Exogenous Melatonin Alleviates Selenium Stress and Promotes Its Uptake in Cyphomandra betacea Sendt. (Solanum betaceum Cav.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Determination of the Parameters
2.4. Statistical Analysis
3. Results
3.1. Biomass
3.2. Contents of the Photosynthetic Pigments
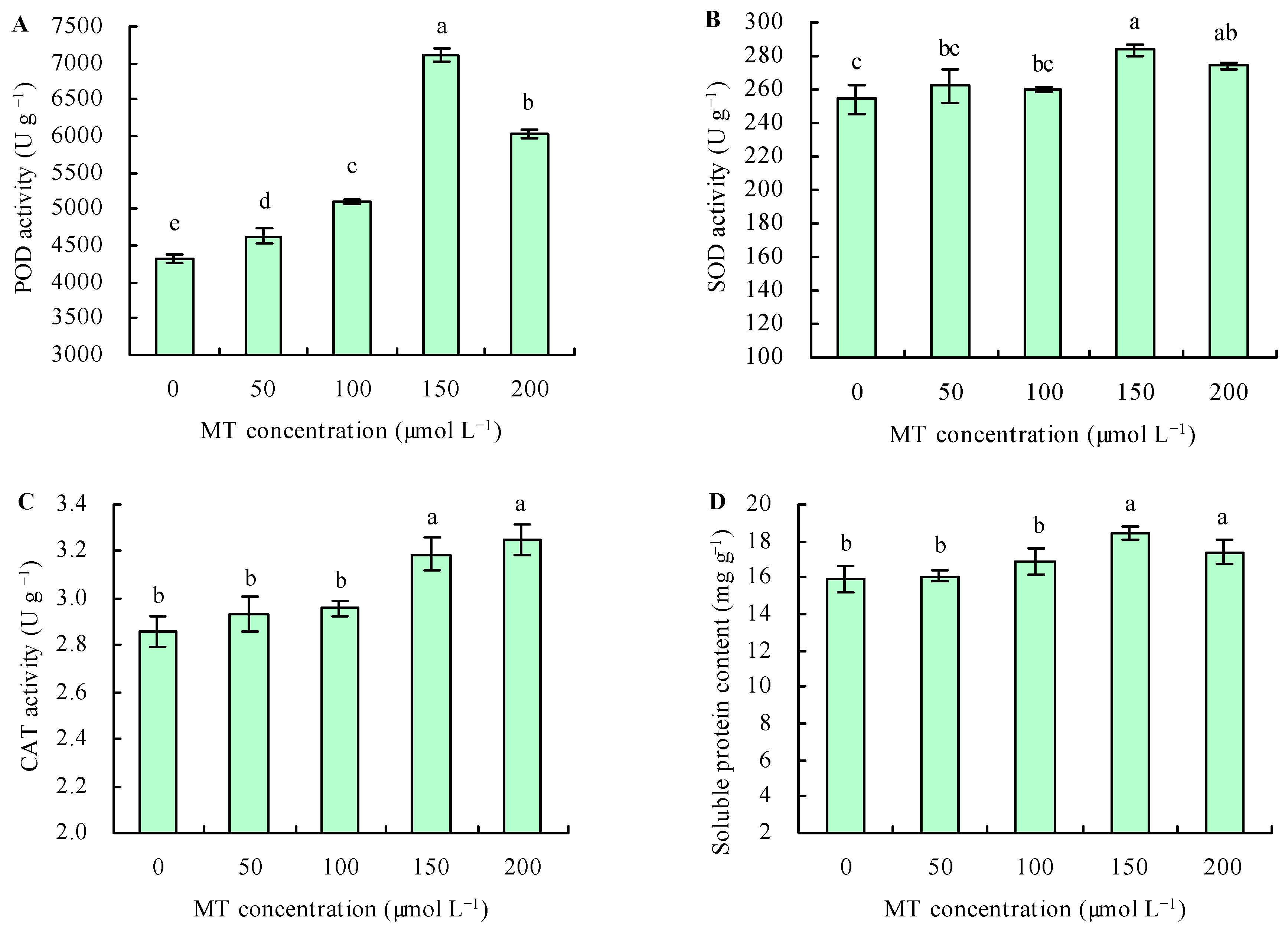
3.3. Activities of Antioxidant Enzymes and the Contents of Soluble Protein
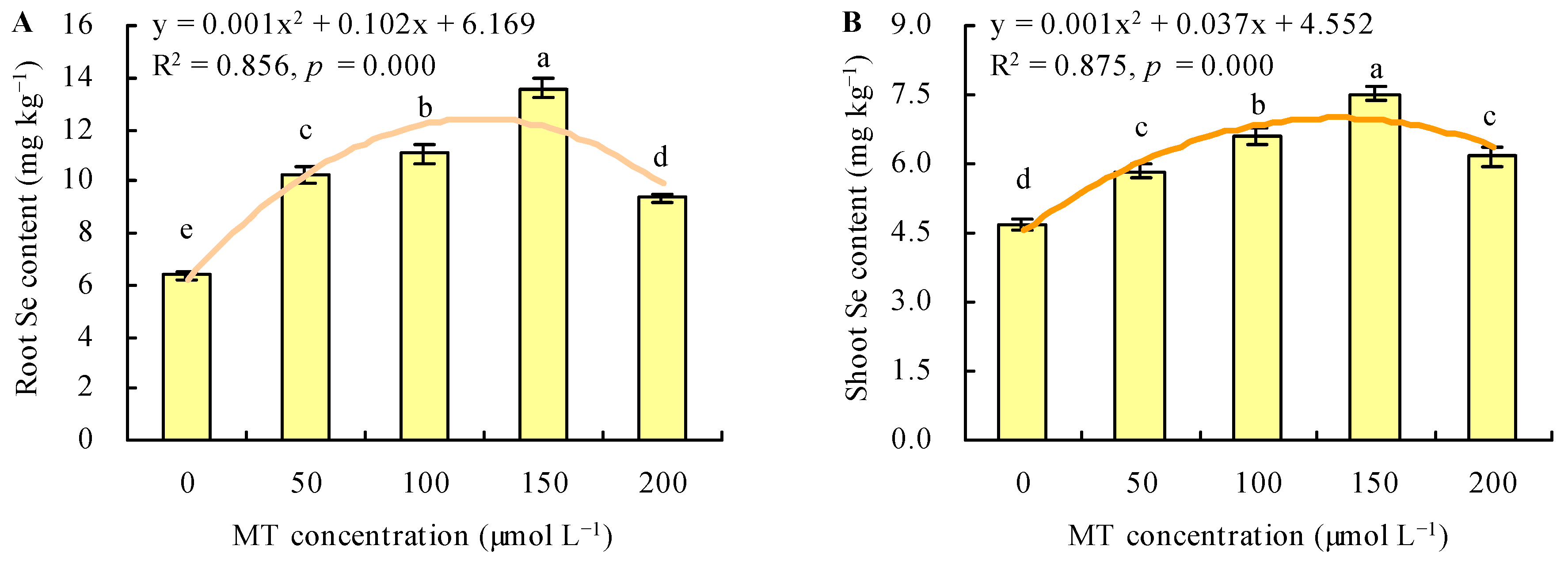
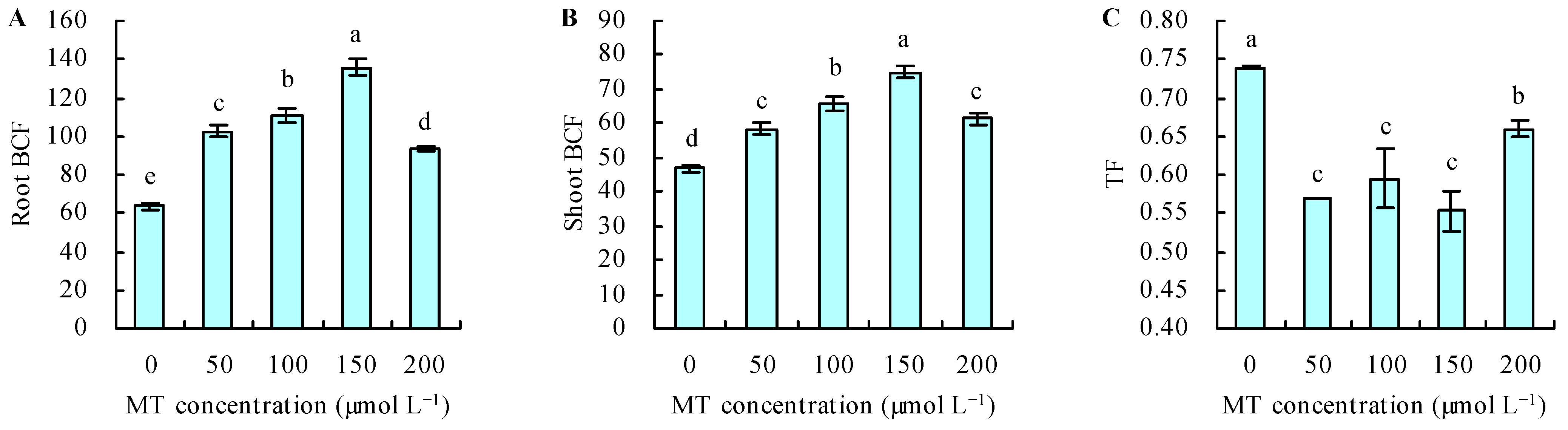
3.4. Content and Transport of Se
3.5. Correlation Analysis
3.6. PCA and Cluster Analysis
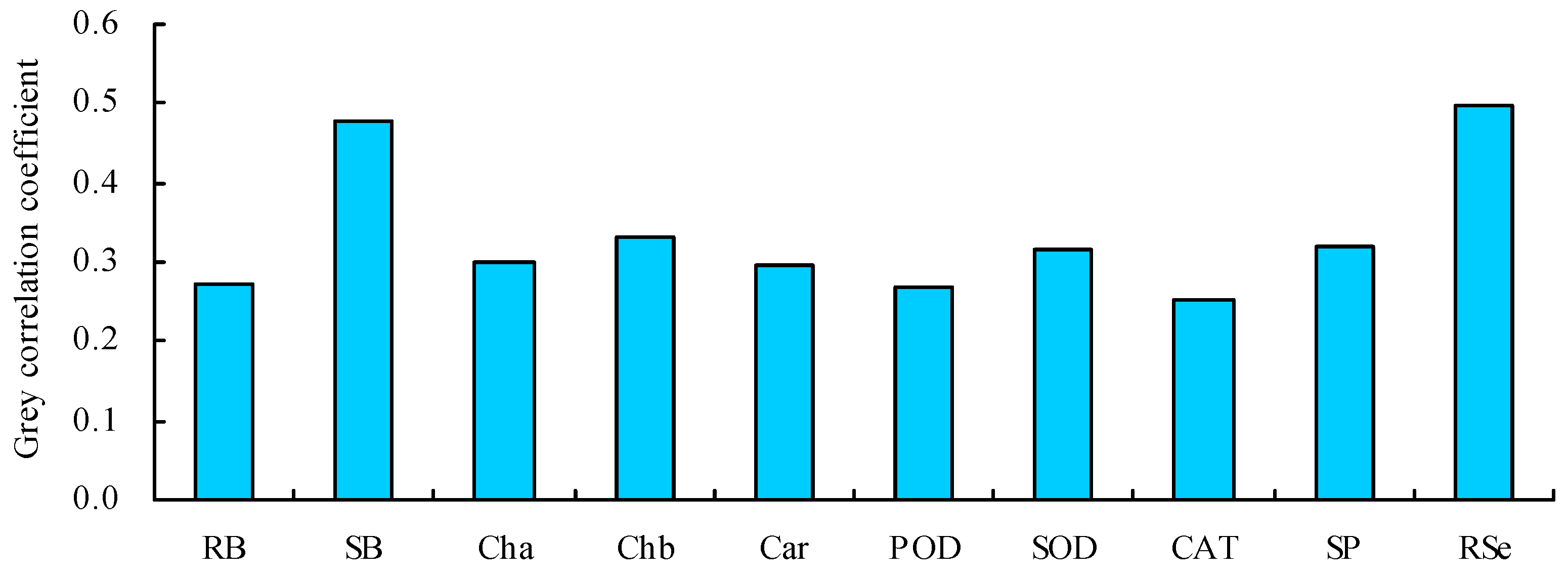
3.7. Grey and Path Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Thiry, C.; Ruttens, A.D.; Temmerman, L.; Schneider, Y.J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, C.; Zhang, T. Selenium transformation and selenium-rich foods. Food Biosci. 2021, 40, 100875. [Google Scholar] [CrossRef]
- El Kassis, E.; Cathala, N.; Rouached, H.; Fourcroy, P.; Berthomieu, P.; Terry, N.; Davidian, J.-C. Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 2007, 143, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits EA, H.; Quinn, C.F. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients; Springer: Berlin/Heidelberg, Germany, 2010; pp. 225–241. [Google Scholar]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Van Hoewyk, D. A tale of two toxicities: Malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann. Bot. 2013, 112, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y.; Wei, X.; Bie, Y.; Zhou, H.; Deng, L.; Lin, L.; Liao, M. Effects of mutual grafting Solanum photeinocarpum from two ecosystems on physiology and selenium absorption of their offspring under selenium stress. Acta Physiol. Plant. 2021, 43, 96. [Google Scholar] [CrossRef]
- Huan, Y.; Yang, L.; Liu, Q.; Lin, L.; Liao, M.; Wang, Z.; Liang, D.; Xia, H.; Tang, Y.; Lv, X.; et al. Effects of indole acetic acid on the growth and selenium absorption characteristics of Cyphomandra betacea seedlings. Acta Physiol. Plant. 2021, 43, 74. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Burkhardt, S.; Manchester, L.C. Melatonin in Plants. Nutr. Rev. 2001, 59, 286–290. [Google Scholar] [CrossRef]
