Authenticity Identification of F1 Hybrid Offspring and Analysis of Genetic Diversity in Pineapple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and DNA Extraction
2.2. Establishment of System for Authenticity Verification
2.3. Authenticity Verification of Hybrids
2.4. Genetic Diversity Analysis
3. Results
3.1. Screening of Pure Co-Dominant SNP Markers in Hybrid Parents
3.2. Identification of Hybrid Authenticity
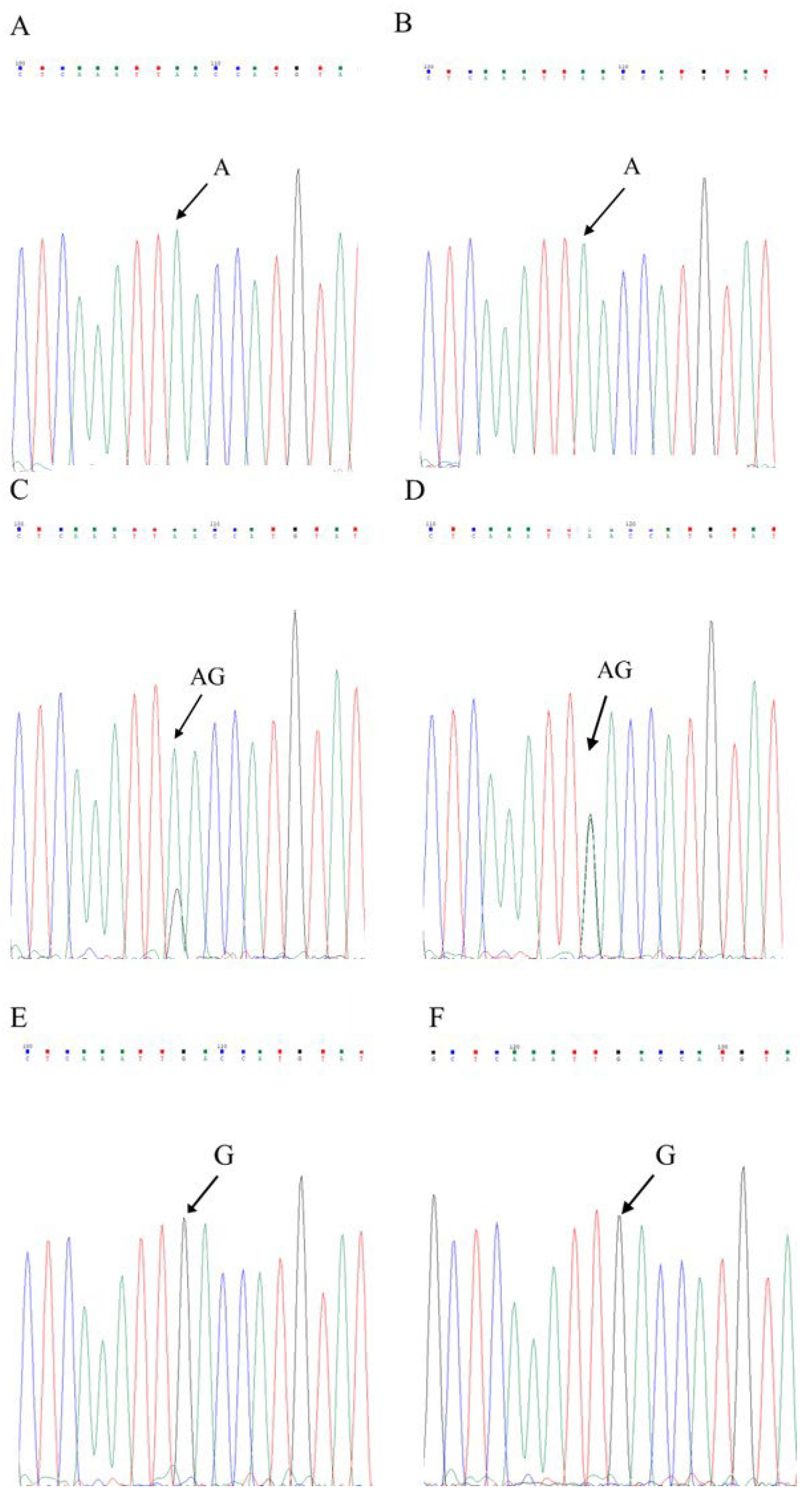
3.3. Sequencing Verification of True Hybrids and Pseudohybrids
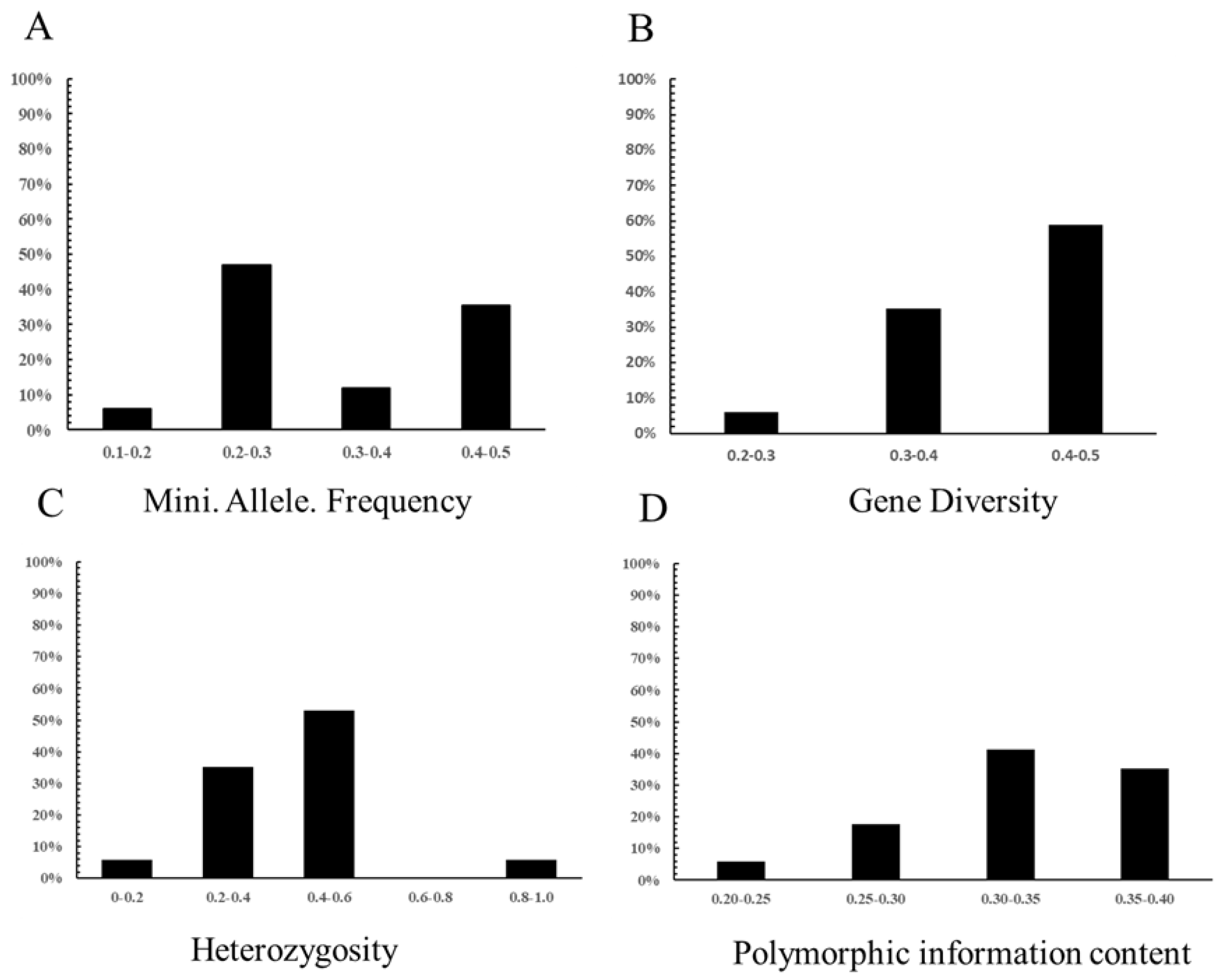
3.4. Genetic Diversity Analysis
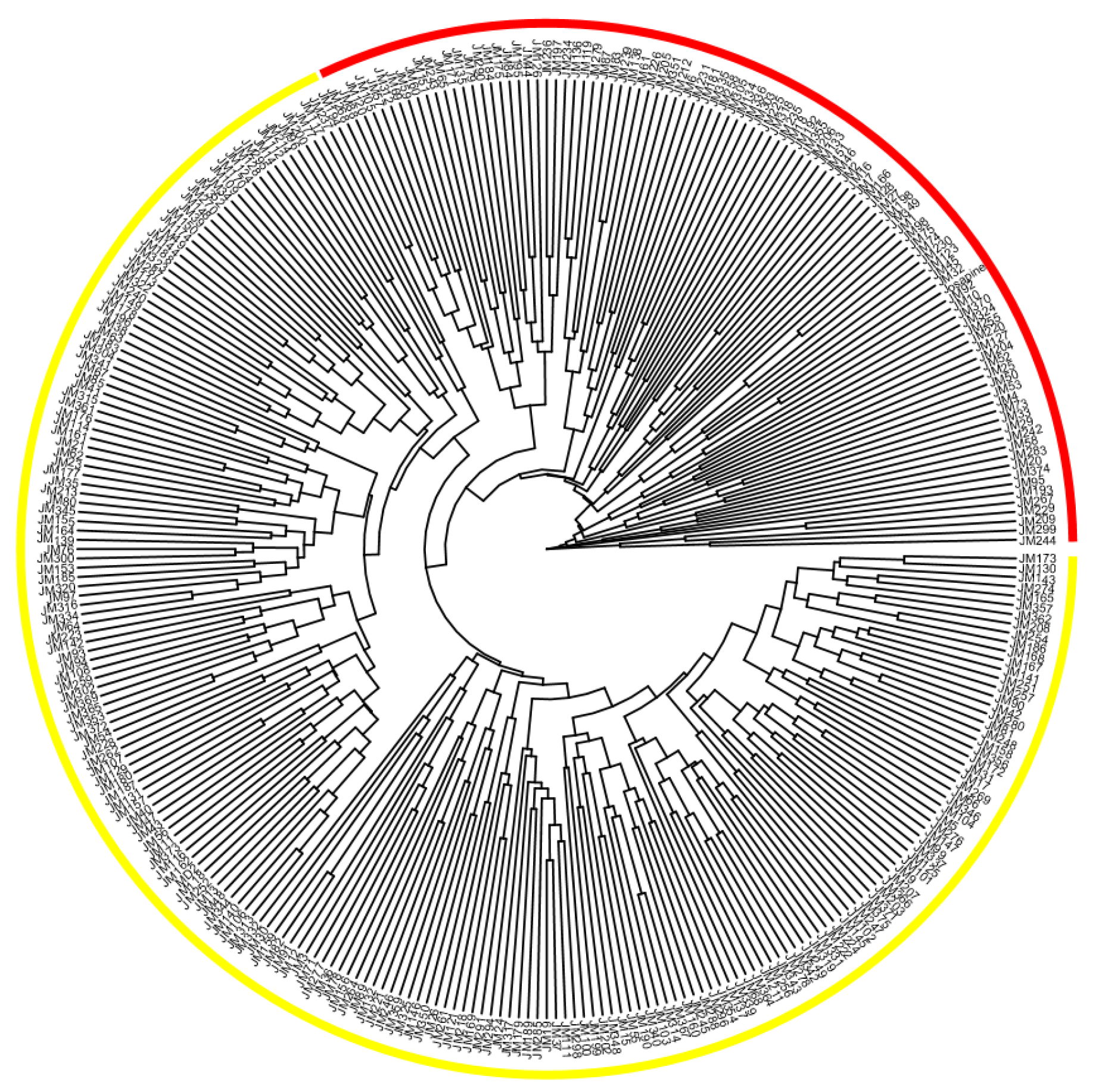
3.5. Clustering Patterns and Class Composition among Genotypes of Parents and Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fassinou, H.V.N.; Lommen, W.J.; Agbossou, E.K.; Struik, P.C. Trade-offs of flowering and maturity synchronisation for pineapple quality. PLoS ONE 2015, 10, e0143290. [Google Scholar] [CrossRef]
- Li, D.; Jing, M.; Dai, X.; Chen, Z.; Ma, C.; Chen, J. Current status of pineapple breeding, industrial development, and genetics in China. Euphytica 2022, 218, 85. [Google Scholar] [CrossRef]
- Deng, C.; Yuping, L.I.; Liang, W.; Lu, Y.E. Present situation and countermeasures of pineapple industry in China. J. Agric. Sci 2018, 46, 1031–1034. [Google Scholar]
- Chan, Y.K. Breeding of seed and vegetatively propagated tropical fruits using papaya and pineapple as examples. Acta Hortic. 2008, 787, 69–76. [Google Scholar] [CrossRef]
