Nanoparticle Uptake and Bioaccumulation in Pisum sativum L. (Green Pea) Analyzed via Dark-Field Microscopy, Infrared Spectroscopy, and Principal Component Analysis Combined with Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphological Testing: Seedlings Development and Extraction
2.2. Anatomical Testing
2.3. Spectroscopic Testing: Data Collection and Analysis
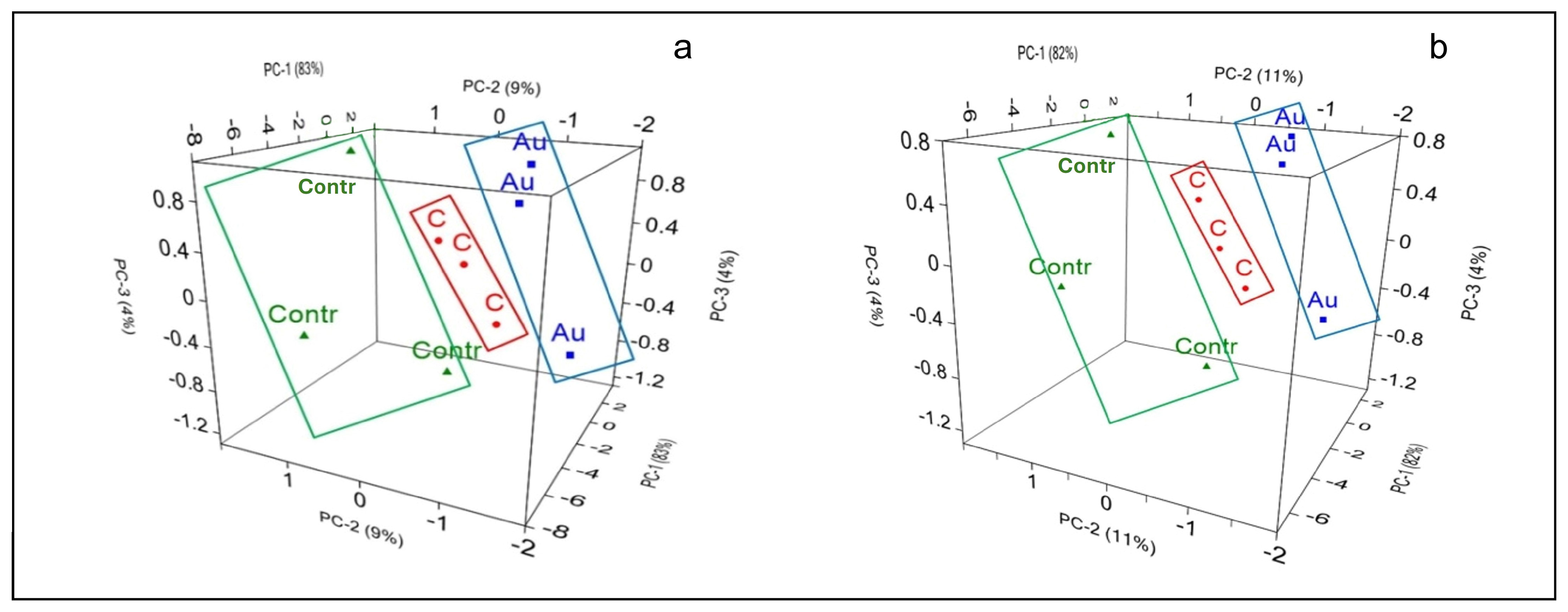
3. Results
3.1. Morphological Results
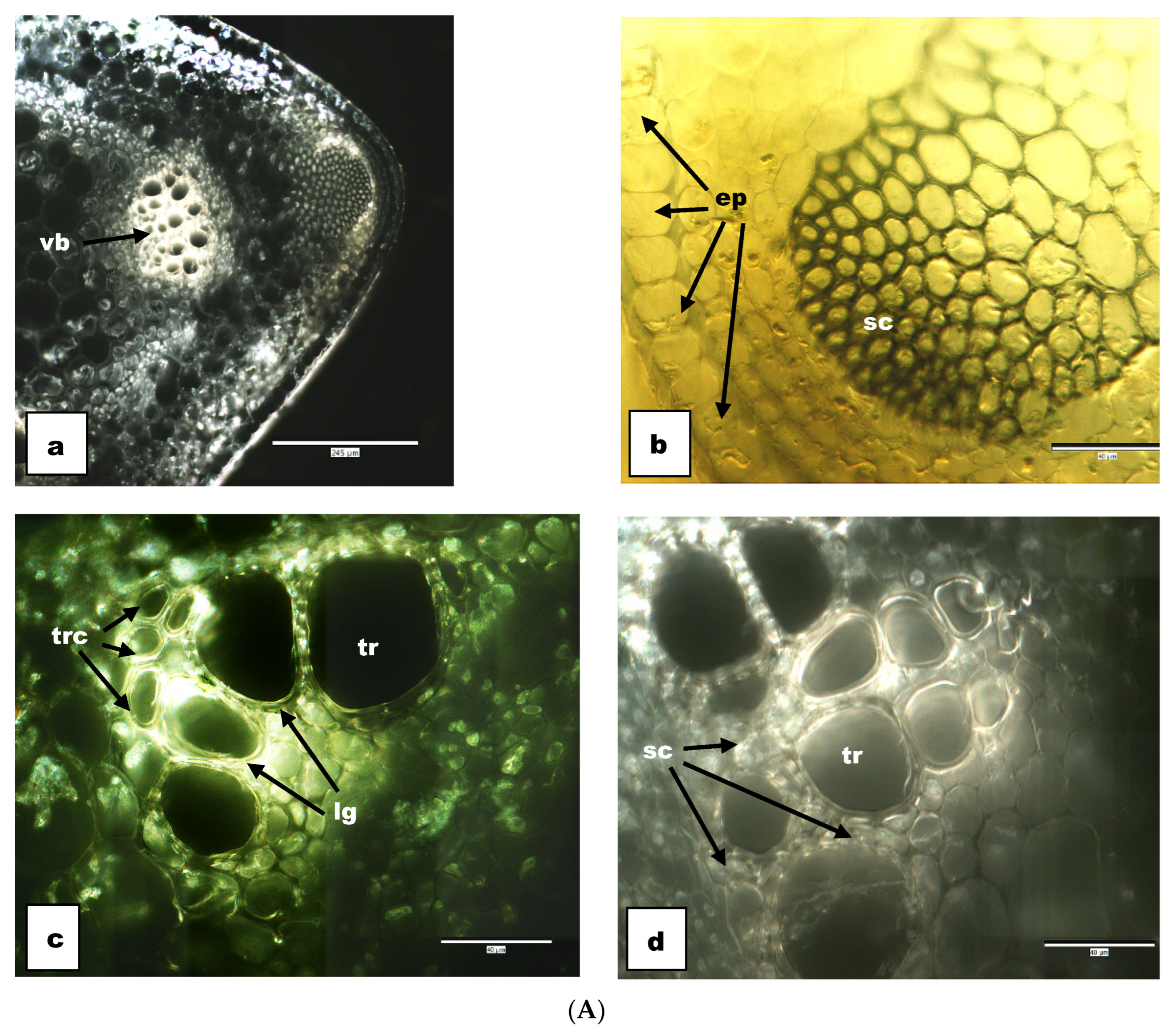
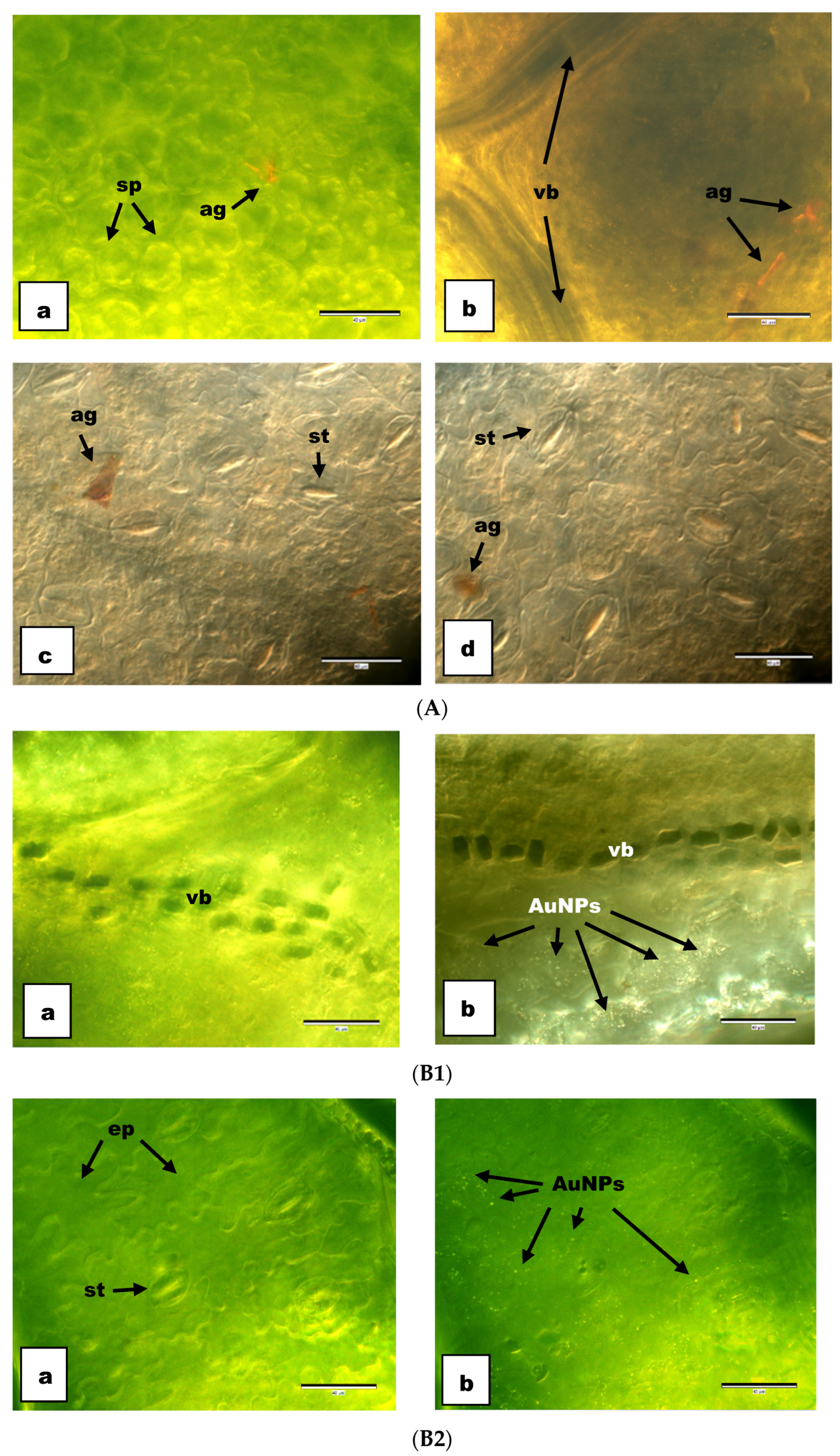
3.2. Anatomical Results
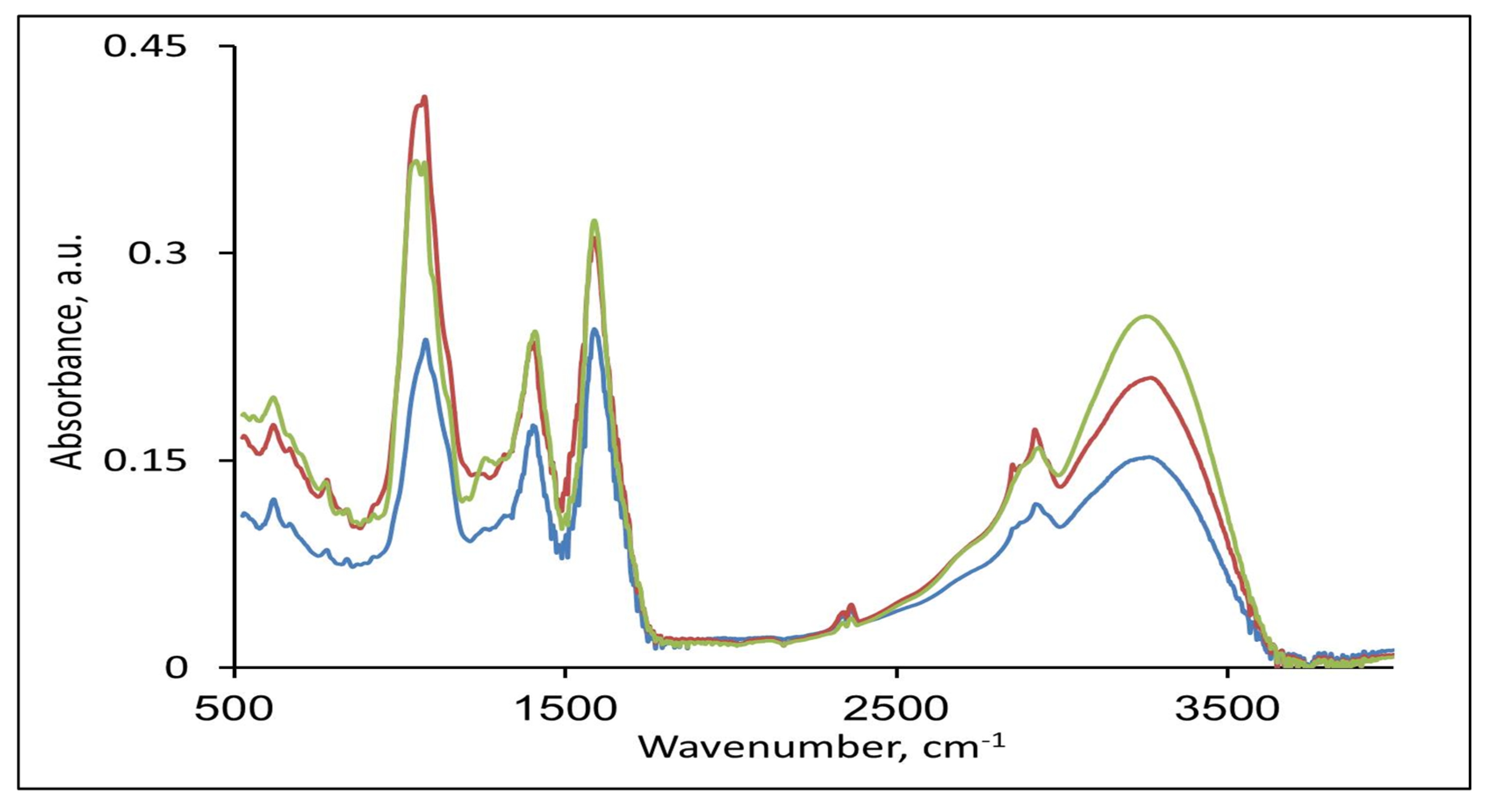
3.3. Spectroscopic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ju, Y.; Li, X.; Ju, L.; Feng, H.; Tan, F.; Cui, Y.; Yang, Y.; Wang, X.; Cao, J.; Qiao, P.; et al. Nanoparticles in the Earth surface systems and their effects on the environment and resource. Gondwana Res. 2022, 110, 370–392. [Google Scholar] [CrossRef]
- Saber, N.B.; Mezni, A.; Alrooqi, A.; Altalhi, T. A review of ternary nanostructures based noble metal/semiconductor for en-vironmental and renewable energy applications. J. Mater. Res. Technol. 2020, 9, 15233–15262. [Google Scholar] [CrossRef]
- Mubayi, A.; Chatterji, S.; Rai, P.M.; Watal, G. The present study represents a clean, non-toxic as well as eco-friendly procedure for synthesizing AgNPs. Adv. Mater. Lett. 2012, 3, 519–525. [Google Scholar] [CrossRef]
- De Dios, A.S.; Diaz-Garcia, M.E. Multifunctional Nanoparticles: Analytical Prospects. Anal. Chim. Acta 2010, 666, 1–22. [Google Scholar] [CrossRef]
- McNeil, S.E. Nanotechnology for the biologist. J. Leukoc. Biol. 2005, 78, 585–594. [Google Scholar] [CrossRef]
- Iijima, S. Synthesis of Carbon Nanotubes. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-Shell Carbon Nanotubes of 1-nm Diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Dinadayalane, T.C.; Leszczynski, J. Remarkable diversity of carbon–carbon bonds: Structures and properties of fullerenes, carbon nanotubes, and graphene. Struct. Chem. 2010, 21, 1155–1169. [Google Scholar] [CrossRef]