- Zou, J.N. Effects of Exogenous Melatonin on Photosynthesis and Growth of Soybean under Drought Stress; Heilongjiang Bayi Agricultural University: Daqing, China, 2019. [Google Scholar]
- Wang, P.; Sun, X.; Wang, N.; Tan, D.X.; Ma, F. Melatonin enhances the occurrence of autophagy induced by oxidative stress in Arabidopsis seedlings. J. Pineal Res. 2015, 58, 479–489. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using exogenous melatonin, glutathione, proline, and glycine betaine treatments to combat abiotic stresses in crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A Small Molecule but Important for Salt Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- He, J.; Zhuang, X.; Zhou, J.; Sun, L.; Wan, H.; Li, H.; Lyu, D. Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Kamiab, F. Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J. Plant Nutr. 2020, 43, 1468–1484. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Altaf, M.M.; Jahan, M.S.; Khan, L.U. Melatonin mitigates nickel toxicity by improving nutrient uptake fluxes, root architecture system, photosynthesis, and antioxidant potential in tomato seedling. J. Soil Sci. Plant Nutr. 2021, 21, 1842–1855. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; You, X.Y.; Bao, R.F.; Wang, J.; Lv, X.L.; Liang, D.; Xia, H.; Liao, M.A.; Lin, L.J. Effects of melatonin on growth and selenium accumulation of grape seedlings. Chin. J. Soil Sci. 2022, 53, 1453–1460. [Google Scholar] [CrossRef]
- Liao, R.Y.; Huang, K.W.; Li, K.Q. Effect of different concentrations of melatonin on selenium accumulation in Plantago asiatica L. J. Chin. Med. Mater. 2018, 41, 1539–1542. [Google Scholar] [CrossRef]