- Cabral, J.R.S.; de Matos, A.P.; Junghans, D.T.; Souza, F.V.D. Pineapple genetic improvement in Brazil. VI Int. Pineapple Symp. 2007, 822, 39–46. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Y.; Meng, X.C. Investigation on the variability of tissue culture seedling of “Yue crisp” pineapple. South China Fruits 2006, 35, 38. [Google Scholar]
- Liu, C.H.; Liu, Y. Phenotype Analysis of Pineapple Hybrid Line Obtained by Mixed-pollen Cross and Its Paternal Origin Analysis. J. Agric. 2018, 8, 39–45. [Google Scholar]
- Brewbaker, J.L.; Gorrez, D.D. Genetics of Self-Incompatibility in the Monocot Genera, Ananas (Pineapple) and Gasteria. Am. J. Bot. 1967, 54, 611–616. [Google Scholar]
- Cascante-Marín, A.; Núñez-Hidalgo, S. A Review of Breeding Systems in the Pineapple Family (Bromeliaceae, Poales). Bot. Rev. 2023, 89, 308–329. [Google Scholar] [CrossRef]
- Jin, S.B.; Yun, S.H.; Park, J.H.; Park, S.M.; Koh, S.W.; Lee, D.H. Early identification of citrus zygotic seedlings using pollen-specific molecular markers. Hortic. Sci. Technol. 2015, 33, 598–604. [Google Scholar] [CrossRef]
- Su, M.; Zhang, C.; Feng, S. Identification and genetic diversity analysis of hybrid offspring of azalea based on EST-SSR markers. Sci. Rep. 2022, 12, 15239. [Google Scholar] [CrossRef]
- Hong, H.; Lee, J.; Chae, W. An economic method to identify cultivars and elite lines in radish (Raphanus sativus L.) for small seed companies and independent breeders. Horticulture 2023, 9, 140. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Doğan, Y.; Comertpay, G.; Yıldız, M.; Baloch, F.S. DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2018, 32, 261–285. [Google Scholar] [CrossRef]
- Zhou, W.; Tian, Q.Q.; Li, T.; Huang, B.; Wen, Q. Phenotypic traits and SSR molecular identification of hybrid progenies of Camellia chekiangoleosa × C. semiserrata. Guihaia 2014, 1–11. [Google Scholar]
