The Internal Extra Sequence Regions in Satellite RNA TA-Tb Are Important for Suppressing RNA Accumulations of Cucumber Mosaic Virus to Attenuate the Virulence of the Helper Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Virus Inoculation
2.2. Construction of satRNA TA-Tb Infectious cDNA Clone and Its Mutant Derivatives
2.3. RNA Extraction and Northern Blot Analysis
2.4. Western Blot Analysis
2.5. Sequence Analysis
3. Results
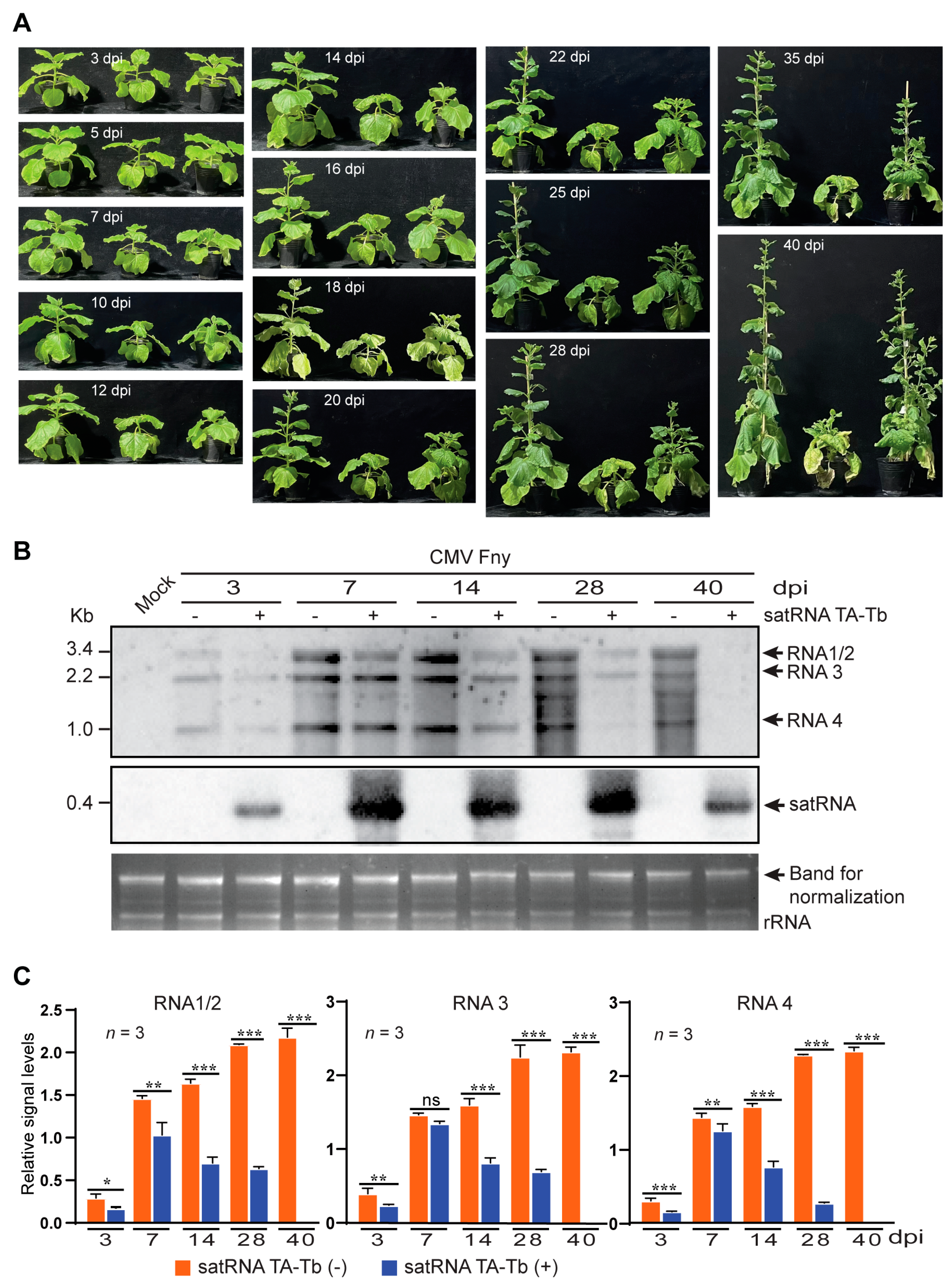
3.1. Replication of satRNA TA-Tb Is Associated with Reduced Accumulation of CMV RNA Genome
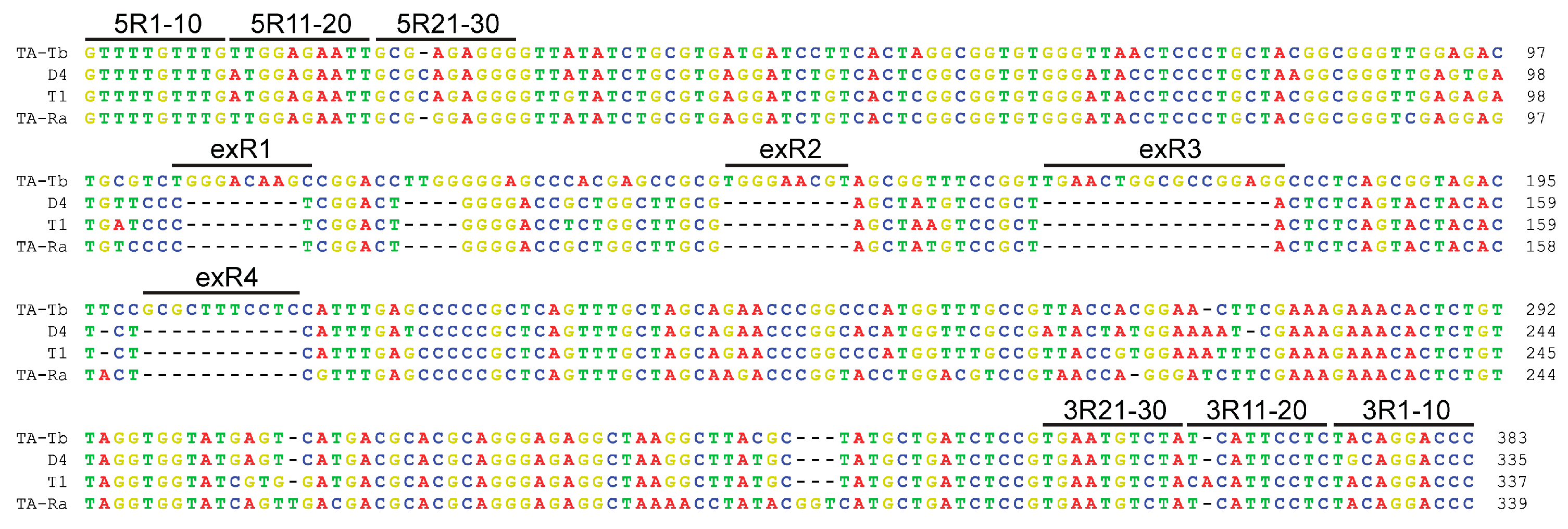
3.2. Deletions of Ten Nucleotides of 5′ and 3′ Termini Abolish Replication of satRNA TA-Tb
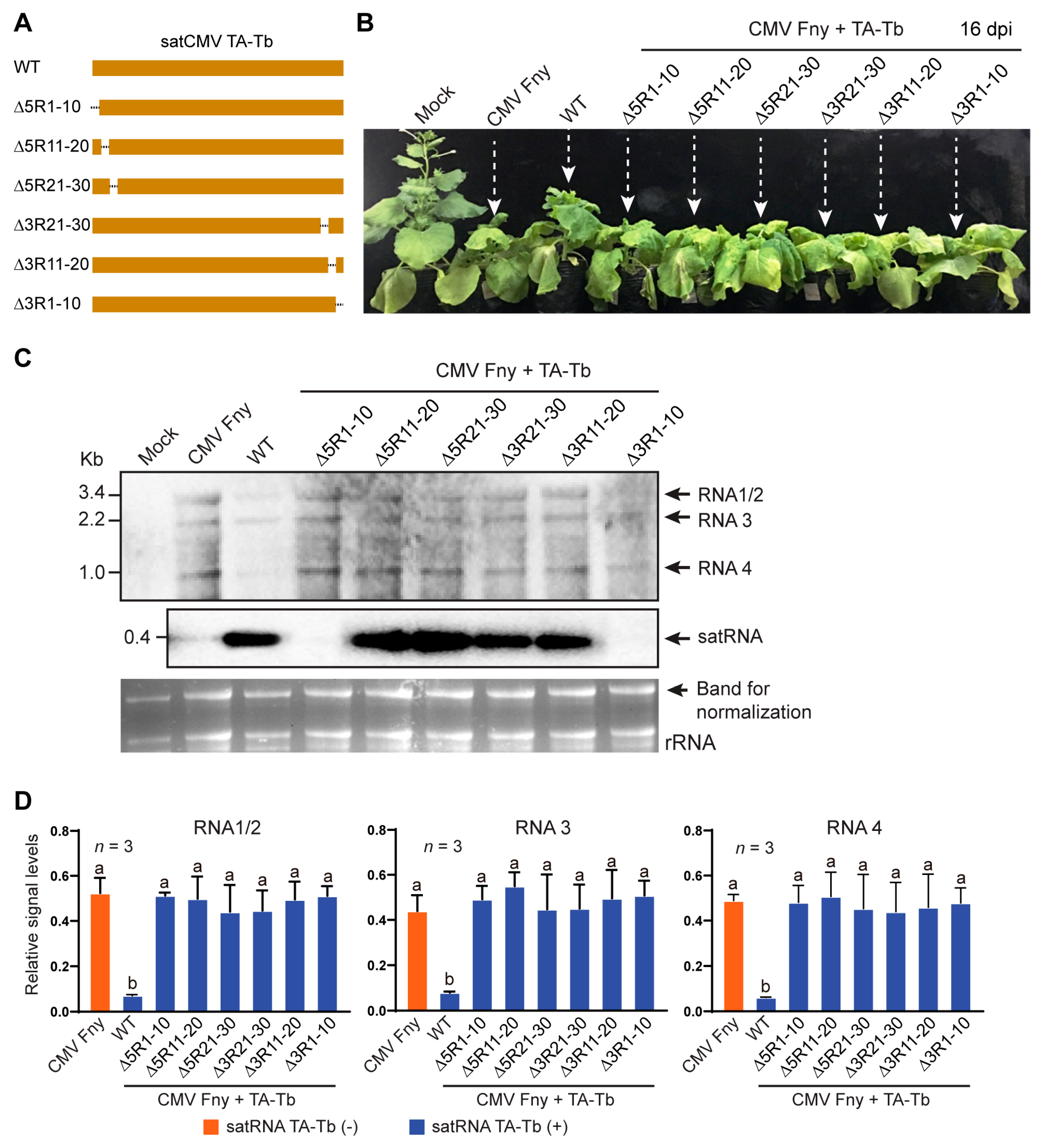
3.3. Internal Extra Sequence Regions of satRNA TA-Tb Are Important for Attenuation of CMV Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, A.E.; Roossinck, M.J.; Havelda, Z. Plant virus satellite and defective interfering RNAs: New paradigms for a new century. Annu. Rev. Phytopathol. 2004, 42, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Roossinck, M.; Sleat, D.; Palukaitis, P. Satellite RNAs of plant viruses: Structures and biological effects. Microbiol. Rev. 1992, 56, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-C.; Hsu, Y.-H.; Lin, N.-S. Satellite RNAs and satellite viruses of plants. Viruses 2009, 1, 1325–1350. [Google Scholar] [CrossRef] [PubMed]
- Palukaitis, P. Satellite RNAs and satellite viruses. Mol. Plant-Microbe Interact. 2016, 29, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Badar, U.; Venkataraman, S.; AbouHaidar, M.; Hefferon, K. Molecular interactions of plant viral satellites. Virus Genes 2021, 57, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tien, P.; Wu, G. Satellite RNA for the biocontrol of plant disease. Adv. Virus Res. 1991, 39, 321–339. [Google Scholar]