- Subramoney, S. Novel Nanocarbons-Structure, Properties, and Potential Applications. Adv. Mater. 1998, 10, 1157–1171. [Google Scholar] [CrossRef]
- Lin, Y.; Taylor, S.; Li, H.; Fernando, K.A.S.; Qu, L.; Wang, W.; Gu, L.; Zhou, B.; Sun, Y.-P. Advances toward bioapplications of carbon nanotubes. J. Mater. Chem. 2004, 14, 527–541. [Google Scholar] [CrossRef]
- Kiran, A.R.; Kumari, G.K.; Krishnamurthy, P.T. Carbon nanotubes in drug delivery: Focus on anticancer therapies. J. Drug Deliv. Sci. Technol. 2020, 59, 101892. [Google Scholar] [CrossRef]
- Collins, P.G.; Arnold, M.S.; Avouris, P. Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown. Science 2001, 292, 706–709. [Google Scholar] [CrossRef]
- Mintmire, J.; White, C. Electronic and structural properties of carbon nanotubes. Carbon 1995, 33, 893–902. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Lorents, D.C. Mechanical and thermal properties of carbon nanotubes. Carbon 1995, 33, 925–930. [Google Scholar] [CrossRef]
- Hassan, H.; Sharma, P.; Hasan, M.R.; Singh, S.; Thakur, D.; Narang, J. Gold nanomaterials—The golden approach from synthesis to applications. Mater. Sci. Energy Technol. 2022, 5, 375–390. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostruc-tured materials for advanced electrochemical energy devices. Prog. Mater. Sci. J. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. J. 2008, 60, 1289–1306. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Papasani, M.R.; Wang, G.; Hill, R.A. Gold nanoparticles: The importance of physiological principles to devise strategies for targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 804–814. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Li, J.-C.; Chen, S.