- Cholastova, T.; Soldanova, M.; Pokorny, R. Random amplified polymorphic DNA (RAPD) and simple sequence repeat (SSR) marker efficacy for maize hybrid identification. Afr. J. Biotechnol. 2011, 10, 4794–4801. [Google Scholar]
- Ali, A.; Jin, D.W.; Yong, B.P.; Zu, H.D.; Zhi, W.C.; Ru, K.C.; San, J.G. Molecular identification and genetic diversity analysis of Chinese sugarcane (Saccharum spp. hybrids) varieties using SSR markers. Trop. Plant Biol. 2017, 10, 194–203. [Google Scholar] [CrossRef]
- Wang, L.P.; Dai, D.L.; Wu, X.H.; Wang, B.G.; Li, G.J. Application of AFLP markers in fast determination of seed purity in gourd, Lagenaria siceraria cv. Zhepu No. 2. Acta Agriculturae Zhejiangensis 2008, 20, 84–87. [Google Scholar]
- Zhang, Y.; An, R.; Song, M.; Xie, C.; Wei, S.; Wang, D.; Mu, J. A set of molecular markers to accelerate breeding and determine seed purity of CMS three-line hybrids in Brassica napus. Plants 2023, 12, 1514. [Google Scholar] [CrossRef] [PubMed]
- Golein, B.; Fifaei, R.; Ghasemi, M. Identification of zygotic and nucellar seedlings in citrus interspecific crosses by inter simple sequence repeats (ISSR) markers. Afr. J. Biotechnol. 2011, 10, 18965–18970. [Google Scholar]
- Krishna, T.A.; Maharajan, T.; Roch, G.V.; Ramakrishnan, M.; Ceasar, S.A.; Ignacimuthu, S. Hybridization and hybrid detection through molecular markers in finger millet [Eleusine coracana (L.) Gaertn.]. J. Crop Improv. 2020, 34, 335–355. [Google Scholar] [CrossRef]
