A Reasonable Rotation Fallow Mode Enhances the Complexity of the Soil Bacterial Network and Enriches Nitrogen-Cycling-Related Taxa
Abstract
:1. Introduction
2. Materials and Methods
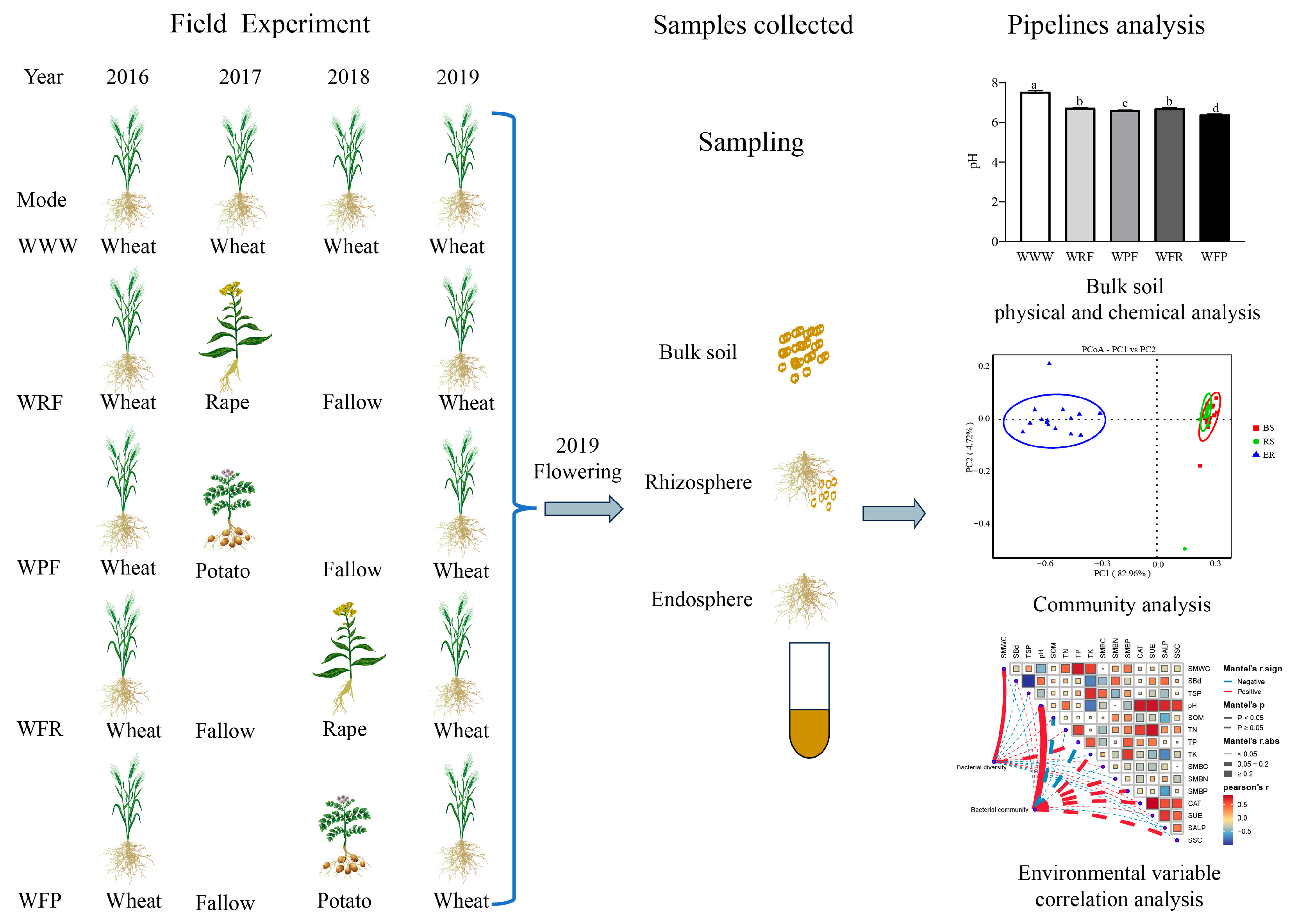
2.1. Site Description and Experimental Design
2.2. Sample Collection
2.3. Determination of Soil Biological and Physicochemical Properties
2.4. DNA Extraction, Amplification, and Construction of Illumina MiSeq Sequencing Library
2.5. Data Processing Analysis
3. Results
3.1. Analysis of Soil Physicochemical Properties and Biological Properties
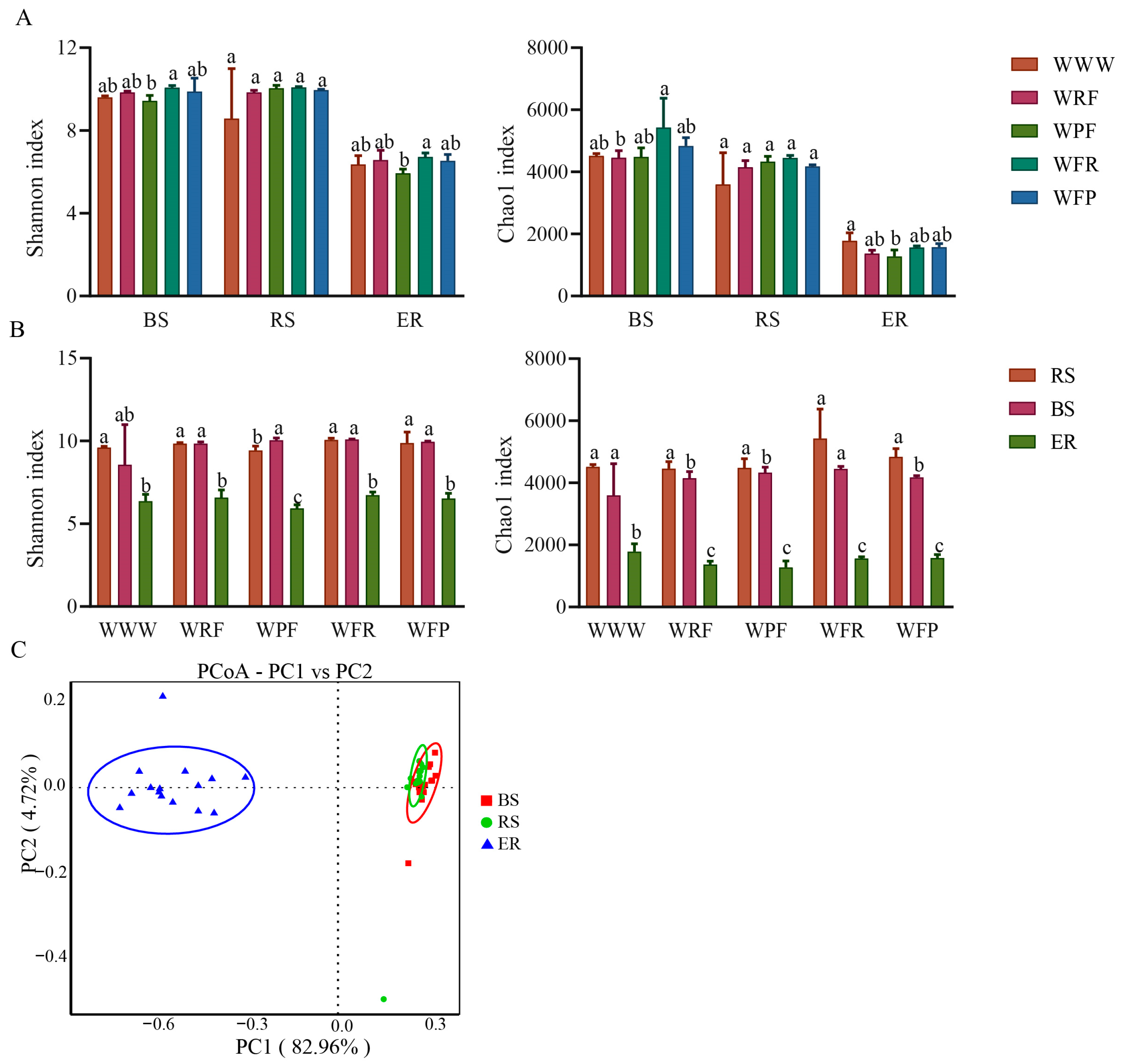
3.2. Analysis of Soil Bacterial Diversity
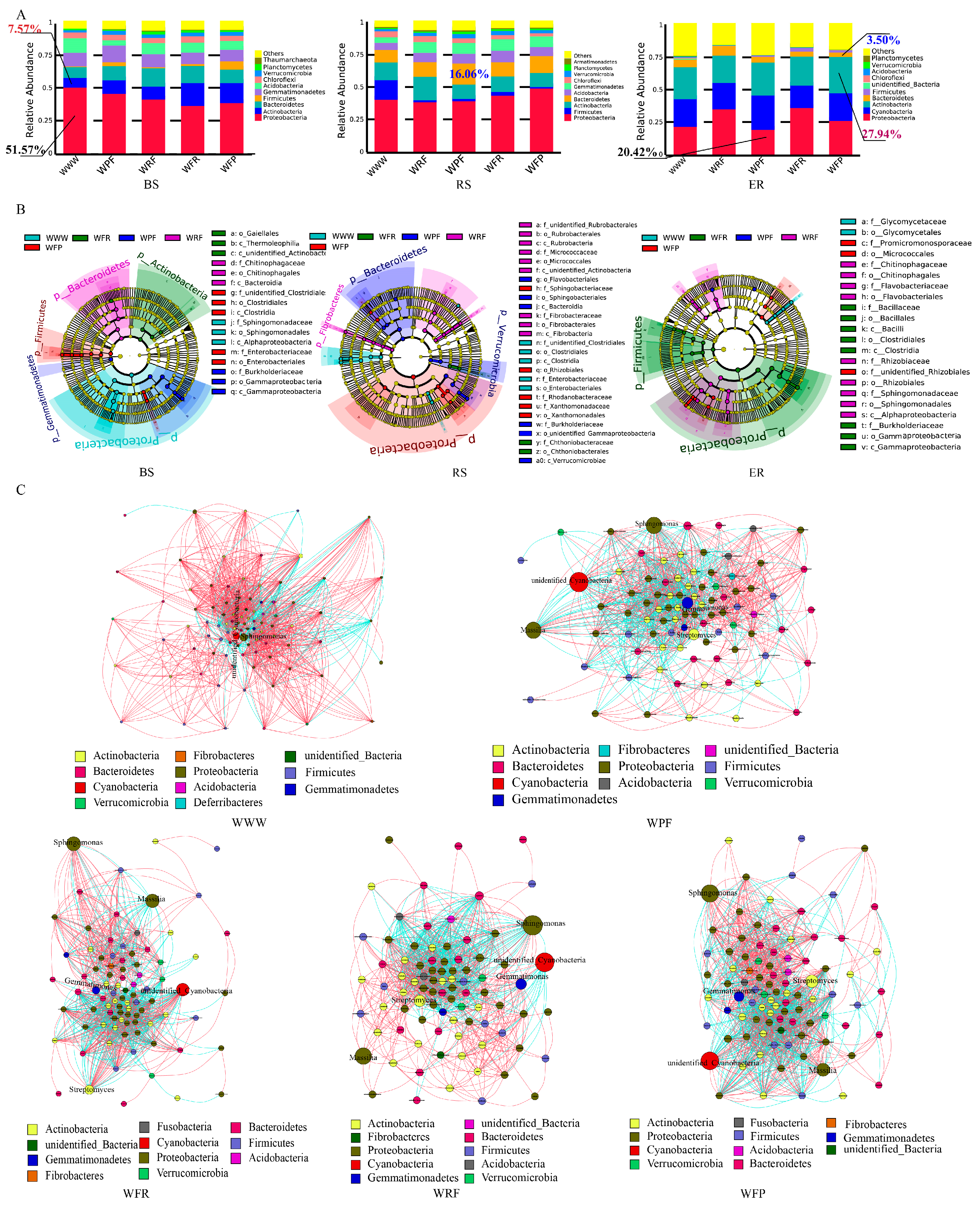
3.3. Analysis of Differences in Soil Bacterial Community Composition
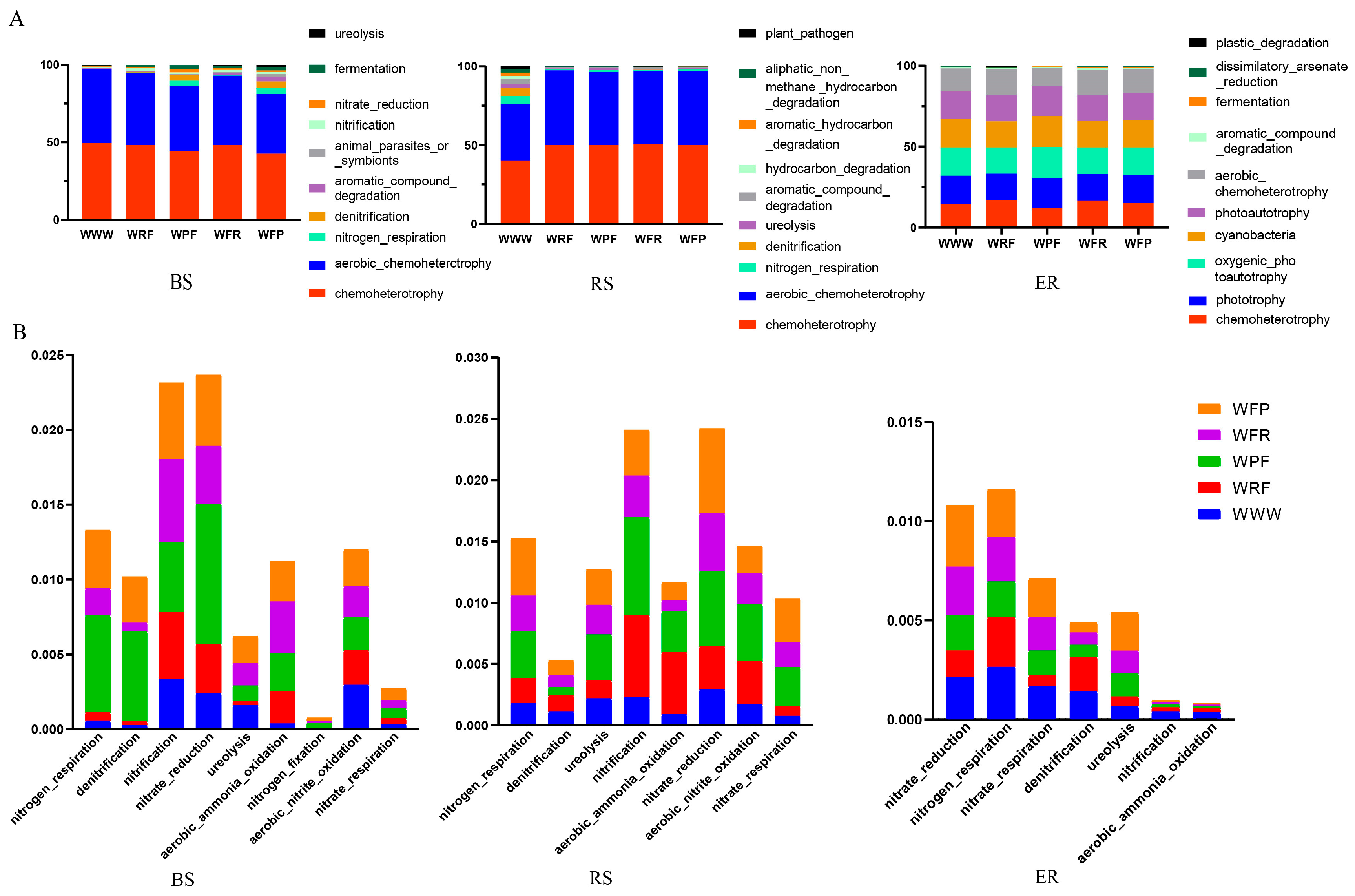
3.4. Analysis of Functional Differences between Soil Dominant Bacteria
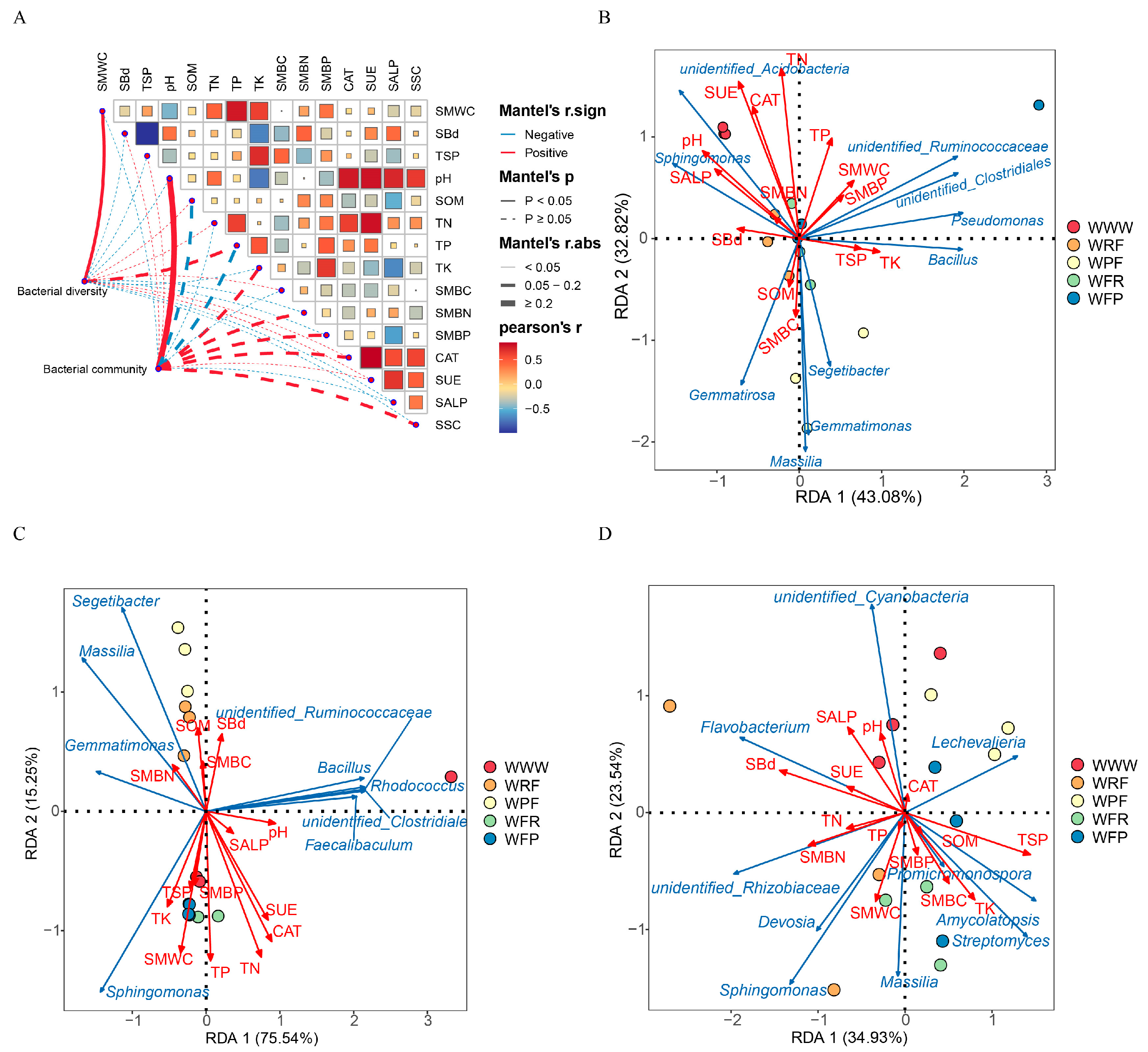
3.5. Analysis of the Correlation between Dominant Bacteria and Environmental Factors
3.6. The Impact of Key Environmental Variables on the Complexity of Microbial Networks and Nitrogen-Related Functional Taxa
4. Discussion
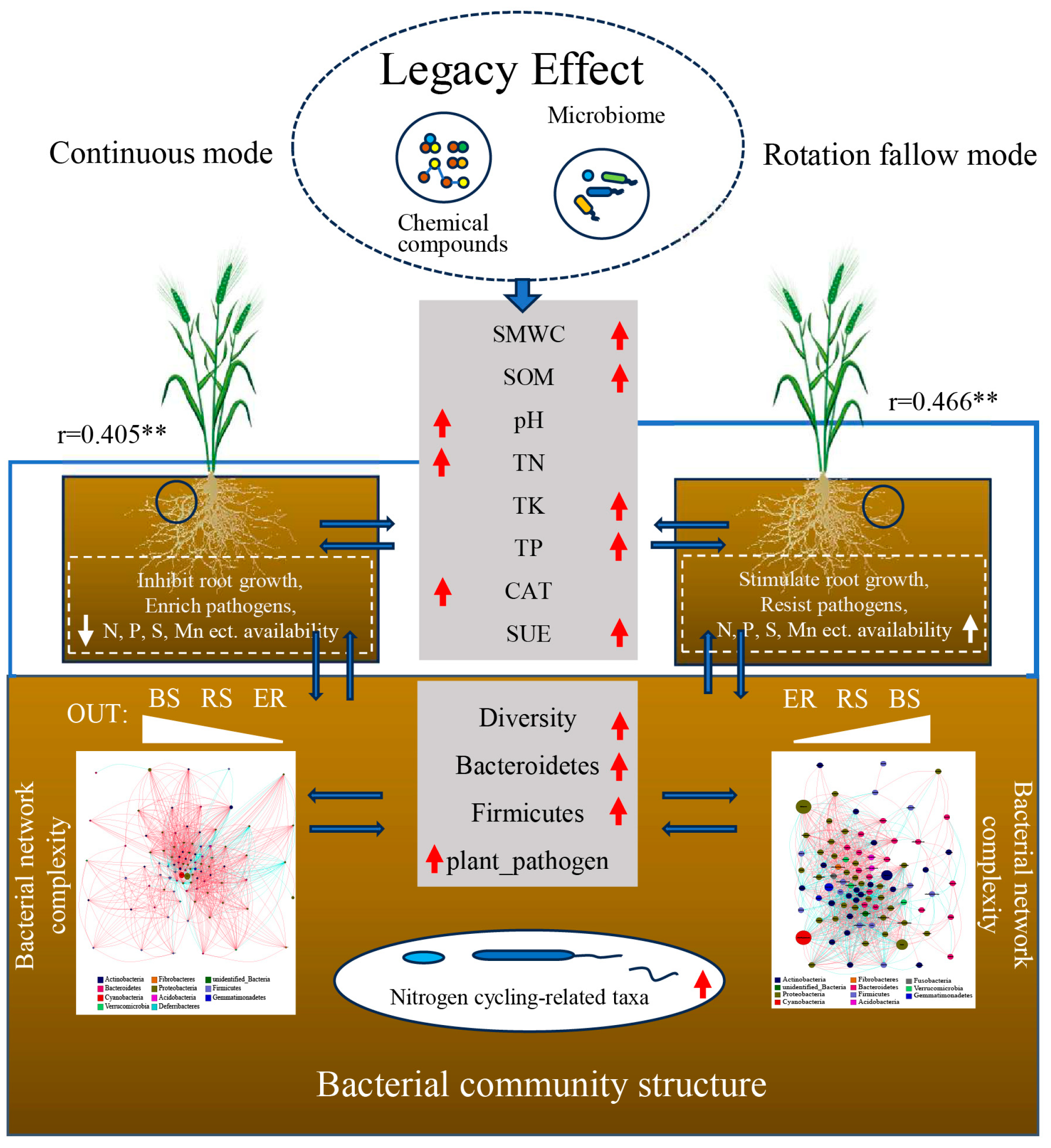
4.1. Reasonable Rotation Fallow Has a Positive Impact on Soil Nutrient Maintenance and Soil Function
4.2. Rotation Fallow Mode Can Affect the Diversity of Bacterial Communities, but Its Effect Is Weaker Than That of Ecological Niches
4.3. Reasonable Rotation Fallow Cultivation Is Beneficial for the Colonization and Growth of Beneficial Micro-organisms
4.4. Rotation Fallow Enhances the Complexity, Stability, and Relative Abundance of Nitrogen-Cycling Functional Bacterial Taxa in Bacterial Communities
4.5. Key Environmental Variables Affecting Microbial Community Assembly
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, G.; Wang, T.; Li, K.; Li, L.; Zhang, J.; Guo, S.; Ling, N.; Shen, Q. Historical Nitrogen Deposition and Straw Addition Facilitate the Resistance of Soil Multifunctionality to Drying-Wetting Cycles. Appl. Environ. Microbiol. 2019, 85, e02251-18. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.S.; Isbell, F.; Fujii, S.; Makoto, K.; Matsuoka, S.; Osono, T. Low Multifunctional Redundancy of Soil Fungal Diversity at Multiple Scales. Ecol. Lett. 2016, 19, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, G.; Teixeira, P.J.P.L.; Paredes, S.H.; Law, T.F.; de Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root Microbiota Drive Direct Integration of Phosphate Stress and Immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Copeland, J.; Wang, P.W.; Guttman, D.S.; Kotanen, P.M.; Johnson, M.T.J. Assembly and Ecological Function of the Root Microbiome across Angiosperm Plant Species. Proc. Natl. Acad. Sci. USA 2018, 115, E1157–E1165. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Wang, Y.; Huang, Y.; Xu, J.; Zhang, P.; Wang, N.; Liu, X.; Chu, H.; Liu, G.; Jiang, H.; et al. Taxonomic Structure and Functional Association of Foxtail Millet Root Microbiome. Gigascience 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Garbeva, P.; van Elsas, J.D.; van Veen, J.A. Rhizosphere Microbial Community and Its Response to Plant Species and Soil History|Plant and Soil. Available online: https://link.springer.com/article/10.1007/s11104-007-9432-0 (accessed on 14 April 2024).
- Schlemper, T.R.; Leite, M.F.A.; Lucheta, A.R.; Shimels, M.; Bouwmeester, H.J.; van Veen, J.A.; Kuramae, E.E. Rhizobacterial Community Structure Differences among Sorghum Cultivars in Different Growth Stages and Soils. FEMS Microbiol. Ecol. 2017, 93, fix096. [Google Scholar] [CrossRef]
- Liu, H.; Pan, F.; Han, X.; Song, F.; Zhang, Z.; Yan, J.; Xu, Y. A Comprehensive Analysis of the Response of the Fungal Community Structure to Long-Term Continuous Cropping in Three Typical Upland Crops. J. Integr. Agric. 2020, 19, 866–880. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Arafat, Y.; Lin, W. Studies on Fungal Communities and Functional Guilds Shift in Tea Continuous Cropping Soils by High-Throughput Sequencing. Ann. Microbiol. 2020, 70, 7. [Google Scholar] [CrossRef]
- Bansal, S.; Yin, X.; Schneider, L.; Sykes, V.; Jagadamma, S.; Lee, J. Carbon Footprint and Net Carbon Gain of Major Long-Term Cropping Systems under No-Tillage. J. Environ. Manag. 2022, 307, 114505. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.E.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant Host Habitat and Root Exudates Shape Soil Bacterial Community Structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Zuppinger-Dingley, D.; Schmid, B.; Petermann, J.S.; Yadav, V.; De Deyn, G.B.; Flynn, D.F.B. Selection for Niche Differentiation in Plant Communities Increases Biodiversity Effects. Nature 2014, 515, 108–111. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.-J. The Impact of Crop Rotation on Soil Microbial Diversity: A Meta-Analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Yu, Z.; Yao, Q.; Li, Y.; Liang, A.; Zhang, W.; Mi, G.; Jin, J.; Liu, X.; et al. Long-Term Continuous Cropping of Soybean Is Comparable to Crop Rotation in Mediating Microbial Abundance, Diversity and Community Composition. Soil Tillage Res. 2020, 197, 104503. [Google Scholar] [CrossRef]
