Bioindicators for Assessing Soil Quality in Ecuador’s Jun Jun Micro-Watershed
Abstract
:1. Introduction
2. Materials and Methods
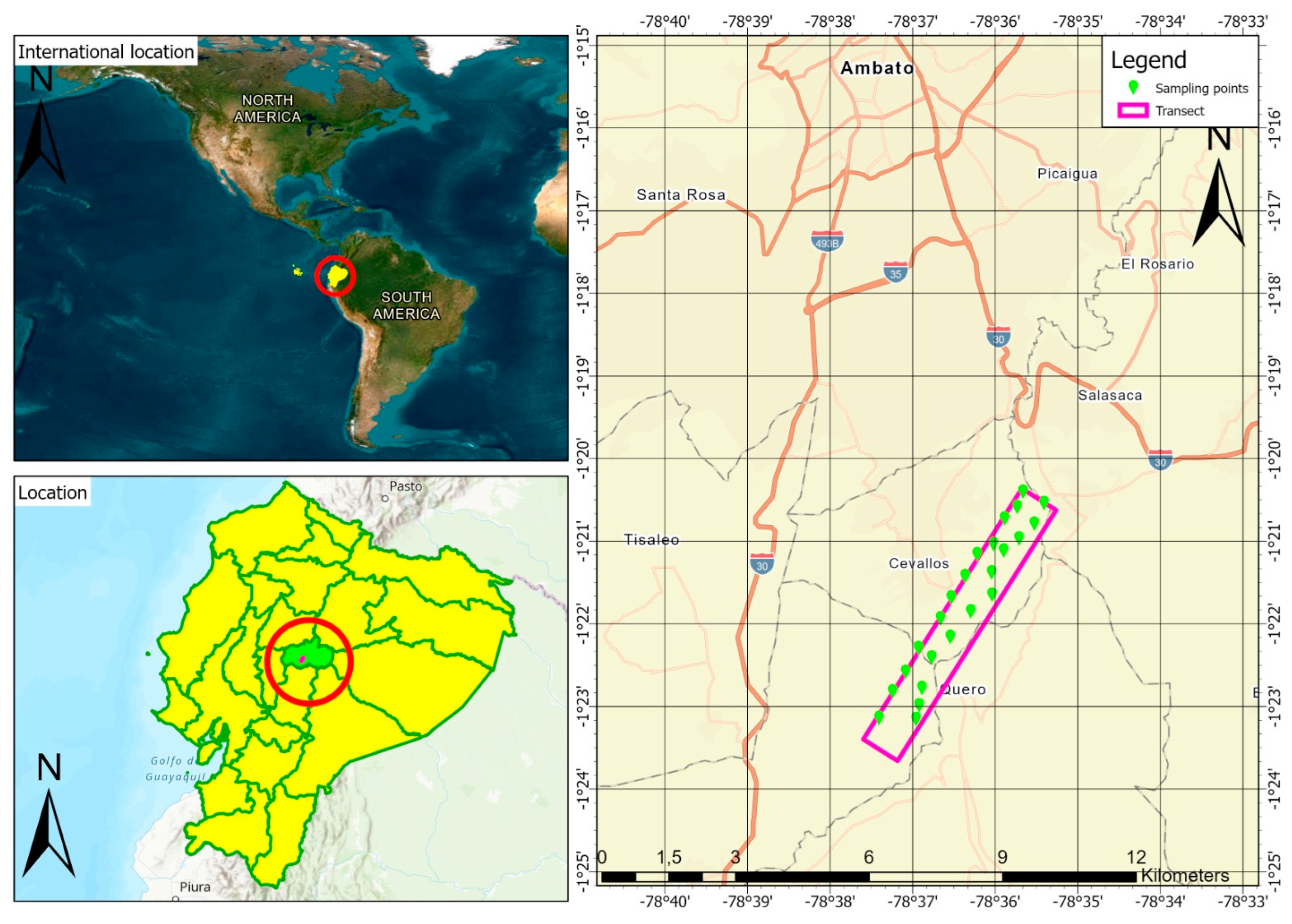
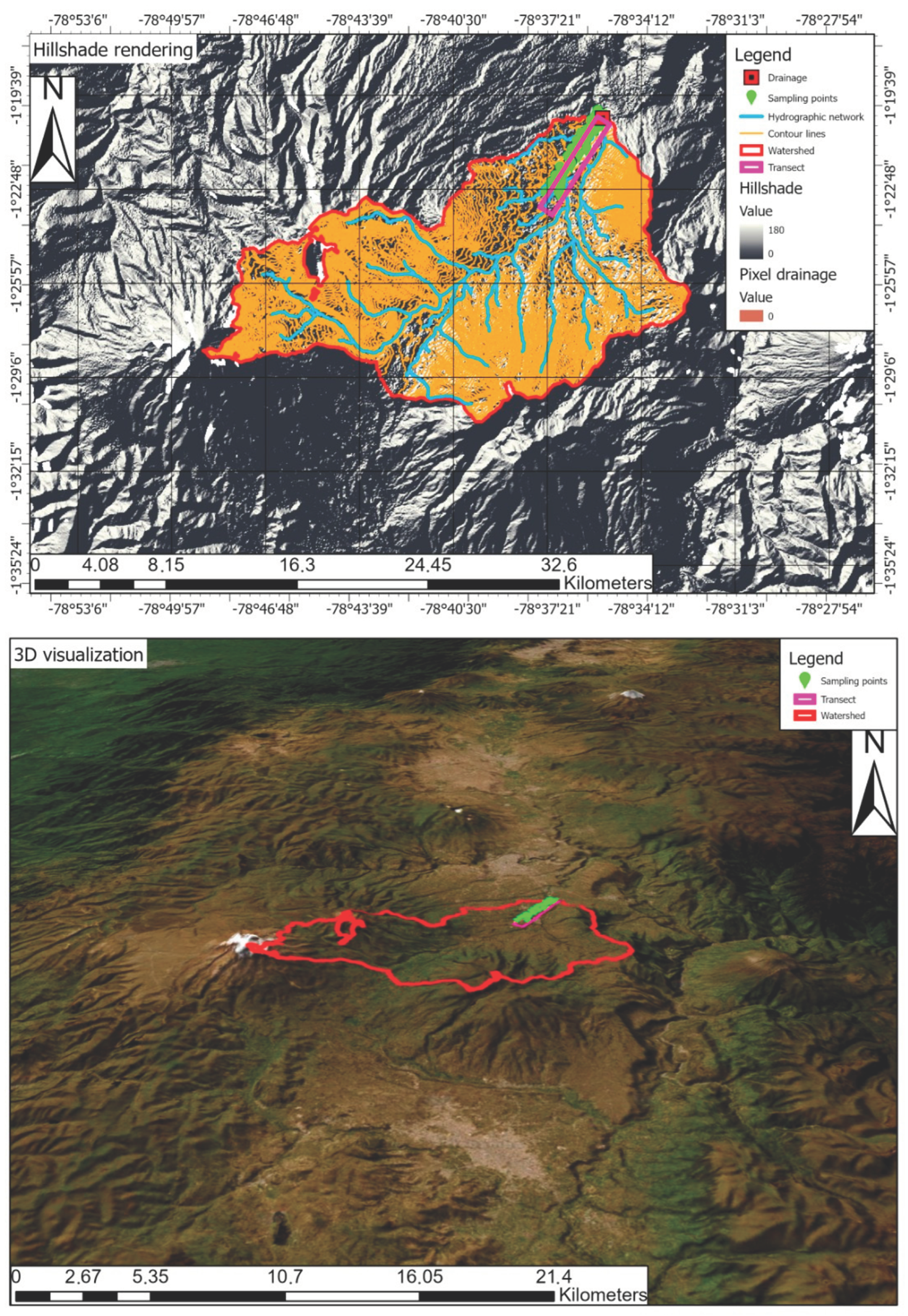
2.1. Study Area
2.2. Soil Quality Index Calculations
2.3. Normalizing Soil Indicators
2.4. Sensitivity Index
2.5. Sampling and Determination of Soil Quality Indicators
2.6. Statistical Analysis
3. Results and Discussion
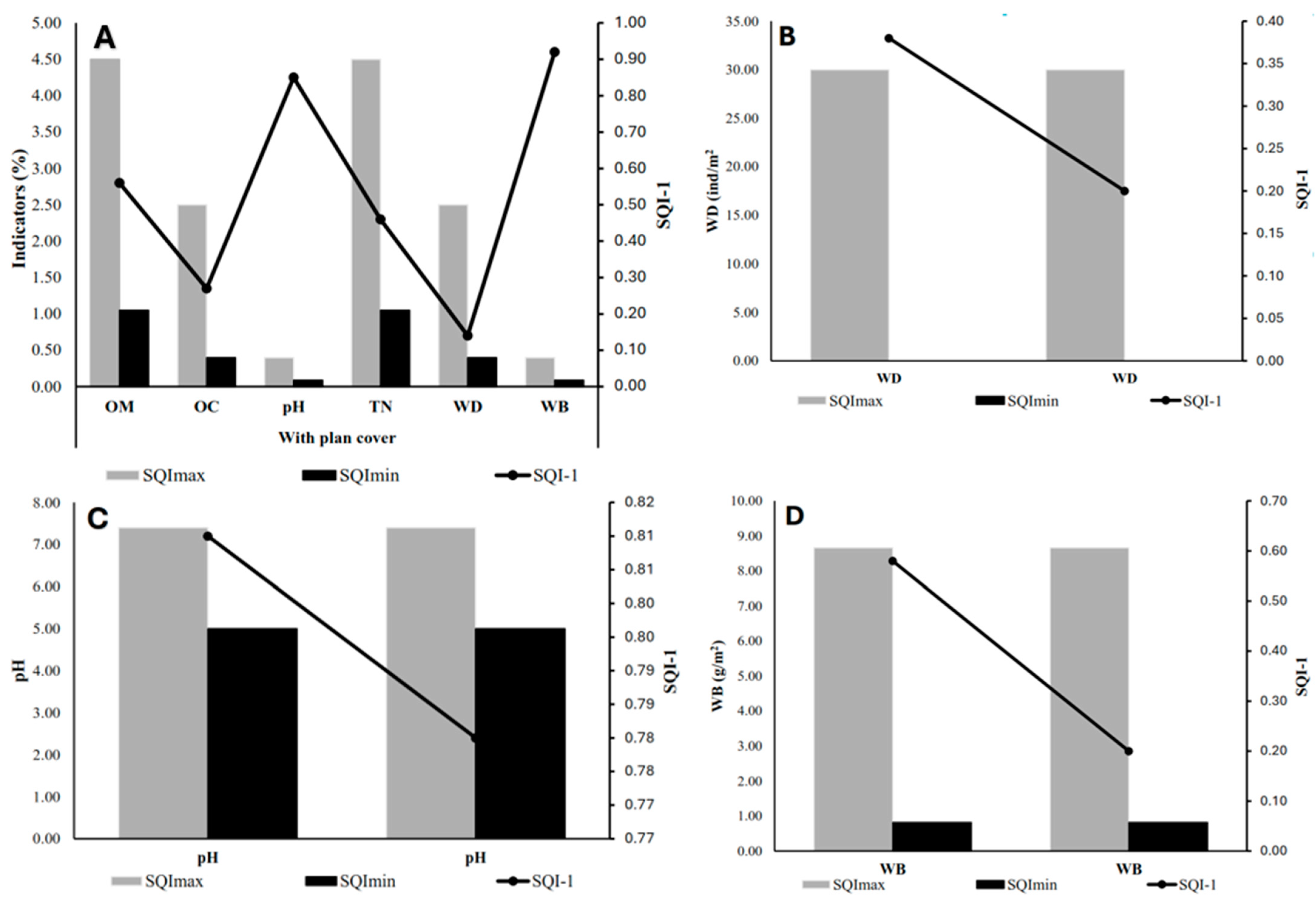
3.1. Organic Carbon (OC)
3.2. Total Nitrogen (TN)
3.3. pH Analysis
3.4. Organic Matter (OM)
3.5. Bioindicators
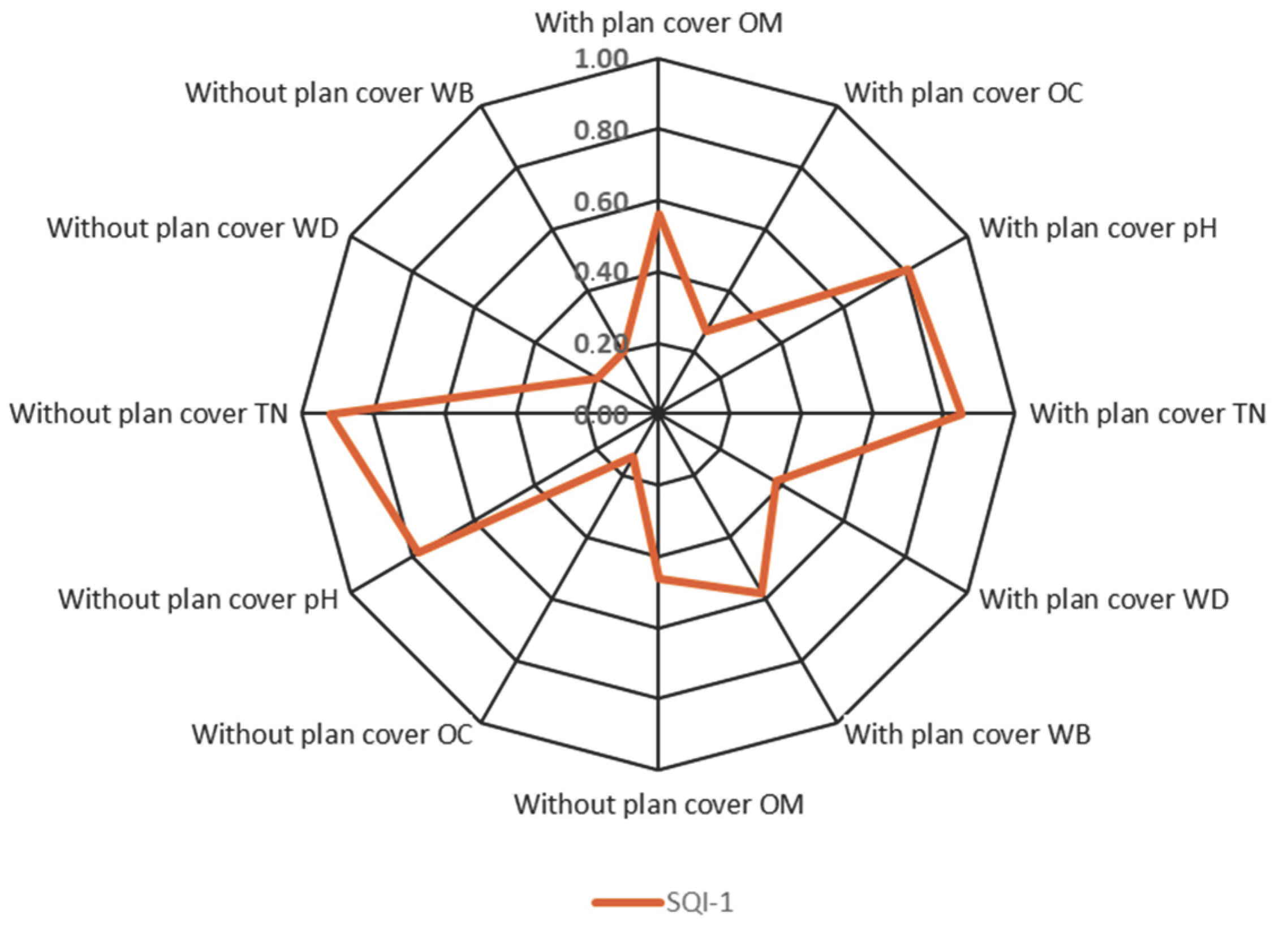
3.6. Soil Quality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, L.; Jiang, J.; Zhang, J.; Zhang, Z.; Zhang, M. Application of Soil Quality Index to Determine the Effects of Different Vegetation Types on Soil Quality in the Yellow River Delta Wetland. Ecol. Indic. 2022, 141, 109116. [Google Scholar] [CrossRef]
- Bagnall, D.K.; Shanahan, J.F.; Flanders, A.; Morgan, C.L.S.; Honeycutt, C.W. Soil Health Considerations for Global Food Security. Agron. J. 2021, 113, 4581–4589. [Google Scholar] [CrossRef]
- Li, Y.; You, S. Chapter 8-Biochar Soil Application: Soil Improvement and Pollution Remediation. In Biochar in Agriculture for Achieving Sustainable Development Goals; Tsang, D.C.W., Ok, Y.S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 97–102. ISBN 978-0-323-85343-9. [Google Scholar]
- Stocking, M.A. Tropical Soils and Food Security: The next 50 Years. Science 2003, 302, 1356–1359. [Google Scholar] [CrossRef]
- Ozsahin, E.; Eroglu, I.; Pektezel, H. Soil Quality Index (SQI) ANALYSIS of Tekirdag Province Using GIS (Thrace, Turkey). Fresen. Environ. Bull. 2017, 26, 3005–3014. [Google Scholar]
- Fazekašová, D. Evaluation of Soil Quality Parameters Development in Terms of Sustainable Land Use. In Sustainable Development; Curkovic, S., Ed.; IntechOpen: Rijeka, Croatia, 2012; p. Ch. 19. [Google Scholar]
- AbdelRahman, M.A.E.; Shalaby, A.; Mohamed, E.S. Comparison of Two Soil Quality Indices Using Two Methods Based on Geographic Information System. Egypt. J. Remote Sens. Space Sci. 2019, 22, 127–136. [Google Scholar] [CrossRef]