- Agarwal, M.; Shrivastava, N.; Padh, H. Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep. 2008, 27, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Bardakci, F. Random amplified polymorphic DNA (RAPD) markers. Turk. J. Biol. 2001, 25, 185–196. [Google Scholar]
- Althoff, D.M.; Gitzendanner, M.A.; Segraves, K.A. The utility of amplified fragment length polymorphisms in phylogenetics: A comparison of homology within and between genomes. Syst. Biol. 2007, 56, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Sarwat, M. ISSR: A reliable and cost-effective technique for detection of DNA polymorphism. Plant DNA Fingerprint. Barcod. Methods Protoc. 2012, 862, 103–121. [Google Scholar]
- Santhy, V.; Sandra, N.; Ravishankar, K.V.; Chidambara, B. Molecular Techniques for Testing Genetic Purity and Seed Health. In Seed Science and Technology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 365–389. [Google Scholar] [CrossRef]
- Al-Samarai, F.R.; Al-Kazaz, A.A. Molecular markers: An introduction and applications. Eur. J. Mol. Biotechnol. 2015, 9, 118–130. [Google Scholar] [CrossRef]
- Ott, A.; Liu, S.; Schnable, J.C.; Yeh, C.T.E.; Wang, K.S.; Schnable, P.S. tGBS® genotyping-by-sequencing enables reliable genotyping of heterozygous loci. Nucleic Acids Res. 2017, 45, e178. [Google Scholar] [CrossRef]
- Rafalski, A. Applications of single nucleotide polymorphisms in crop genetics. Curr. Opin. Plant Biol. 2002, 5, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Paux, E.; Sourdille, P.; Mackay, I.; Feuillet, C. Sequence-based marker development in wheat: Advances and applications to breeding. Biotechnol. Adv. 2012, 30, 1071–1088. [Google Scholar] [CrossRef]