- Cao, X.; Liu, S.; Yu, C.; Li, X.; Yuan, X. A new strategy of using satellite RNA to control viral plant diseases: Post-inoculation with satellite RNA attenuates symptoms derived from pre-infection with its helper virus. Plant Biotechnol. J. 2019, 17, 1856. [Google Scholar] [CrossRef] [PubMed]
- Bujarski, J.; Gallitelli, D.; García-Arenal, F.; Pallás, V.; Palukaitis, P.; Reddy, M.K.; Wang, A.; Consortium, I.R. ICTV virus taxonomy profile: Bromoviridae. J. Gen. Virol. 2019, 100, 1206–1207. [Google Scholar] [CrossRef]
- Jacquemond, M. Cucumber mosaic virus. Adv. Virus Res. 2012, 84, 439–504. [Google Scholar]
- Nitta, N.; Takanami, Y.; Kuwata, S.; Kubo, S. Inoculation with RNAs 1 and 2 of cucumber mosaic virus induces viral RNA replicase activity in tobacco mesophyll protoplasts. J. Gen. Virol. 1988, 69, 2695–2700. [Google Scholar] [CrossRef]
- Lucy, A.P.; Guo, H.-S.; Li, W.-X.; Ding, S.-W. Suppression of post-transcriptional gene silencing by a plant viral protein localized in the nucleus. EMBO J. 2000, 19, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-W.; Anderson, B.J.; Haase, H.R.; Symons, R.H. New overlapping gene encoded by the cucumber mosaic virus genome. Virology 1994, 198, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kuwata, S.; Kataoka, J.; Masuta, C.; Nitta, N.; Takanami, Y. Functional analysis of deletion mutants of cucumber mosaic virus RNA3 using an in vitro transcription system. Virology 1991, 183, 106–113. [Google Scholar] [CrossRef]
- Schwinghamer, M.W.; Symons, R.H. Translation of the four major RNA species of cucumber mosaic virus in plant and animal cell-free systems and in toad oocytes. Virology 1977, 79, 88–108. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. A Top Ten list for economically important plant viruses. Arch. Virol. 2015, 160, 17–20. [Google Scholar] [CrossRef]
- Scholthof, K.B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Palukaitis, P.; García-Arenal, F. Cucumber Mosaic Virus; American Phytopathological Society (APS Press):, St. Paul, MN, USA, 2018.