- Pershina, E.V.; Ivanova, E.A.; Korvigo, I.O.; Chirak, E.L.; Sergaliev, N.H.; Abakumov, E.V.; Provorov, N.A.; Andronov, E.E. Investigation of the Core Microbiome in Main Soil Types from the East European Plain. Sci. Total Environ. 2018, 631–632, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. High Throughput Sequencing Analysis of Biogeographical Distribution of Bacterial Communities in the Black Soils of Northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Bai, L.; Wang, Y.; Li, Y.; Zhang, X.; Lu, Z.; Zhang, D.; Sun, F.; Zhao, X. Changes in the Microbial Community in Maize (Zea mays L.) Root Spatial Structure Following Short-Term Nitrogen Application. ACS Omega 2023, 8, 208–218. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S rRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4516–4522. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling Function and Taxonomy in the Global Ocean Microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, G.; Wang, Y.; Fei, J.; Xiangmin, R.; Peng, J.; Tian, C.; Zhang, Y. Crop Rotation-Driven Change in Physicochemical Properties Regulates Microbial Diversity, Dominant Components, and Community Complexity in Paddy Soils. Agric. Ecosyst. Environ. 2023, 343, 108278. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Narsing Rao, M.P.; Shi, Y.; Lian, Z.-H.; Jin, P.-J.; Wang, W.; Li, Y.-M.; Wang, K.-K.; Banerjee, A.; et al. The Shift of Soil Microbial Community Induced by Cropping Sequence Affect Soil Properties and Crop Yield. Front. Microbiol. 2023, 14, 1095688. [Google Scholar] [CrossRef]
- Meier, M.A.; Lopez-Guerrero, M.G.; Guo, M.; Schmer, M.R.; Herr, J.R.; Schnable, J.C.; Alfano, J.R.; Yang, J. Rhizosphere Microbiomes in a Historical Maize-Soybean Rotation System Respond to Host Species and Nitrogen Fertilization at the Genus and Subgenus Levels. Appl. Environ. Microbiol. 2021, 87, e0313220. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil Health and Sustainability: Managing the Biotic Component of Soil Quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the Global Distribution of Dominant Archaeal Populations in Soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, J.; Wu, L.; Lin, S.; Wang, J.; Li, Z.; Zhang, Z.; Lin, W. Effects of Continuous Monoculture of Achyranthes bidentata on Microbial Community Structure and Functional Diversity in Soil. Allelopath. J. 2015, 36, 197–211. [Google Scholar]