- Suárez-Montenegro, Z.J.; Ballesteros-Vivas, D.; Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Parada-Alfonso, F.; Ibáñez, E.; Cifuentes, A. Neuroprotective potential of tamarillo (Cyphomandra betacea) epicarp extracts obtained by sustainable extraction process. Front. Nutr. 2021, 8, 769617. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Sun, J.; Cui, T.; Zhou, X.; Liao, M.; Huan, Y.; Yang, L.; Wu, C.; Xia, X.; Wang, Y.; et al. Selenium accumulation characteristics of Cyphomandra betacea (Solanum betaceum) seedlings. Physiol. Mol. Biol. Plants 2020, 26, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Wang, J.; Liang, D.; Xia, H.; Lv, X.; Deng, Q.; Wang, X.; Luo, X.; Liao, M.; et al. Gibberellic acid promotes selenium accumulation in Cyphomandra betacea under selenium stress. Front. Plant Sci. 2022, 13, 968768. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, J.; Deng, L.; Zhou, H.; Bie, Y.; Jing, Q.; Lin, L.; Wang, J.; Liao, M. Effects of diethyl aminoethyl hexanoate on the physiology and selenium absorption of grape seedlings. Acta Physiol. Plant. 2021, 43, 115. [Google Scholar] [CrossRef]
- Xia, H.; Yang, C.; Liang, Y.; He, Z.; Guo, Y.; Lang, Y.; Wei, J.; Tian, X.; Lin, L.; Deng, H.; et al. Melatonin and arbuscular mycorrhizal fungi synergistically improve drought toleration in kiwifruit seedlings by increasing mycorrhizal colonization and nutrient uptake. Front. Plant Sci. 2022, 13, 1073917. [Google Scholar] [CrossRef]
- Hao, Z.B.; Cang, J.; Xu, Z. Plant Physiology Experiment; Harbin Institute of Technology Press: Harbin, China, 2004. [Google Scholar]
- Lin, L.; Wu, C.; Jiang, W.; Liao, M.; Tang, Y.; Wang, J.; Lv, X.; Liang, D.; Xia, H.; Wang, X.; et al. Grafting increases cadmium accumulation in the post-grafting generations of the potential cadmium-hyperaccumulator Solanum photeinocarpum. Chem. Ecol. 2020, 36, 685–704. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soils, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Rastmanesh, F.; Moore, F.; Keshavarzi, B. Speciation and phytoavailability of heavy metals in contaminated soils in Sarcheshmeh area, Kerman Province, Iran. Bull. Environ. Contam. Toxicol. 2010, 85, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, Z.; Wang, J.; Liang, D.; Xia, H.; Lv, X.; Tang, Y.; Wang, X.; Deng, Q.; Liao, M. 24-epibrassinolide promotes selenium uptake in grapevine under selenium stress. Sci. Hortic. 