Zhang, J.B.; Cai, Z.C. Distinct impacts of reductive soil disinfestation and chemical soil disinfestation on soil fungal communities and memberships. Appl. Microbiol. Biotechnol. 2018, 102, 7623–7634. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.S.; Song, Z.X.; Tao, S.; Zhang, D.Q.; Huang, B.; Ren, L.R.; Cheng, H.Y.; Yan, D.D.; Li, Y.; Cao, A.C.; et al. Biochar mitigates the negative effect of chloropicrin fumigation on beneficial soil microorganisms. Sci. Total Environ. 2020, 738, 139880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.P.; Hu, R.J.; Bocharnikova, E.; Matichenkov, V. Co-treatment with silicon and quicklime in pig manure application as a promising option of environmental management. J. Environ. Manag. 2022, 309, 114684. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Zhao, J.; Zhou, X.; Zhang, J.B.; Cai, Z.C. Differential responses of soil bacterial community and functional diversity to reductive soil disinfestation and chemical soil disinfestation. Geoderma 2019, 348, 124–134. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Koike, S.; Baird, G.; Fennimore, S.; Samtani, J.; Bolda, M.; Dara, S.; Daugovish, O.; Lazarovits, G.; et al. Anaerobic soil disinfestation is an alternative to soil fumigation for control of some soil-borne pathogens in strawberry production. Plant Pathol. 2018, 67, 51–66. [Google Scholar] [CrossRef]
- Márquez-Caro, A.; Borrero, C.; Hernández-Muñiz, P.; Avilés, M. Use optimization of organic wastes in anaerobic soil disinfestation against strawberry charcoal rot root. Horticulturae 2022, 8, 841. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, Y.H.; Li, C.; Xu, R.S.; Pei, Z.R.; Li, F.C.; Wu, Y.H.; Chen, F.; Liang, Y.R.; Li, Z.H.; et al. Linking soil organic carbon dynamics to microbial community and enzyme activities in degraded soil remediation by reductive soil disinfestation. Appl. Soil. Ecol. 2023, 189, 104931. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, Y.H.; Xu, R.S.; Song, J.X.; Chen, A.L.; Chen, F.; Liang, Y.R.; Guo, D.; Tang, X.; Qin, Z.M.; et al. Short-term responses of soil organic carbon and chemical composition of particle-associated organic carbon to anaerobic soil disinfestation in degraded greenhouse soils. Land Degrad. Dev. 2023, 34, 4428–4440. [Google Scholar] [CrossRef]
- Jat, R.S.; Singh, H.V.; Dotaniya, M.L.; Choudhary, R.L.; Meena, M.K.; Rai, P.K. Biological and chemical vicissitudes in soil rhizosphere arbitrated under different tillage, residues recycling and Oilseed Brassica-based cropping systems. Sustainability 2024, 16, 2027. [Google Scholar] [CrossRef]
- Ueki, A.; Kaku, N.; Ueki, K. Role of anaerobic bacteria in biological soil disinfestation for elimination of soil-borne plant pathogens in agriculture. Appl. Microbiol. Biotechnol. 2018, 102, 6309–6318. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Cantarella, H.; Kuramae, E.E. Carbon and nutrients from organic residues modulate the dynamics of prokaryotic and fungal communities. Microorganisms 2023, 11, 2905. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Bo, H.; Xue, D.; Li, Z.; Wang, H.; Jin, D.; Wang, H. Soil microbial residual carbon accumulation affected by reclamation period and straw incorporation in reclaimed soil from coal mining area. Agronomy 2024, 14, 742. [Google Scholar] [CrossRef]