- Zhong, S.; Mo, Y.; Guo, G.; Zeng, H.; Jin, Z. Effect of Continuous Cropping on Soil Chemical Properties and Crop Yield in Banana Plantation. J. Agric. Sci. Technol. 2014, 16, 239–250. [Google Scholar]
- Qin, L.; Freeman, C.; Jia, X.; Zhang, Z.; Liu, B.; Zhang, S.; Jiang, M. Microbial Enzyme Activity and Stoichiometry Signal the Effects of Agricultural Intervention on Nutrient Cycling in Peatlands. Ecol. Indic. 2021, 122, 107242. [Google Scholar] [CrossRef]
- Gonnety, J.T.; Assémien, E.F.L.; Guéi, A.M.; N’Dri, A.A.; Djina, Y.; Koné, A.W.; Tondoh, J.E. Effect of Land-Use Types on Soil Enzymatic Activities and Chemical Properties in Semi-Deciduous Forest Areas of Central-West Côte d’Ivoire. Biotechnol. Agron. Société Environ. 2012, 16, 478–485. [Google Scholar]
- Borase, D.N.; Nath, C.P.; Hazra, K.K.; Senthilkumar, M.; Singh, S.S.; Praharaj, C.S.; Singh, U.; Kumar, N. Long-Term Impact of Diversified Crop Rotations and Nutrient Management Practices on Soil Microbial Functions and Soil Enzymes Activity. Ecol. Indic. 2020, 114, 106322. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Niu, J.; Dang, K.; Zhang, S.; Wang, S.; Wang, Z. Changes in Physicochemical Properties, Enzymatic Activities, and the Microbial Community of Soil Significantly Influence the Continuous Cropping of Panax quinquefolius L. (American Ginseng). Plant Soil 2021, 463, 427–446. [Google Scholar] [CrossRef]
- Malobane, M.E.; Nciizah, A.D.; Nyambo, P.; Mudau, F.N.; Wakindiki, I.I.C. Microbial Biomass Carbon and Enzyme Activities as Influenced by Tillage, Crop Rotation and Residue Management in a Sweet Sorghum Cropping System in Marginal Soils of South Africa. Heliyon 2020, 6, e05513. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Abaidoo, R.C.; Iwuafor, E.N.O.; Olufajo, O.O.; Sanginga, N. Rotation Effects of Grain Legumes and Fallow on Maize Yield, Microbial Biomass and Chemical Properties of an Alfisol in the Nigerian Savanna. Agric. Ecosyst. Environ. 2009, 129, 325–331. [Google Scholar] [CrossRef]
- Chinnadurai, C.; Gopalaswamy, G.; Balachandar, D. Long Term Effects of Nutrient Management Regimes on Abundance of Bacterial Genes and Soil Biochemical Processes for Fertility Sustainability in a Semi-Arid Tropical Alfisol. Geoderma 2014, 232–234, 563–572. [Google Scholar] [CrossRef]
- Yang, X.; Hu, H.-W.; Yang, G.-W.; Cui, Z.-L.; Chen, Y.-L. Crop Rotational Diversity Enhances Soil Microbiome Network Complexity and Multifunctionality. Geoderma 2023, 436, 116562. [Google Scholar] [CrossRef]