- Song, L.; Wang, R.; Yang, X.; Zhang, A.; Liu, D. Molecular markers and their applications in marker-assisted selection (MAS) in bread wheat (Triticum aestivum L.). Agriculture 2023, 13, 642. [Google Scholar] [CrossRef]
- Josia, C.; Mashingaidze, K.; Amelework, A.B.; Kondwakwenda, A.; Musvosvi, C.; Sibiya, J. SNP-based assessment of genetic purity and diversity in maize hybrid breeding. PLoS ONE 2021, 16, e0249505. [Google Scholar] [CrossRef]
- Utami, D.W.; Rosdianti, I.; Dewi, I.S.; Ambarwati, D.; Sisharmini, A.; Apriana, A.; Somantri, I.H. Utilization of 384 SNP genotyping technology for seed purity testing of new Indonesian rice varieties Inpari Blas and Inpari HDB. SABRAO J. Breed. Genet. 2016, 48, 416–424. [Google Scholar]
- Kishor, D.S.; Noh, Y.; Song, W.H.; Lee, G.P.; Jung, J.K.; Shim, E.J.; Chung, S.M. Identification and purity test of melon cultivars and F1 hybrids using fluidigm-based snp markers. Hortic. Sci. Technol. 2020, 38, 686–694. [Google Scholar] [CrossRef]
- Ongom, P.O.; Fatokun, C.; Togola, A.; Salvo, S.; Oyebode, O.G.; Ahmad, M.S.; Boukar, O. Molecular fingerprinting and hybridity authentication in cowpea using single nucleotide polymorphism based kompetitive allele-specific PCR assay. Front. Plant Sci. 2021, 12, 734117. [Google Scholar] [CrossRef]
- Aboul-Maaty, N.A.F.; Oraby, H.A.S. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull. Natl. Res. Cent. 2019, 43, 25. [Google Scholar] [CrossRef]
- Ayalew, H.; Tsang, P.W.; Chu, C.; Wang, J.; Liu, S.; Chen, C.; Ma, X.F. Comparison of TaqMan, KASP and rhAmp SNP genotyping platforms in hexaploid wheat. PLoS ONE 2019, 14, e0217222. [Google Scholar] [CrossRef] [PubMed]