- Bonilla-Bedoya, S.; Valencia, K.; Herrera, M.Á.; López-Ulloa, M.; Donoso, D.A.; Macedo Pezzopane, J.E. Mapping 50 Years of Contribution to the Development of Soil Quality Biological Indicators. Ecol. Indic. 2023, 148, 110091. [Google Scholar] [CrossRef]
- Armenise, E.; Redmile-Gordon, M.A.; Stellacci, A.M.; Ciccarese, A.; Rubino, P. Developing a Soil Quality Index to Compare Soil Fitness for Agricultural Use under Different Managements in the Mediterranean Environment. Soil Tillage Res. 2013, 130, 91–98. [Google Scholar] [CrossRef]
- Maaz, T.M.; Heck, R.H.; Glazer, C.T.; Loo, M.K.; Zayas, J.R.; Krenz, A.; Beckstrom, T.; Crow, S.E.; Deenik, J.L. Measuring the Immeasurable: A Structural Equation Modeling Approach to Assessing Soil Health. Sci. Total Environ. 2023, 870, 161900. [Google Scholar] [CrossRef] [PubMed]
- Bahena-Osorio, Y.; Franco-Hernández, M.O.; Pueyo, J.J.; Vásquez-Murrieta, M.S. Development of a Quality Index to Evaluate the Impact of Abiotic Stress in Saline Soils in the Geothermal Zone of Los Negritos, Michoacán, Mexico. Agronomy 2023, 13, 1650. [Google Scholar] [CrossRef]
- Zuber, S.M.; Veum, K.S.; Myers, R.L.; Kitchen, N.R.; Anderson, S.H. Role of Inherent Soil Characteristics in Assessing Soil Health across Missouri. Agric. Environ. Lett. 2020, 5, e20021. [Google Scholar] [CrossRef]
- Prior, T.; Hagmann, J. Measuring Resilience: Methodological and Political Challenges of a Trend Security Concept. J. Risk Res. 2014, 17, 281–298. [Google Scholar] [CrossRef]
- Hubanks, H.L.; Deenik, J.L.; Crow, S.E. Getting the Dirt on Soil Health and Management. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-409548-9. [Google Scholar]
- Magalhães, W.d.A.; Amorim, R.S.S.; Hunter, M.O.; Bocuti, E.D.; Di Loreto Di Raimo, L.A.; da Silva, W.M.; Hoshide, A.K.; de Abreu, D.C. Using the GeoWEPP Model to Predict Water Erosion in Micro-Watersheds in the Brazilian Cerrado. Sustainability 2023, 15, 4711. [Google Scholar] [CrossRef]