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agricultural Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Eudy, L.W.; Walla, M.D.; Morgan, S.L.; Fox, A. Gas chromatographic-mass spectrometric determination of muramic acid content and pyrolysis profiles for a group of gram-positive and gram-negative bacteria. Analyst 1985, 110, 381–385. [Google Scholar] [CrossRef]
- Amelung, W.; Lobe, I.; Du Preez, C.C. Fate of microbial residues in sandy soils of South African Highveld as influenced by prolonged arable cropping. Eur. J. Soil Sci. 2002, 53, 29–35. [Google Scholar] [CrossRef]
- Engelking, B.; Flessa, H.; Joergensen, R.G. Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil. Biol. Biochem. 2007, 39, 2111–2118. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Fungal and bacterial growth in soil with plant materials of different C/N ratios. FEMS Microbiol. Ecol. 2007, 62, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS microbiol. ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Moche, M.; Gutknecht, J.; Schulz, E.; Langer, U.; Rinklebe, J. Monthly dynamics of microbial community structure and their controlling factors in three floodplain soils. Soil Biol. Biochem. 2015, 90, 169–178. [Google Scholar] [CrossRef]
- Marshall, C.B.; McLaren, J.R.; Turkington, R. Soil microbial communities resistant to changes in plant functional group composition. Soil Biol. Biochem. 2011, 43, 78–85. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhang, Y.N.; Hu, Y.; Wu, J.H.; Fu, X.H.; Le, Y.Q. The variability and causes of organic carbon retention ability of different agricultural straw types returned to soil. Environ. Technol. 2016, 38, 538–548. [Google Scholar] [CrossRef]
- Fornara, D.A.; Steinbeiss, S.; McNamara, N.P.; Gleixner, G.; Oakley, S.; Poulton, P.R.; Macdonald, A.J.; Bardgett, R.D. Increases in soil organic carbon sequestration can reduce the global warming potential of long-term liming to permanent grassland. Glob. Change Biol. 2011, 17, 1925–1934. [Google Scholar] [CrossRef]
- Li, Q.J.; Zhang, D.Q.; Cheng, H.Y.; Song, Z.X.; Ren, L.R.; Hao, B.Q.; Zhu, J.H.; Fang, W.S.; Yan, D.D.; Li, Y.; et al. Chloropicrin alternated with dazomet improved the soil’s physical and chemical properties, changed microbial communities and increased strawberry yield. Ecotoxicol. Environ. Saf. 2021, 220, 112362. [Google Scholar] [CrossRef]
- Nicolaou, S.A.; Gaida, S.M.; Papoutsakis, E.T. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: From biofuels and chemicals, to biocatalysis and bioremediation. Metab. Eng. 2010, 12, 307–331. [Google Scholar] [CrossRef]