- Andika, I.B.; Wei, S.; Cao, C.; Salaipeth, L.; Kondo, H.; Sun, L. Phytopathogenic fungus hosts a plant virus: A naturally occurring cross-kingdom viral infection. Proc. Natl. Acad. Sci. USA 2017, 114, 12267–12272. [Google Scholar] [CrossRef]
- Cao, X.; Liu, J.; Pang, J.; Kondo, H.; Chi, S.; Zhang, J.; Sun, L.; Andika, I.B. Common but nonpersistent acquisitions of plant viruses by plant-associated fungi. Viruses 2022, 14, 2279. [Google Scholar] [CrossRef]
- Garcia-Arenal, F.; Palukaitis, P. Structure and functional relationships of satellite RNAs of cucumber mosaic virus. Satell. Defective Viral RNAs 1999, 239, 37–63. [Google Scholar]
- Masuta, C. Molecular biology of Cucumber mosaic virus and its satellite RNA. J. Gen. Plant Pathol. 2014, 80, 514–518. [Google Scholar] [CrossRef]
- Kouadio, K.T.; De Clerck, C.; Agneroh, T.A.; Parisi, O.; Lepoivre, P.; Jijakli, H. Role of satellite RNAs in cucumber mosaic virus-host plant interactions. A review. Biotechnol. Agron. Société Environ. 2013, 17, 644–650. [Google Scholar]
- Xu, P.; Roossinck, M.J. Cucumber mosaic virus D satellite RNA–induced programmed cell death in tomato. Plant Cell 2000, 12, 1079–1092. [Google Scholar]
- Shimura, H.; Pantaleo, V.; Ishihara, T.; Myojo, N.; Inaba, J.-I.; Sueda, K.; Burgyán, J.; Masuta, C. A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. PLoS Pathog. 2011, 7, e1002021. [Google Scholar] [CrossRef]
- Smith, N.A.; Eamens, A.L.; Wang, M.-B. Viral small interfering RNAs target host genes to mediate disease symptoms in plants. PLoS Pathog. 2011, 7, e1002022. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, W.H.; Kim, H.; Nakada, Y.; Masuta, C. A plant virus satellite RNA directly accelerates wing formation in its insect vector for spread. Nat. Commun. 2021, 12, 7087. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, Q.; Gu, Z.; Liao, Q.; Palukaitis, P.; Du, Z. A conserved RNA structure is essential for a satellite RNA-mediated inhibition of helper virus accumulation. Nucleic Acids Res. 2019, 47, 8255–8271. [Google Scholar] [CrossRef]