- Isong, I.A.; John, K.; Okon, P.B.; Ogban, P.I.; Afu, S.M. Soil Quality Estimation Using Environmental Covariates and Predictive Models: An Example from Tropical Soils of Nigeria. Ecol. Process. 2022, 11, 66. [Google Scholar] [CrossRef]
- Zehetner, F.; Miller, W.P. Erodibility and Runoff-Infiltration Characteristics of Volcanic Ash Soils along an Altitudinal Climosequence in the Ecuadorian Andes. Catena 2006, 65, 201–213. [Google Scholar] [CrossRef]
- Villacís, M.; Vimeux, F.; Taupin, J.D. Analysis of the Climate Controls on the Isotopic Composition of Precipitation (δ18O) at Nuevo Rocafuerte, 74.5°W, 0.9°S, 250 m, Ecuador. Comptes Rendus Geosci. 2008, 340, 1–9. [Google Scholar] [CrossRef]
- Buytaert, W.; Deckers, J.; Wyseure, G. Regional Variability of Volcanic Ash Soils in South Ecuador: The Relation with Parent Material, Climate and Land Use. Catena 2007, 70, 143–154. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Comparison of Soil Quality Index Using Three Methods. PLoS ONE 2014, 9, 0105981. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Herrera, I.R.; Hidalgo-Moreno, C.; Guzmán-Plazola, R.; Almaraz Suárez, J.J.; Navarro-Garza, H.; Etchevers-Barra, J.D. Indicadores de Calidad de Suelo Para Evaluar Su Fertilidad. Agrociencia 2017, 51, 813–831. [Google Scholar]
- Desta, K.G. Soil Quality Monitoring: A Practical Guide; Oklahoma Cooperative Extension Service: Norman, OK, USA, 2010. [Google Scholar]
- Akram, Z.; Hussain, S.; Mansoor, M.; Afzal, M.; Waqar, A.; Shabbir, I. Soil Fertility and Salinity Status of Muzaffargarh District, Punjab Pakistan. Univers. J. Agric. Res. 2014, 2, 242–249. [Google Scholar] [CrossRef]
- Amacher, M.C.; O’Neil, K.P.; Perry, C.H. Soil Vital Signs: A New Soil Quality Index (SQI) for Assessing Forest Soil Health; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007. [Google Scholar]
- Cantú, M.P.; Becker, A.; Bedano, J.C.; Schiavo, H.F. Evaluación de La Calidad de Suelos Mediante El Uso de Indicadores e Índices. Cienc. Del Suelo 2007, 25, 173–178. [Google Scholar]