-H.; Liu, T.-Y.; Kuo, C.-H.; Huang, W.-T.; Lin, C.-S. An optical biosensing platform for proteinase activity using gold nanoparticles. Biomaterials 2010, 31, 6087–6095. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.M.; Fayad, Z.A.; Cormode, D.P. Multifunctional Gold Nanoparticles for Diagnosis and Therapy of Disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Candan, F.; Markushin, Y.; Ozbay, G. Uptake and Presence Evaluation of Nanoparticles in Cicer arietinum L. by Infrared Spectroscopy and Machine Learning Techniques. Plants 2022, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Sreelakshmy, V.; Deepa, M.K.; Mridula, P. Green synthesis of silver nanoparticles from Glycyrrhiza glabra root extract for the treatment of gastric ulcer. J. Dev. Drugs Open Access 2016, 5, 152. [Google Scholar] [CrossRef]
- Toledo, A.C.O.; De, D.P.D.S.F.; Boscardin, P.M.D.; De Paula, J.D.F.P. Biosynthesis and characterization of silver nanoparticles pro-duced with aqueous extract of Pterodon emarginatus Vogel-Fabaceae seeds associated with gentamicin sulfate and hyaluronic acid with potential antimicrobial activity. Braz. J. Dev. 2020, 6, 100655–100677. [Google Scholar] [CrossRef]
- Ghosh, B.; Datta, A.K.; Pramanik, A.; Kumbhakar, D.V.; Das, D.; Paul, R.; Biswas, J. Mutagenic Effectivity of Cadmium Sulphide and Copper Oxide Nanoparticles on Some Physiological and Cytological Attributes of Lathyrus sativus L. Cytologia 2017, 82, 267–271. [Google Scholar] [CrossRef]
- Bapi, G.; Kumar, D.A.; Debadrito, D.; Vishambhar, K.D.; Ankita, P. Assessment of nanoparticles (copper, cadmium sulphide, copper oxide and zinc oxide) mediated toxicity in a plant system (Indigofera tinctoria L.; Fabaceae). Res. J. Chem. Environ. 2018, 22, 34–48. [Google Scholar]