- Liao, Q.; Zhu, L.; Du, Z.; Zeng, R.; Feng, J.; Chen, J. Satellite RNA-mediated reduction of cucumber mosaic virus genomic RNAs accumulation in Nicotiana tabacum. Acta Biochim. Biophys. Sin. 2007, 39, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Escriu, F.; Perry, K.L.; García-Arenal, F. Transmissibility of Cucumber mosaic virus by Aphis gossypii correlates with viral accumulation and is affected by the presence of its satellite RNA. Phytopathology 2000, 90, 1068–1072. [Google Scholar] [CrossRef]
- Wu, G.; Kaper, J. Competition of viral and satellite RNAs of cucumber mosaic virus for replication in vitro by viral RNA-dependent RNA polymerase. Res. Virol. 1995, 146, 61–67. [Google Scholar] [CrossRef]
- Hou, W.N.; Duan, C.G.; Fang, R.X.; Zhou, X.Y.; Guo, H.S. Satellite RNA reduces expression of the 2b suppressor protein resulting in the attenuation of symptoms caused by Cucumber mosaic virus infection. Mol. Plant Pathol. 2011, 12, 595–605. [Google Scholar] [CrossRef]
- Shen, W.-X.; Au, P.C.K.; Shi, B.-J.; Smith, N.A.; Dennis, E.S.; Guo, H.-S.; Zhou, C.-Y.; Wang, M.-B. Satellite RNAs interfere with the function of viral RNA silencing suppressors. Front. Plant Sci. 2015, 6, 281. [Google Scholar] [CrossRef] [PubMed]
- Mascia, T.; Gallitelli, D. Satellites as Viral Biocontrol Agents. In Viroids and Satellites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 681–688. [Google Scholar]
- Liu, S.; Yu, C.; Cao, X.; Geng, G.; Li, X.; Shi, K.; Yuan, X. Analysis of sequences and structures of two Cucumber mosaic virus satellite RNAs from Shandong province of China and the effect on virulence of helper virus. Acta Phytopathol. Sin. 2018, 48, 365–372. [Google Scholar]