- Prieto Méndez, J.; Prieto-Garcia, F.; Acevedo, O.; Méndez-Marzo, M. Indicadores e Índices de Calidad de Los Suelos (ICS) Cebaderos Del Sur Del Estado de Hidalgo, México. Agron. Mesoam. 2013, 24, 83–91. [Google Scholar] [CrossRef]
- Cantú, M.; Becker, A.R.; Bedano, J.; Schiavo, H.F.; Parra, B. Evaluation of the Impact of Land Use and Management Change by Means of Soil Quality Indicators, Córdoba, Argentina. Cad. Lab. Xeol. Laxe. Coruna 2009, 34, 203–214. [Google Scholar]
- Feiza, V.; Feiziene, D.; Kadziene, G.; Lazauskas, S.; Deveikyte, I.; Slepetiene, A.; Seibutis, V. Soil State in the 11th Year of Three Tillage Systems Application on a Cambisol. J. Food Agric. Environ. 2011, 9, 1088–1095. [Google Scholar]
- Anup, K.C.; Bhandari, G.; Wagle, S.P.; Banjade, Y. Status of Soil Fertility in a Community Forest of Nepal. Int. J. Environ. 2013, 1, 56–67. [Google Scholar] [CrossRef]
- Demetrio, W.C.; Dionísio, J.A.; Maceda, A. Negative Effects of Earthworms on Soil Nematodes Are Dependent on Earthworm Density, Ecological Category and Experimental Conditions. Pedobiologia 2019, 76, 150568. [Google Scholar] [CrossRef]
- Morel, A.; Acosta, O.O. Calidad Del Suelo En Diferentes Usos y Manejo Por Medio de La Macrofauna Como Indicador Biológico. Braz. J. Anim. Environ. Res. 2022, 5, 996–1006. [Google Scholar] [CrossRef]
- Sheidai Karkaj, E.; Sepehry, A.; Barani, H.; Motamedi, J.; Shahbazi, F. Establishing a Suitable Soil Quality Index for Semi-Arid Rangeland Ecosystems in Northwest of Iran. J. Soil Sci. Plant Nutr. 2019, 19, 648–658. [Google Scholar] [CrossRef]
- Eyherabide, M.; Saínz Rozas, H.; Barbieri, P.; Echeverría, H.E. Ciencia del Suelo; Asociación Argentina de la Ciencia del Suelo: Buenos Aires, Argentina, 2014; Volume 32. [Google Scholar]
- Bellomonte, G.; Costantini, A.; Giammarioli, S. Comparison of Modified Automatic Dumas Method and the Traditional Kjeldahl Method for Nitrogen Determination in Infant Food. J. Assoc. Off. Anal. Chem. 1987, 70, 227–229. [Google Scholar] [CrossRef] [PubMed]
- USDA. Soil Quality Test Kit Guide; Soil Quality Institute, National Resources Conservation Service, United States Department of Agriculture: Washington, DC, USA, 1999. [Google Scholar]