- Chatterjee, A.; Ajantha, M.; Talekar, A.; Revathyr, N.; Abraham, J. Biosynthesis, antimicrobial and cytotoxic effects of titanium dioxide nanoparticles using Vigna unguiculata seeds. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 95–99. [Google Scholar] [CrossRef]
- Tauseef, A.; Hisamuddin; Khalilullah, A.; Uddin, I. Role of MgO nanoparticles in the suppression of Meloidogyne incognita, infecting cowpea and improvement in plant growth and physiology. Exp. Parasitol. 2021, 220, 108045. [Google Scholar] [CrossRef] [PubMed]
- Tariverdizadeh, N.; Mohebodini, M.; Chamani, E.; Ebadi, A. Iron and zinc oxide nanoparticles: An efficient elicitor to enhance trigonelline alkaloid production in hairy roots of fenugreek. Ind. Crop. Prod. 2021, 162, 113240. [Google Scholar] [CrossRef]
- Mukherjee, A.; Pokhrel, S.; Bandyopadhyay, S.; Mädler, L.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. A soil mediated phy-to-toxicological study of iron doped zinc oxide nanoparticles (Fe@ZnO) in green peas (Pisum sativum L.). Chem. Eng. J. 2014, 258, 394–401. [Google Scholar] [CrossRef]
- Ochoa, L.; Medina-Velo, I.A.; Barrios, A.C.; Bonilla-Bird, N.J.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Modulation of CuO nanoparticles toxicity to green pea (Pisum sativum Fabaceae) by the phytohormone indole-3-acetic acid. Sci. Total. Environ. 2017, 598, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.H.; Pisum, L. Flora of Turkey and East Aegean Islands; Edinburgh University Press: Great Britain, UK, 1970; Volume 3, pp. 370–372. [Google Scholar]
- Jing, R.; Ambrose, M.A.; Knox, M.R.; Smykal, P.; Hybl, M.; Ramos, Á.; Caminero, C.; Burstin, J.; Duc, G.; van Soest, L.J.M.; et al. Genetic diversity in European Pisum germplasm collections. Theor. Appl. Genet. 2012, 125, 367–380. [Google Scholar] [CrossRef]
- Makasheva, R.X. Peas. The Cultural Flora of the USSR; Cereal Legumes; Kolos: Leningrad, Russia, 1979; Volume 4, p. 324. [Google Scholar]