- Ji, N.; Liang, D.; Clark, L.V.; Sacks, E.J.; Kent, A.D. Host Genetic Variation Drives the Differentiation in the Ecological Role of the Native Miscanthus Root-Associated Microbiome. Microbiome 2023, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, Y.S.; Zhan, Y.; Zhang, Z.; Liu, Y.; Wei, Y.; Xu, T.; Li, J. Tomato Microbiome under Long-Term Organic and Conventional Farming. iMeta 2022, 1, e48. [Google Scholar] [CrossRef] [PubMed]
- Gdanetz, K.; Trail, F. The Wheat Microbiome Under Four Management Strategies, and Potential for Endophytes in Disease Protection. Phytobiomes J. 2017, 1, 158–168. [Google Scholar] [CrossRef]
- Shi, Y.; Delgado-Baquerizo, M.; Li, Y.; Yang, Y.; Zhu, Y.-G.; Peñuelas, J.; Chu, H. Abundance of Kinless Hubs within Soil Microbial Networks Are Associated with High Functional Potential in Agricultural Ecosystems. Environ. Int. 2020, 142, 105869. [Google Scholar] [CrossRef] [PubMed]
- Donn, S.; Kirkegaard, J.A.; Perera, G.; Richardson, A.E.; Watt, M. Evolution of Bacterial Communities in the Wheat Crop Rhizosphere. Environ. Microbiol. 2015, 17, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct Soil Microbial Diversity under Long-Term Organic and Conventional Farming. ISME J. 2014, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Nacke, H.; Thürmer, A.; Wollherr, A.; Will, C.; Hodac, L.; Herold, N.; Schöning, I.; Schrumpf, M.; Daniel, R. Pyrosequencing-Based Assessment of Bacterial Community Structure along Different Management Types in German Forest and Grassland Soils. PLoS ONE 2011, 6, e17000. [Google Scholar] [CrossRef]
- Shen, C.; Liang, W.; Shi, Y.; Lin, X.; Zhang, H.; Wu, X.; Xie, G.; Chain, P.; Grogan, P.; Chu, H. Contrasting Elevational Diversity Patterns between Eukaryotic Soil Microbes and Plants. Ecology 2014, 95, 3190–3202. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, M.; Yao, F.; Ye, J.; Bu, R.; Ma, R.; Lin, J.; Kurganova, I.; Wang, X.; et al. Temperature Sensitivity of SOM Decomposition Is Linked with a K-selected Microbial Community. Glob. Chang. Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative Metagenomic, Phylogenetic and Physiological Analyses of Soil Microbial Communities across Nitrogen Gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; He, X.; Baer, M.; Beirinckx, S.; Tian, T.; Moya, Y.A.T.; Zhang, X.; Deichmann, M.; Frey, F.P.; Bresgen, V.; et al. Plant Flavones Enrich Rhizosphere Oxalobacteraceae to Improve Maize Performance under Nitrogen Deprivation. Nat. Plants 2021, 7, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lu, Y. Decomposition of Soil Polymeric Organic Matter by Bacteroidetes and Clostridia:Progress and Perspectives. Microbiol. China 2022, 49, 1147–1157. [Google Scholar] [CrossRef]