- Beltrán-Dávalos, A.A.; Ayala Izurieta, J.E.; Echeverria Guadalupe, M.M.; Van Wittenberghe, S.; Delegido, J.; Otero Pérez, X.L.; Merino, A. Evaluation of Soil Organic Carbon Storage of Atillo in the Ecuadorian Andean Wetlands. Soil Syst. 2022, 6, 92. [Google Scholar] [CrossRef]
- Pedroza-Parga, E.; Velásquez-Valle, M.A.; Pedroza-Sandoval, A.; Sánchez-Cohen, I.; Yáñez-Chávez, L.G. The Impact of Vegetation Cover on Soil Erosion and Soil Deposition Due to Runoff. Ing. Agrícola Y Biosist. 2022, 144, 17–31. [Google Scholar] [CrossRef]
- He, X.; Hou, E.; Liu, Y.; Wen, D. Altitudinal Patterns and Controls of Plant and Soil Nutrient Concentrations and Stoichiometry in Subtropical China. Sci. Rep. 2016, 6, 24261. [Google Scholar] [CrossRef] [PubMed]
- Beniston, M. Climatic Change in Mountain Regions: A Review of Possible Impacts. In Climate Variability and Change in High Elevation Regions: Past, Present & Future; Diaz, H.F., Ed.; Springer: Dordrecht, The Netherlands, 2003; pp. 5–31. ISBN 978-94-015-1252-7. [Google Scholar]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and Ecosystem Responses to Elevational Gradients: Processes, Mechanisms, and Insights for Global Change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef]
- Oliveras, I.; Bentley, L.; Fyllas, N.M.; Gvozdevaite, A.; Shenkin, A.F.; Peprah, T.; Morandi, P.; Peixoto, K.S.; Boakye, M.; Adu-Bredu, S.; et al. The Influence of Taxonomy and Environment on Leaf Trait Variation Along Tropical Abiotic Gradients. Front. For. Glob. Change 2020, 3, 18. [Google Scholar] [CrossRef]
- Llambí, L.D.; Durbecq, A.; Cáceres-Mago, K.; Cáceres, A.; Ramírez, L.; Torres, E.; Méndez, Z. Interactions between Nurse-Plants and an Exotic Invader along a Tropical Alpine Elevation Gradient: Growth-Form Matters. Alp. Bot. 2020, 130, 59–73. [Google Scholar] [CrossRef]
- Li, L.; Vogel, J.; He, Z.; Zou, X.; Ruan, H.; Huang, W.; Wang, J.; Bianchi, T.S. Association of Soil Aggregation with the Distribution and Quality of Organic Carbon in Soil along an Elevation Gradient on Wuyi Mountain in China. PLoS ONE 2016, 11, e0150898. [Google Scholar] [CrossRef]