- Poltoretskyi, S.; Karpenko, V.; Liubych, V.; Poltoretska, N.; Bilonozhko, V.; Demydas, H. Morphological and ecological features of green pea (Pisum sativum L.). Ukranian J. Ecol. Melitopol 2022, 12, 12–19. [Google Scholar] [CrossRef]
- Dyachenko, E.A.; Ryzhova, N.N.; Vishnyakova, M.A.; Kochieva, E.Z. Molecular genetic diversity of the pea (Pisum sativum L.) from the Vavilov Research Institute collection detected by the AFLP analysis. Russ. J. Genet. 2014, 50, 916–924. [Google Scholar] [CrossRef]
- Vaughan, J.C.; Geissler, C.A. The New Oxford Book of Foodplants; Oxford University Press: New York, NY, USA, 2009; p. 249. [Google Scholar]
- Marcano, A.; Pokrajac, D.; Lazarevic, A.; Smith, M.; Markushin, Y.; Melikechi, N. Statistical Analysis for Automatic Identification of Ovarian Cancer Protein-Biomarkers Based on Fast Fourier Transform Infrared Spectroscopy: Fourier Transforms-New Analytical Approaches and FTIR Strategies; InTech–Open Press: London, UK, 2011; Chapter 8; pp. 147–166. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis; Springer: New York, NY, USA, 2002; p. 518. [Google Scholar]
- Pokrajac, D.; Lazarevic, A.; Kecman, V.; Marcano, A.; Markushin, Y.; Vance, T.; Reljin, N.; McDaniel, S.; Melikechi, N. Automatic classification of laser-induced breakdown spectroscopy (LIBS) data of protein biomarker solutions. Appl. Spectrosc. 2014, 68, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 27, 3485–3498. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Barriers, W. Pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Shekhawat, G.S.; Mahawar, L.; Rajput, P.; Rajput, V.D.; Minkina, T.; Singh, R.K. Role of engineered carbon nanoparticles (CNPs) in promoting growth and metabolism of Vigna radiata (L.) Wilczek: Insights into the biochemical and physiological responses. Plants 2021, 10, 1317. [Google Scholar] [CrossRef]