- Liu, Z.; Cao, X.; Yu, C.; Yuan, X. Probing the RNA Structure of a Satellite RNA of Cucumber Mosaic Virus Using SHAPE Method. Agronomy 2023, 13, 1990. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, T.; Tian, Z.; Wang, Y.; Tao, X. Construction of Agrobacterium-mediated Cucumber mosaic virus infectious cDNA clones and 2b deletion viral vector. Sci. Agric. Sin. 2011, 44, 3060–3068. [Google Scholar]
- Zhu, M.; Liu, S.; Wang, Z.; Yu, C.; Yuan, X. A New Strategy of Cross-Protection Based on Attenuated Vaccines: RNA Viruses Are Used as Vectors to Control DNA Viruses. Agronomy 2023, 13, 2334. [Google Scholar] [CrossRef]
- Guo, L.-H.; Cao, Y.-H.; Li, D.-W.; Niu, S.-N.; Cai, Z.-N.; Han, C.-G.; Zhai, Y.-F.; Yu, J.-L. Analysis of nucleotide sequences and multimeric forms of a novel satellite RNA associated with Beet black scorch virus. J. Virol. 2005, 79, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-M.; Geng, G.-W.; Cao, X.-R.; Yang, C.; Qi, Z.; Liu, S.-S.; Zhu, C.-X.; Yuan, X.-F. First identification of cucumber mosaic virus infecting six fruit crops in china. J. Plant Pathol. 2019, 101, 373–376. [Google Scholar] [CrossRef]
- Fraile, A.; Garcia-Arenal, F. Secondary structure as a constraint on the evolution of a plant viral satellite RNA. J. Mol. Biol. 1991, 221, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Jacquemond, M.; Leroux, J.-P. L’ARN satellite du virus de la mosaïque du concombre II. Etude de la relation virus-ARN satellite chez divers hôtes. Agronomie 1982, 2, 55–62. [Google Scholar] [CrossRef]
- Waterworth, H.; Kaper, J.; Tousignant, M. CARNA 5, the small cucumber mosaic virus—Dependent replicating RNA, regulates disease expression. Science 1979, 204, 845–847. [Google Scholar] [CrossRef]
- Du, Q.-S.; Duan, C.-G.; Zhang, Z.-H.; Fang, Y.-Y.; Fang, R.-X.; Xie, Q.; Guo, H.-S. DCL4 targets Cucumber mosaic virus satellite RNA at novel secondary structures. J. Virol. 2007, 81, 9142–9151. [Google Scholar] [CrossRef] [PubMed]