- Peters, M.K.; Hemp, A.; Appelhans, T.; Becker, J.N.; Behler, C.; Classen, A.; Detsch, F.; Ensslin, A.; Ferger, S.W.; Frederiksen, S.B.; et al. Climate–Land-Use Interactions Shape Tropical Mountain Biodiversity and Ecosystem Functions. Nature 2019, 568, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; He, K.; Chen, Y.; Zhao, J.; Hu, H.; Zhu, B. Changes of Soil Organic Matter Stability along Altitudinal Gradients in Tibetan Alpine Grassland. Plant Soil 2021, 458, 21–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Ai, J.; Sun, Q.; Li, Z.; Hou, L.; Song, L.; Tang, G.; Li, L.; Shao, G. Soil Organic Carbon and Total Nitrogen Stocks as Affected by Vegetation Types and Altitude across the Mountainous Regions in the Yunnan Province, South-Western China. Catena 2021, 196, 104872. [Google Scholar] [CrossRef]
- Zool, T.; Popovic, F.; Stojanovic, M.; Radosavljević, S.; Trakić, T.; Sekulić, J. Turkish Journal of Zoology Earthworm Community Structure along Altitudinal Gradients on the Western Slopes of Kopaonik Mountain in Serbia. Turk. J. Zool. 2022, 46, 103–114. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, Y.; Wang, W.; Wu, D. Earthworms Promote the Accumulation of Maize Root-Derived Carbon in a Black Soil of Northeast China, Especially in Soil from Long-Term No-Till. Geoderma 2019, 340, 124–132. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Al-Taey, D.K.A.; Al-Mammori, H.Z. Earthworms and Eco-Consequences: Considerations to Soil Biological Indicators and Plant Function: A Review. Acta Ecol. Sin. 2021, 41, 512–523. [Google Scholar] [CrossRef]
- Puig-Girones, R.; Real, J. A Comprehensive but Practical Methodology for Selecting Biological Indicators for Long-Term Monitoring. PLoS ONE 2022, 17, e0265246. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xue, Z.; Li, B.; An, S. Soil Organic Carbon Distribution in Relation to Land Use and Its Storage in a Small Watershed of the Loess Plateau, China. Catena 2012, 88, 6–13. [Google Scholar] [CrossRef]