- Fahn, A. Plant Anatomy, 3rd ed.; Bjorn Walles; Nordic Society Oikos: Oxford, UK; Pergamon, Turkey, 1982; ISBN 0-08-0280293. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Pereira, A. Plant Abiotic Stress Challenges from the Changing Environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Rashmi, R.; Surya Ulhas, R.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Aldaej, M.I.; Rezk, A.A.S.; Shehata, W.F.; Almaghasla, M.I. The Role of Nanoparticles in Response of Plants to Abiotic Stress at Physiological, Biochemical, and Molecular Levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Laurette, J.; Herlin-Boime, N.; Khodja, H.; Fayard, B.; Flank, A.-M.; Brisset, F.; Carriere, M. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): Influence of diameter and crystal phase. Sci. Total Environ. 2012, 431, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kummerová, M.; Zezulka, S.; Babula, P.; Váňová, L. Root response in Pisum sativum and Zea mays under fluoranthenestress: Morphological and anatomical traits. Chemosphere 2013, 90, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Labeeb, M.; Badr, A.; Haroun, S.A.; Mattar, M.Z.; El-Kholy, A.S.; El-Mehasseb, I.M. Ecofriendly Synthesis of Silver Nanoparticles and Their Effects on Early Growth and Cell Division in Roots of Green Pea (Pisum sativum L.). Gesunde Pflanz. 2020, 72, 113–127. [Google Scholar] [CrossRef]
- El-Okkiah, S.A.F.; El-Tahan, A.M.; Ibrahim, O.M.; Taha, M.A.; Korany, S.M.; Alsherif, E.A.; AbdElgawad, H.; Sen, E.Z.F.A.; Sharaf-Eldin, M.A. Under cadmium stress, silicon has a defensive effect on the morphology, physiology, and anatomy of pea (Pisum sativum L.) plants. Front. Plant Sci. 2022, 13, 997475. [Google Scholar] [CrossRef] [PubMed]
- Smoliñski, M.; Saniewski, M.; PieniaZek, J. The changes in anatomical structures in Pisum sativum L. seedlings after treatment with morphactin IT 3456 and benzyladenine. Acta Soc. Bot. Pol. 1969, XXXVIII, 303–308. [Google Scholar]