- Buck, K.W. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 1996, 47, 159–251. [Google Scholar] [PubMed]
- Masuta, C.; Takanami, Y. Determination of sequence and structural requirements for pathogenicity of a cucumber mosaic virus satellite RNA (Y-satRNA). Plant Cell 1989, 1, 1165–1173. [Google Scholar] [PubMed]
- Shang, J.; Xi, D.-H.; Huang, Q.-R.; Xu, M.-Y.; Yuan, S.; Wang, S.-D.; Jia, S.-D.; Cao, S.; Zhou, Z.-L.; Lin, H.-H. Effect of two satellite RNAs on Nicotiana glutinosa infected with Cucumber mosaic virus (CMV). Physiol. Mol. Plant Pathol. 2009, 74, 184–190. [Google Scholar] [CrossRef]
- Canto, T.; Prior, D.A.; Hellwald, K.-H.; Oparka, K.J.; Palukaitis, P. Characterization of Cucumber mosaic virus. Virology 1997, 237, 237–248. [Google Scholar] [CrossRef]
- Schmitz, I.; Rao, A. Deletions in the conserved amino-terminal basic arm of cucumber mosaic virus coat protein disrupt virion assembly but do not abolish infectivity and cell-to-cell movement. Virology 1998, 248, 323–331. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Liu, Z.; Yu, C.; Andika, I.B.; Yuan, X. The Internal Extra Sequence Regions in Satellite RNA TA-Tb Are Important for Suppressing RNA Accumulations of Cucumber Mosaic Virus to Attenuate the Virulence of the Helper Virus. Agronomy 2024, 14, 1451. https://doi.org/10.3390/agronomy14071451
Cao X, Liu Z, Yu C, Andika IB, Yuan X. The Internal Extra Sequence Regions in Satellite RNA TA-Tb Are Important for Suppressing RNA Accumulations of Cucumber Mosaic Virus to Attenuate the Virulence of the Helper Virus. Agronomy. 2024; 14(7):1451. https://doi.org/10.3390/agronomy14071451
Chicago/Turabian StyleCao, Xinran, Zhifei Liu, Chengming Yu, Ida Bagus Andika, and Xuefeng Yuan. 2024. "The Internal Extra Sequence Regions in Satellite RNA TA-Tb Are Important for Suppressing RNA Accumulations of Cucumber Mosaic Virus to Attenuate the Virulence of the Helper Virus" Agronomy 14, no. 7: 1451. https://doi.org/10.3390/agronomy14071451