- Gangopadhyay, S.K.; Bhattacharyya, T.; Mishra, T.K.; Banerjee, S.K. Chapter 7-Organic Carbon Stock in the Forest Soils of Himalayas and Other Areas in India. In Forest Resources Resilience and Conflicts; Kumar Shit, P., Pourghasemi, H.R., Adhikary, P.P., Bhunia, G.S., Sati, V.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 93–116. ISBN 978-0-12-822931-6. [Google Scholar]
- Barrow, N.J.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Sofo, A.; Mininni, A.N.; Ricciuti, P. Soil Macrofauna: A Key Factor for Increasing Soil Fertility and Promoting Sustainable Soil Use in Fruit Orchard Agrosystems. Agronomy 2020, 10, 456. [Google Scholar] [CrossRef]
- Singh, J.; Schädler, M.; Demetrio, W.; Brown, G.G.; Eisenhauer, N. Climate Change Effects on Earthworms—A Review. Soil Org. 2019, 91, 114–138. [Google Scholar] [CrossRef]
- Xiang, T.; Qiang, F.; Liu, G.; Liu, C.; Liu, Y.; Ai, N.; Ma, H. Soil Quality Evaluation of Typical Vegetation and Their Response to Precipitation in Loess Hilly and Gully Areas. Forests 2023, 14, 1909. [Google Scholar] [CrossRef]
- Koudahe, K.; Allen, S.C.; Djaman, K. Critical Review of the Impact of Cover Crops on Soil Properties. Int. Soil Water Conserv. Res. 2022, 10, 343–354. [Google Scholar] [CrossRef]
- Sekaran, U.; Sagar, K.L.; Kumar, S. Soil Aggregates, Aggregate-Associated Carbon and Nitrogen, and Water Retention as Influenced by Short and Long-Term No-till Systems. Soil Tillage Res. 2021, 208, 104885. [Google Scholar] [CrossRef]
- Rahman, N.S.N.A.; Hamid, N.W.A.; Nadarajah, K. Effects of Abiotic Stress on Soil Microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef]

| With Plant Cover | Without Plant Cover | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indicators | Units | <2800 Masl | SD | 2800–2900 Masl | SD | >2900 Masl | SD | <2800 Masl | SD | 2800–2900 Masl | SD | >2900 Masl | SD |
| pH | 7.09 | 0.11 | 6.96 | 0.65 | 6.80 | 0.31 | 7.08 | 0.31 | 6.76 | 0.60 | 6.77 | 0.59 | |
| OM | % | 3.28 | 0.19 | 3.04 | 0.24 | 2.60 | 0.13 | 2.57 | 0.34 | 2.95 | 0.25 | 2.43 | 0.22 |