- Melby, E.S.; Mensch, A.C.; Lohse, S.E.; Hu, D.; Orr, G.; Murphy, C.J.; Hamers, R.J.; Pedersen, J.A. Formation of supported lipid bilayers containing phase-segregated domains and their interaction with gold nanoparticles. Environ. Sci. Nano 2016, 3, 45–55. [Google Scholar] [CrossRef]
- Kutík, J.; Beneš, K. The anatomical study of heterotrophic starch formation in leaf segments of maize and pea. Biol. Plant. 1981, 23, 52–57. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Lv, H.; Jia, Q.; Tang, Z.; Zheng, X. Green synthesis of silver nanoparticles by Chrysanthemum morifolium Ramat. Extract and their application in clinical ultrasound gel. Int. J. Nanomed. 2013, 8, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.R.; Kumar, C.S.; Selvamani, P.; Subramanian, N.; Ruckmani, K. Synthesis and characterization of Arabic gum capped gold nanoparticles for tumor-targeted drug delivery. Mater. Lett. 2015, 139, 241–244. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Bhagat, E.; Sadras, S.R. Green synthesis of silver and gold nanoparticles from Gymnema sylvestre leaf extract: Study of antioxidant and anticancer activities. J. Nanopart. Res. 2015, 17, 151. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Rai, A.; Sastry, M. Rapid synthesis of Au, Ag and bimetallic Au core-Ag shell nanoparticles by using neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Safaepour, M.; Shahverdi, A.R.; Shahverdi, H.R.; Khorramizadeh, M.R.; Gohari, A.R. Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against fibrosarcoma-wehi 164. Avicenna J. Med. Biotechnol. (AJMB) 2009, 1, 111–115. [Google Scholar] [PubMed]
- Parashar, V.; Parashar, R.; Sharma, B.; Pandey, A.C. Parthenium leaf extract mediated synthesis of silver nanoparticles: A novel approach towards weed utilization. Dig. J. Nanomater. Biostructures 2009, 4, 45–50. [Google Scholar]