- Breitkreuz, C.; Buscot, F.; Tarkka, M.; Reitz, T. Shifts Between and Among Populations of Wheat Rhizosphere Pseudomonas, Streptomyces and Phyllobacterium Suggest Consistent Phosphate Mobilization at Different Wheat Growth Stages Under Abiotic Stress. Front. Microbiol. 2019, 10, 3109. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Gan, L.-Z.; Xu, Z.-B.; Yang, F.; Li, Y.; Fan, X.-L.; Liu, X.-F.; Tian, Y.-Q.; Dai, Y.-M. Pedobacter chitinilyticus Sp. Nov., a Chitin-Degrading Bacterium Isolated from Wheat Leaf Tissue. Int. J. Syst. Evol. Microbiol. 2018, 68, 3713–3719. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.-J.; Kong, H.G.; Choi, K.; Kwon, S.-K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere Microbiome Structure Alters to Enable Wilt Resistance in Tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Zhang, Y.; Chen, G.; Li, Z.; Zhang, M. Straw Return Drives Soil Microbial Community Assemblage to Change Metabolic Processes for Soil Quality Amendment in a Rice-Wheat Rotation System. Soil Biol. Biochem. 2023, 185, 109131. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, X.; Yang, J.; Yang, Y.; Jv, H.; Li, R.; Jia, Z.; Ruan, Y. Selection of Rhizosphere Communities of Diverse Rotation Crops Reveals Unique Core Microbiome Associated with Reduced Banana Fusarium Wilt Disease. New Phytol. 2023, 238, 2194–2209. [Google Scholar] [CrossRef]
- Kan, Z.-R.; Chen, Z.; Wei, Y.-X.; Virk, A.L.; Bohoussou, Y.N.; Lal, R.; Zhao, X.; Zhang, H.-L. Contribution of Wheat and Maize to Soil Organic Carbon in a Wheat-Maize Cropping System: A Field and Laboratory Study. J. Appl. Ecol. 2022, 59, 2716–2729. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zhang, F. Abundant and Rare Bacteria Possess Different Diversity and Function in Crop Monoculture and Rotation Systems across Regional Farmland. Soil Biol. Biochem. 2022, 171, 108742. [Google Scholar] [CrossRef]
- Xie, Y.; Ouyang, Y.; Han, S.; Se, J.; Tang, S.; Yang, Y.; Ma, Q.; Wu, L. Crop Rotation Stage Has a Greater Effect than Fertilisation on Soil Microbiome Assembly and Enzymatic Stoichiometry. Sci. Total Environ. 2022, 815, 152956. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate Warming Enhances Microbial Network Complexity and Stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Melillo, J.M.; Frey, S.D.; DeAngelis, K.M.; Werner, W.J.; Bernard, M.J.; Bowles, F.P.; Pold, G.; Knorr, M.A.; Grandy, A.S. Long-Term Pattern and Magnitude of Soil Carbon Feedback to the Climate System in a Warming World. Science 2017, 358, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Steinauer, K.; Chatzinotas, A.; Eisenhauer, N. Root Exudate Cocktails: The Link between Plant Diversity and Soil Microorganisms? Ecol. Evol. 2016, 6, 7387–7396. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, M.D.; Grandy, A.S.; Tiemann, L.K.; Weintraub, M.N. Crop Rotation Complexity Regulates the Decomposition of High and Low Quality Residues. Soil Biol. Biochem. 2014, 78, 243–254. [Google Scholar] [CrossRef]
- Xiong, W.; Jousset, A.; Li, R.; Delgado-Baquerizo, M.; Bahram, M.; Logares, R.; Wilden, B.; de Groot, G.A.; Amacker, N.; Kowalchuk, G.A.; et al. A Global Overview of the Trophic Structure within Microbiomes across Ecosystems. Environ. Int. 2021, 151, 106438. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Cong, W.-F.; Bezemer, T.M. Legacies at Work: Plant-Soil-Microbiome Interactions Underpinning Agricultural Sustainability. Trends Plant Sci. 2022, 27, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jin, L.; Zhang, C.; Li, S.; Zhou, T.; Hua, Z.; Wang, L.; Ji, S.; Wang, Y.; Gan, Y.; et al. Destabilized Microbial Networks with Distinct Performances of Abundant and Rare Biospheres in Maintaining Networks under Increasing Salinity Stress. iMeta 2023, 2, e79. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental Stress Destabilizes Microbial Networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- Hao, J.; Feng, Y.; Wang, X.; Yu, Q.; Zhang, F.; Yang, G.; Ren, G.; Han, X.; Wang, X.; Ren, C. Soil Microbial Nitrogen-Cycling Gene Abundances in Response to Crop Diversification: A Meta-Analysis. Sci. Total Environ. 2022, 838, 156621. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, F.; Hu, C.; Liu, B. Metagenomics Reveals Taxon-Specific Responses of the Nitrogen-Cycling Microbial Community to Long-Term Nitrogen Fertilization. Soil Biol. Biochem. 2021, 156, 108214. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling Mechanisms That Mediate the Balance between Stochastic and Deterministic Processes in Microbial Succession. Proc. Natl. Acad. Sci. USA 2015, 112, E1326–E1332. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, J.; Luan, X.; Yang, L.; Chen, W.; Ma, X.; Yang, Z.; Shangguan, Z. Biogeographical Patterns of Rhizosphere Microbial Communities in Robinia pseudoacacia Forests along a North–South Transect in the Loess Plateau. Geoderma 2023, 435, 116516. [Google Scholar] [CrossRef]