- Lopes, M.; Leite, F.L.; Valente, B.S.; Heres, T.; Dai Prá, M.A.; Xavier, E.G.; Roll, V.F.B. An assessment of the effectiveness of four in-house treatments to reduce the bacterial levels in poultry litter. Poult. Sci. 2015, 94, 2094–2098. [Google Scholar] [CrossRef]
- Daccò, C.; Girometta, C.; Asemoloye, M.D.; Carpani, G.; Picco, A.M.; Tosi, S. Key fungal degradation patterns, enzymes and their applications for the removal of aliphatic hydrocarbons in polluted soils: A review. Int. Biodeterior. Biodegrad. 2020, 147, 104866. [Google Scholar] [CrossRef]
- Pasquina-Lemonche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef]
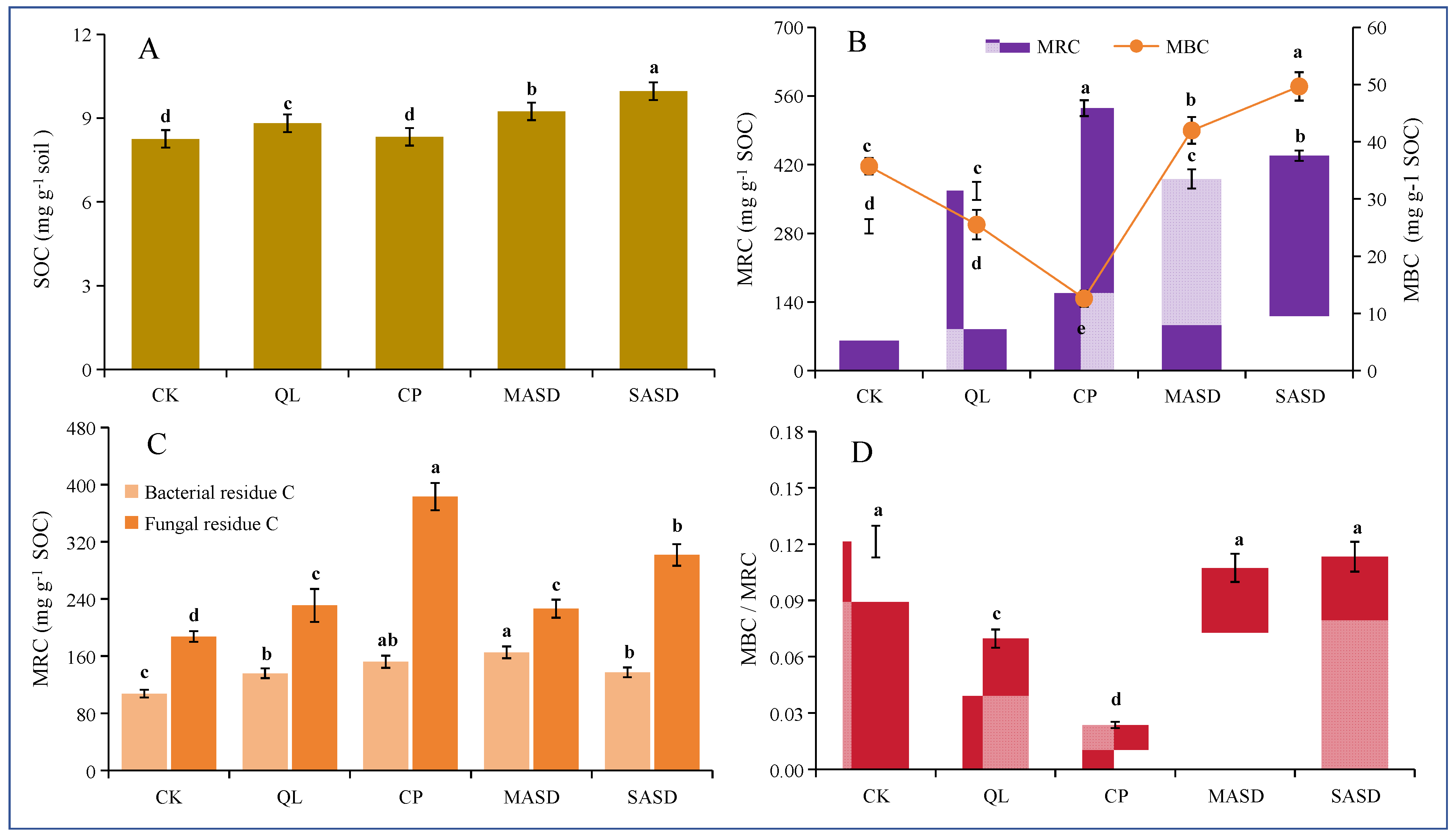


| Microorganisms | Markers of PLFAs | References |
|---|---|---|
| Fungi | 18:1w9c | [20] |
| Arbuscular mycorrhiza | 16:1ω5c | [21] |
| Saprophytic fungi | 18:2ω6, 18:1ω9 | [21] |
| Bacteria | 11:0; 12:0; 13:0; 14:0; 15:0; 16:1; 17:0; 18:1; 19:0; i15:0; a15:0; i16:0; a17:0; i17:0; 16:1ω7c; 18:1ω7c | [20] |
| Anaerobic bacteria | i-15:0; a-15:0; a-17:0; i-17:0; a-17:0 | [22] |
| Aerobic bacteria | 14:1; 18:1ω7c; 15:1ω6c; 16:1ω7c; 16:1ω7t; 18:1ω9c; 18:1ω9t | [22] |
| Gram- positive bacteria | i15:0; a15:0; i16:0; 16:1w9c; 16:1w7; i17:0; a17:0; 18:0; 18:1w9c | [23] |
| Gram- negative bacteria | i16:1w7c; i17:1w8c; cy17:0; 18:1w7c; 18:1w5c; cy19:0 | [23] |
| Soil Parameters (μg g−1 Soil) | CK | QL | CP | MASD | SASD |
|---|---|---|---|---|---|
| Amino sugars | 435 # d | 473 cd | 635 a | 519 c | 576 b |
| Glucosamine | 264 b | 281 b | 395 a | 297 b | 377 a |
| Mannosamine | 9.78 c | 11.3 bc | 12.4 ab | 14.4 a | 9.19 c |
| Galactosamine | 135 c | 147 c | 198 a | 185 ab | 162 bc |
| Muramic acid | 23.0 d | 26.6 c | 30.3 b | 33.9 a | 28.1 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Yan, J.; Wang, X.; She, P.; Li, Z.; Xu, R.; Chen, Y. Insights for Soil Improvements: Unraveling Distinct Mechanisms of Microbial Residue Carbon Accumulation under Chemical and Anaerobic Soil Disinfestation. Agronomy 2024, 14, 1430. https://doi.org/10.3390/agronomy14071430
Yang K, Yan J, Wang X, She P, Li Z, Xu R, Chen Y. Insights for Soil Improvements: Unraveling Distinct Mechanisms of Microbial Residue Carbon Accumulation under Chemical and Anaerobic Soil Disinfestation. Agronomy. 2024; 14(7):1430. https://doi.org/10.3390/agronomy14071430
Chicago/Turabian StyleYang, Kejian, Jiangtao Yan, Xianwei Wang, Pengtao She, Zhonghui Li, Risheng Xu, and Yanlong Chen. 2024. "Insights for Soil Improvements: Unraveling Distinct Mechanisms of Microbial Residue Carbon Accumulation under Chemical and Anaerobic Soil Disinfestation" Agronomy 14, no. 7: 1430. https://doi.org/10.3390/agronomy14071430
APA StyleYang, K., Yan, J., Wang, X., She, P., Li, Z., Xu, R., & Chen, Y. (2024). Insights for Soil Improvements: Unraveling Distinct Mechanisms of Microbial Residue Carbon Accumulation under Chemical and Anaerobic Soil Disinfestation. Agronomy, 14(7), 1430. https://doi.org/10.3390/agronomy14071430