- Chandran, S.P.; Chandhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]



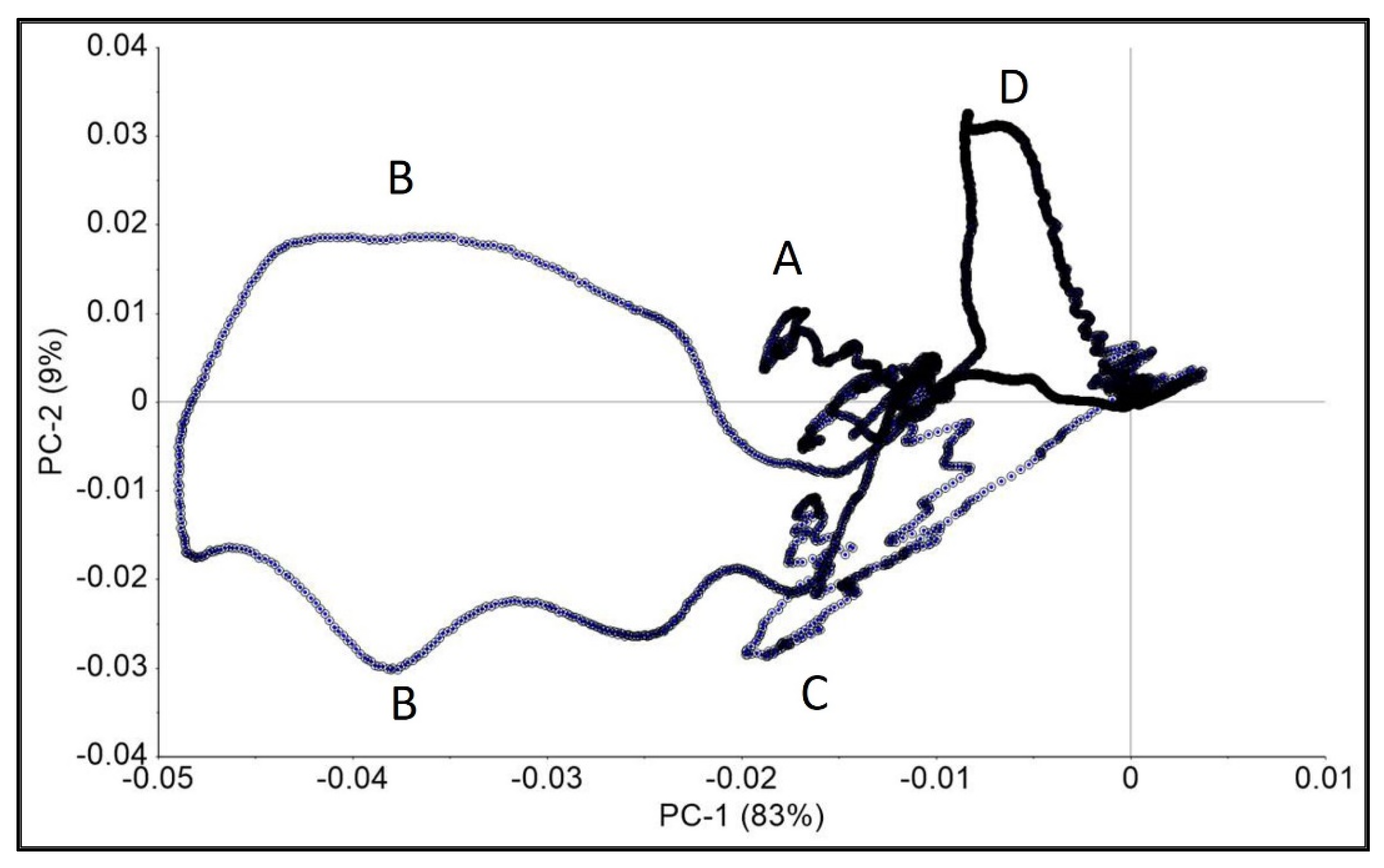
| Spectral Ranges for PCA Loading with Stronger Effects on the PC for Pisum sativum L. Root, Stem and Leaf Samples Grown under Influence of NPs, cm−1 | ATR-FTIR Peaks, AuNPs-Standard, cm−1 [4] | ATR-FTIR Peaks/Valleys (*) CNTs-Standard, cm−1 [4] |
|---|---|---|
| 525–646 (A) | 450–503 | 490–560 * |
| 750–870 | 790–850 * | |
| 962–1203 (B) | 1022–1218 | 1130–1260 |
| 1499–1660 (C), 3075–3461 (D) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candan, F.; Markushin, Y.; Ozbay, G. Nanoparticle Uptake and Bioaccumulation in Pisum sativum L. (Green Pea) Analyzed via Dark-Field Microscopy, Infrared Spectroscopy, and Principal Component Analysis Combined with Machine Learning. Agronomy 2024, 14, 1473. https://doi.org/10.3390/agronomy14071473
Candan F, Markushin Y, Ozbay G. Nanoparticle Uptake and Bioaccumulation in Pisum sativum L. (Green Pea) Analyzed via Dark-Field Microscopy, Infrared Spectroscopy, and Principal Component Analysis Combined with Machine Learning. Agronomy. 2024; 14(7):1473. https://doi.org/10.3390/agronomy14071473
Chicago/Turabian StyleCandan, Feyza, Yuriy Markushin, and Gulnihal Ozbay. 2024. "Nanoparticle Uptake and Bioaccumulation in Pisum sativum L. (Green Pea) Analyzed via Dark-Field Microscopy, Infrared Spectroscopy, and Principal Component Analysis Combined with Machine Learning" Agronomy 14, no. 7: 1473. https://doi.org/10.3390/agronomy14071473
APA StyleCandan, F., Markushin, Y., & Ozbay, G. (2024). Nanoparticle Uptake and Bioaccumulation in Pisum sativum L. (Green Pea) Analyzed via Dark-Field Microscopy, Infrared Spectroscopy, and Principal Component Analysis Combined with Machine Learning. Agronomy, 14(7), 1473. https://doi.org/10.3390/agronomy14071473