2023, 308, 111564. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Q.; Xu, X.; Liao, M.; Lin, L.; Hu, R.; Luo, X.; Wang, Z.; Wang, J.; Deng, Q.; et al. An amino acid fertilizer improves the emergent accumulator plant Nasturtium officinale R. Br. phytoremediation capability for cadmium-contaminated paddy soils. Front. Plant Sci. 2022, 13, 1003743. [Google Scholar] [CrossRef]
- Sun, C.L.; Liu, L.J.; Wang, L.X.; Li, B.H.; Jin, C.W.; Lin, X.Y. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Liu, Z.; Chen, L.; Tang, S.; Ding, C.; Jiang, Y.; Li, G. Melatonin regulates antioxidant strategy in response to continuous salt stress in rice seedlings. Plant Physiol. Biochem. 2021, 165, 239–250. [Google Scholar] [CrossRef] [PubMed]
- He, M.M.; Mei, S.Y.; Zhai, Y.N.; Geng, G.; Yu, L.H.; Wang, Y.G. Effects of melatonin on the growth of sugar beet (Beta vulgaris L.) seedlings under drought stress. J. Plant Growth Regul. 2023, 42, 5116–5130. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Chen, Z.Y.; Yang, B.X.; Komatsu, S.; Zhou, S.L. Proteomic analysis reveals the effects of melatonin on soybean root tips under flooding stress. J. Proteom. 2021, 232, 104064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; An, B.; Wei, Y.; Reiter, R.J.; Shi, H.; Luo, H.; He, C. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis. Front. Plant Sci. 2016, 7, 1882. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; You, J.; Li, J.Z.; Wang, Y.P.; Chan, Z.L. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 2021, 72, 5599–5611. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Rutto, L.; Katuuramu, D. Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS ONE 2019, 14, e0221687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, C.; Xu, L.; Niu, H.; Liu, Q.; Huang, Y.; Lv, G.; Yang, H.; Li, M. Melatonin and indole-3-acetic acid synergistically regulate plant growth and stress resistance. Cells 2022, 11, 3250. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Growth activity, rooting capacity, and tropism, three auxinic precepts fulfilled by melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin promotes adventitious and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Y.; Qin, X.; Ding, C.; Chen, Y.; Tang, Z.; Huang, Y.; Reiter, R.J.; Yuan, S.; Yuan, M. New insights into the role of melatonin in photosynthesis. J. Exp. Bot. 2022, 73, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, D.; Wang, J.; Tian, B.; Li, Y.; Sun, G.; Zhang, H. Exogenous melatonin alleviates NO2 damage in tobacco leaves by promoting antioxidant defense, modulating redox homeostasis, and signal transduction. J. Hazard. Mater. 2022, 424, 127265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cao, S.; Xie, K.; Chi, Z.; Wang, J.; Wang, H.; Wei, Y.; Shao, X.; Zhang, C.; Xu, F.; et al. Melatonin delays yellowing of broccoli during storage by regulating chlorophyll catabolism and maintaining chloroplast ultrastructure. Postharvest Biol. Technol. 2021, 172, 111378. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.R.; Chen, S.C. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Danilova, E.D.; Zlobin, I.E.; Kuznetsov, V.V.; Efimova, M. Exogenic melatonin reduces the toxic effect of polymetallic stress on barley plants. Dokl. Biochem. Biophys. 2021, 499, 228–232. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Yang, X.X.; Ren, J.H.; Lin, X.Y.; Yang, Z.P.; Deng, X.P.; Ke, Q.B. Melatonin alleviates chromium toxicity in maize by modulation of cell wall polysaccharides biosynthesis, glutathione metabolism, and antioxidant capacity. Int. J. Mol. Sci. 2023, 24, 3816. [Google Scholar] [CrossRef]
- Ren, J.H.; Ye, J.; Yin, L.; Li, G.X.; Deng, X.P.; Wang, S.W. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Deng, H.; Wang, J.; Lin, L.; Deng, Q.; Lv, X.; Liang, D.; Hu, R.; Wang, Z.; et al. Melatonin application improves berry coloration, sucrose synthesis, and nutrient absorption in ‘Summer Black’ grape. Food Chem. 2021, 356, 129713. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. Amst. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Wu, C.; Xu, Y.; Wang, J.; Lv, X.; Xia, H.; Liang, D.; Huang, Z.; Tang, Y. Melatonin promotes iron reactivation and reutilization in peach plants under iron deficiency. Int. J. Mol. Sci. 2023, 24, 16133. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, B.; Qiu, D.; Xie, Z.; Dai, S.; Li, C.; Xu, S.; Zheng, Y.; Li, S.; Jiang, M. Melatonin enhances metallic oxide nanoparticle stress tolerance in rice via inducing tetrapyrrole biosynthesis and amino acid metabolism. Environ. Sci. Nano 2021, 8, 2310–2323. [Google Scholar] [CrossRef]
- Dong, Z.; Xiao, Y.Q.; Wu, H. Selenium accumulation, speciation, and its effect on nutritive value of Flammulina velutipes (golden needle mushroom). Food Chem. 2021, 350, 128667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Pan, G.P.; Chen, J.; Hu, Q.H. Uptake and transport of selenite and selenate by soybean seedlings of two genotypes. Plant Soil 2003, 253, 437–443. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]



| Parameter | Root Biomass | Shoot Biomass | Chlorophyll a Content | Chlorophyll b Content | Carotenoid Content | POD Activity | SOD Activity | CAT Activity | Soluble Protein Content | Root Se Content | Shoot Se Content |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Root biomass | 1 | ||||||||||
| Root biomass | 0.863 ** | 1 | |||||||||
| Shoot biomass | 0.935 ** | 0.858 ** | 1 | ||||||||
| Chlorophyll a content | 0.861 ** | 0.935 ** | 0.947 ** | 1 | |||||||
| Chlorophyll b content | 0.935 ** | 0.874 ** | 0.994 ** | 0.952 ** | 1 | ||||||
| POD activity | 0.892 ** | 0.907 ** | 0.927 ** | 0.905 ** | 0.941 ** | 1 | |||||
| SOD activity | 0.948 ** | 0.828 ** | 0.942 ** | 0.841 ** | 0.943 ** | 0.923 ** | 1 | ||||
| CAT activity | 0.894 ** | 0.777 ** | 0.947 ** | 0.898 ** | 0.929 ** | 0.860 ** | 0.853 ** | 1 | |||
| Soluble protein content | 0.845 ** | 0.837 ** | 0.876 ** | 0.867 ** | 0.916 ** | 0.932 ** | 0.842 ** | 0.803 ** | 1 | ||
| Root Se content | 0.683 ** | 0.864 ** | 0.628 * | 0.729 ** | 0.682 ** | 0.759 ** | 0.699 ** | 0.499 | 0.739 ** | 1 | |
| Shoot Se content | 0.782 ** | 0.946 ** | 0.740 ** | 0.837 ** | 0.783 ** | 0.850 ** | 0.758 ** | 0.623 * | 0.828 ** | 0.965 ** | 1 |