| OC | % | 1.26 | 0.39 | 1.03 | 0.22 | 0.58 | 0.55 | 1.14 | 0.34 | 0.90 | 0.14 | 0.58 | 0.12 |
| TN | % | 0.19 | 0.08 | 0.47 | 0.20 | 0.40 | 0.06 | 0.16 | 0.13 | 0.47 | 0.07 | 0.50 | 0.08 |
| WD | Worm/m2 | 15.25 | 1.98 | 13.00 | 1.00 | 6.25 | 0.6 | 6.50 | 0.20 | 6.00 | 0.23 | 5.75 | 0.06 |
| WB | g/m2 | 7.45 | 0.23 | 5.90 | 0.70 | 2.83 | 0.69 | 2.85 | 0.05 | 2.25 | 0.02 | 2.02 | 0.33 |
| Indicator | Units | Range | References |
|---|---|---|---|
| OM | % | 2.0–6.0 | [21] |
| 3.5–5.0 | [22] | ||
| 1.29–4.5 | [23] | ||
| pH | - | 5.5–7.2 | [24] |
| 7.2–8.0 | |||
| 5.5–7.2 | [20] | ||
| 7.2–8.0 | |||
| 5.5–7.0 | [25] | ||
| 5.0–8.5 | [26] | ||
| 5.5–7.0 | [27] | ||
| TN | % | 0.2–0.3 | [28] |
| 0.1–0.5 | [24] | ||
| 0.09–0.12 | [29] | ||
| WD | Number of worms/square foot2 | 5–15 | [22] |
| 15–70 | [30] | ||
| WB | g/m2 | 1.82–9.66 | [31] |
| OC | % | 2.0–3.0 | [28] |
| 0.6–2.5 | [25] | ||
| 0.6–2.5 | [27] | ||
| 1.0–1.5 | [26] | ||
| 1–1.5 | [24] |
| Soil Quality Classes | Scale | Classes |
|---|---|---|
| Very high quality | 0.80–1.00 | 1 |
| High quality | 0.60–0.79 | 2 |
| Moderate quality | 0.40–0.59 | 3 |
| Low quality | 0.20–0.39 | 4 |
| Very low quality | 0.00–0.19 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneses Quelal, O.; Yánez Yánez, W.; Aranguren Carrera, J. Bioindicators for Assessing Soil Quality in Ecuador’s Jun Jun Micro-Watershed. Agronomy 2024, 14, 1436. https://doi.org/10.3390/agronomy14071436
Meneses Quelal O, Yánez Yánez W, Aranguren Carrera J. Bioindicators for Assessing Soil Quality in Ecuador’s Jun Jun Micro-Watershed. Agronomy. 2024; 14(7):1436. https://doi.org/10.3390/agronomy14071436
Chicago/Turabian StyleMeneses Quelal, Orlando, Wilfrido Yánez Yánez, and Jesús Aranguren Carrera. 2024. "Bioindicators for Assessing Soil Quality in Ecuador’s Jun Jun Micro-Watershed" Agronomy 14, no. 7: 1436. https://doi.org/10.3390/agronomy14071436
APA StyleMeneses Quelal, O., Yánez Yánez, W., & Aranguren Carrera, J. (2024). Bioindicators for Assessing Soil Quality in Ecuador’s Jun Jun Micro-Watershed. Agronomy, 14(7), 1436. https://doi.org/10.3390/agronomy14071436