- Zhang, W.; Munkholm, L.J.; Liu, X.; An, T.; Xu, Y.; Ge, Z.; Xie, N.; Li, A.; Dong, Y.; Peng, C.; et al. Soil Aggregate Microstructure and Microbial Community Structure Mediate Soil Organic Carbon Accumulation: Evidence from One-Year Field Experiment. Geoderma 2023, 430, 116324. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion Reduces Soil Microbial Diversity, Network Complexity and Multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Bender, S.F.; Schulz, S.; Martínez-Cuesta, R.; Laughlin, R.J.; Kublik, S.; Pfeiffer-Zakharova, K.; Vestergaard, G.; Hartman, K.; Parladé, E.; Römbke, J.; et al. Simplification of Soil Biota Communities Impairs Nutrient Recycling and Enhances Above- and Belowground Nitrogen Losses. New Phytol. 2023, 240, 2020–2034. [Google Scholar] [CrossRef]


| Layer (cm) | Organic Matter (g/kg) | Available Nitrogen (mg/kg) | Available Phosphorus (mg/kg) | Available Potassium (mg/kg) | Total Nitrogen (g/kg) | Total Phosphorus (g/kg) | pH |
|---|---|---|---|---|---|---|---|
| 0~20 | 57.82 | 37.26 | 22.25 | 195.83 | 2.39 | 0.60 | 6.80 |
| Niche | Variable | pH | TN | TP | TK | CAT | SUE | SSC |
|---|---|---|---|---|---|---|---|---|
| BS | r | 0.455 | 0.499 | 0.363 | 0.291 | 0.272 | 0.430 | 0.372 |
| P | 0.005 | 0.001 | 0.013 | 0.018 | 0.025 | 0.002 | 0.020 | |
| RS | r | 0.477 | 0.311 | 0.066 | 0.200 | 0.539 | 0.472 | 0.299 |
| P | 0.001 | 0.041 | 0.286 | 0.064 | 0.001 | 0.002 | 0.044 | |
| ER | r | 0.003 | 0.009 | 0.151 | 0.016 | 0.090 | 0.000 | 0.060 |
| P | 0.438 | 0.410 | 0.929 | 0.507 | 0.134 | 0.445 | 0.631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.; Fang, J.; Wei, S.; Cheng, Y.; Su, S.; Zhang, X.; Wang, J.; Zhang, F.; Wu, J.; Zhao, L.; et al. A Reasonable Rotation Fallow Mode Enhances the Complexity of the Soil Bacterial Network and Enriches Nitrogen-Cycling-Related Taxa. Agronomy 2024, 14, 1456. https://doi.org/10.3390/agronomy14071456
Shi G, Fang J, Wei S, Cheng Y, Su S, Zhang X, Wang J, Zhang F, Wu J, Zhao L, et al. A Reasonable Rotation Fallow Mode Enhances the Complexity of the Soil Bacterial Network and Enriches Nitrogen-Cycling-Related Taxa. Agronomy. 2024; 14(7):1456. https://doi.org/10.3390/agronomy14071456
Chicago/Turabian StyleShi, Gongfu, Jing Fang, Shuli Wei, Yuchen Cheng, Shaofeng Su, Xiangqian Zhang, Jianguo Wang, Fan Zhang, Jianhui Wu, Lili Zhao, and et al. 2024. "A Reasonable Rotation Fallow Mode Enhances the Complexity of the Soil Bacterial Network and Enriches Nitrogen-Cycling-Related Taxa" Agronomy 14, no. 7: 1456. https://doi.org/10.3390/agronomy14071456