| Factor | Total Effect | Direct Effect | Indirect Effect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | X1→Y | X2→Y | X3→Y | X4→Y | X5→Y | X6→Y | X7→Y | X8→Y | X9→Y | X10→Y | |||
| X1 | 0.783 | 0.954 | −0.171 | −0.420 | −1.124 | 1.081 | 0.332 | 0.690 | −0.299 | −0.542 | −0.180 | 0.291 | |
| X2 | 0.948 | −0.487 | 1.435 | 0.823 | −1.032 | 1.174 | 0.311 | 0.701 | −0.261 | −0.471 | −0.178 | 0.368 | |
| X3 | 0.740 | −1.202 | 1.942 | 0.892 | −0.418 | 1.188 | 0.353 | 0.717 | −0.297 | −0.574 | −0.187 | 0.268 | |
| X4 | 0.837 | 1.255 | −0.418 | 0.822 | −0.456 | −1.138 | 0.338 | 0.700 | −0.265 | −0.544 | −0.185 | 0.310 | |
| X5 | 0.781 | 0.355 | 0.426 | 0.892 | −0.426 | −1.196 | 1.194 | 0.727 | −0.298 | −0.563 | −0.195 | 0.291 | |
| X6 | 0.850 | 0.773 | 0.077 | 0.851 | −0.442 | −1.115 | 1.136 | 0.334 | −0.291 | −0.521 | −0.198 | 0.323 | |
| X7 | 0.758 | −0.316 | 1.074 | 0.904 | −0.404 | −1.133 | 1.055 | 0.335 | 0.714 | −0.516 | −0.179 | 0.298 | |
| X8 | 0.622 | −0.606 | 1.228 | 0.853 | −0.379 | −1.139 | 1.126 | 0.330 | 0.665 | −0.269 | −0.171 | 0.212 | |
| X9 | 0.829 | −0.213 | 1.042 | 0.807 | −0.408 | −1.054 | 1.088 | 0.326 | 0.721 | −0.266 | −0.487 | 0.315 | |
| X10 | 0.966 | 0.426 | 0.540 | 0.652 | −0.420 | −0.755 | 0.914 | 0.242 | 0.587 | −0.221 | −0.302 | −0.157 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Du, R.; Dai, J.; Xiao, Y.; Dai, Z.; Zhang, D.; Lin, L. Exogenous Melatonin Alleviates Selenium Stress and Promotes Its Uptake in Cyphomandra betacea Sendt. (Solanum betaceum Cav.). Agronomy 2024, 14, 1454. https://doi.org/10.3390/agronomy14071454
Wang X, Du R, Dai J, Xiao Y, Dai Z, Zhang D, Lin L. Exogenous Melatonin Alleviates Selenium Stress and Promotes Its Uptake in Cyphomandra betacea Sendt. (Solanum betaceum Cav.). Agronomy. 2024; 14(7):1454. https://doi.org/10.3390/agronomy14071454
Chicago/Turabian StyleWang, Xun, Ruimin Du, Jingtong Dai, Yunying Xiao, Zhen Dai, Dilian Zhang, and Lijin Lin. 2024. "Exogenous Melatonin Alleviates Selenium Stress and Promotes Its Uptake in Cyphomandra betacea Sendt. (Solanum betaceum Cav.)" Agronomy 14, no. 7: 1454. https://doi.org/10.3390/agronomy14071454