- Dipta, B.; Sood, S.; Mangal, V.; Bhardwaj, V.; Thakur, A.K.; Kumar, V.; Singh, B. KASP: A high-throughput genotyping system and its applications in major crop plants for biotic and abiotic stress tolerance. Mol. Biol. Rep. 2024, 51, 508. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, N.; Jiang, Z.C.; Zhang, H.; Zhai, H.; He, S.Z.; Gao, S.P.; Liu, Q.C. Analysis of genetic diversity and population structure in sweetpotato using SSR markers. J. Integr. Agric. 2023, 22, 3408–3415. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knya, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Kai, C.Z.; Rong, Q.L.; Xiao, Y.B.; Shu, P.Y.; Lu, P.W.; Shi, X.J. Sexual hybrid identification in apomictic PingYiTianCha seedlings using RAPD markers. J. Agric. Biotechnol. 1997, 5, 392–396. [Google Scholar]
- Han, G.; Xiang, S.; Wang, W.; Wei, X.; He, B.; Li, X.; Liang, G. Identification and genetic diversity of hybrid progenies from Shatian pummelo by SSR. Sci. Agric. Sin. 2010, 43, 4678–4686. [Google Scholar]
- Li, X.; Zheng, B.; Xu, W.; Ma, X.; Wang, S.; Qian, M.; Wu, H. Identification of F1 hybrid progenies in mango based on Fluorescent SSR markers. Horticulture 2022, 8, 1122. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, Z.X.; Jiang, N.H.; Yang Xiao, Y.; Yuan P, Y.; Qiu, Y.P.; Fan, C.F.; Xiang, X. Identification of Litchi (Litchi chinensis Sonn) Hybrids by SNP Markers. Mol. Plant Breed. 2016, 14, 647–654. [Google Scholar]
- Isabel, N.; Lamothe, M.; Thompson, S.L. A second-generation diagnostic single nucleotide polymorphism (SNP)-based assay, optimized to distinguish among eight poplar (Populus L.) species and their early hybrids. Tree Genet. Genomes 2013, 9, 621–626. [Google Scholar] [CrossRef]



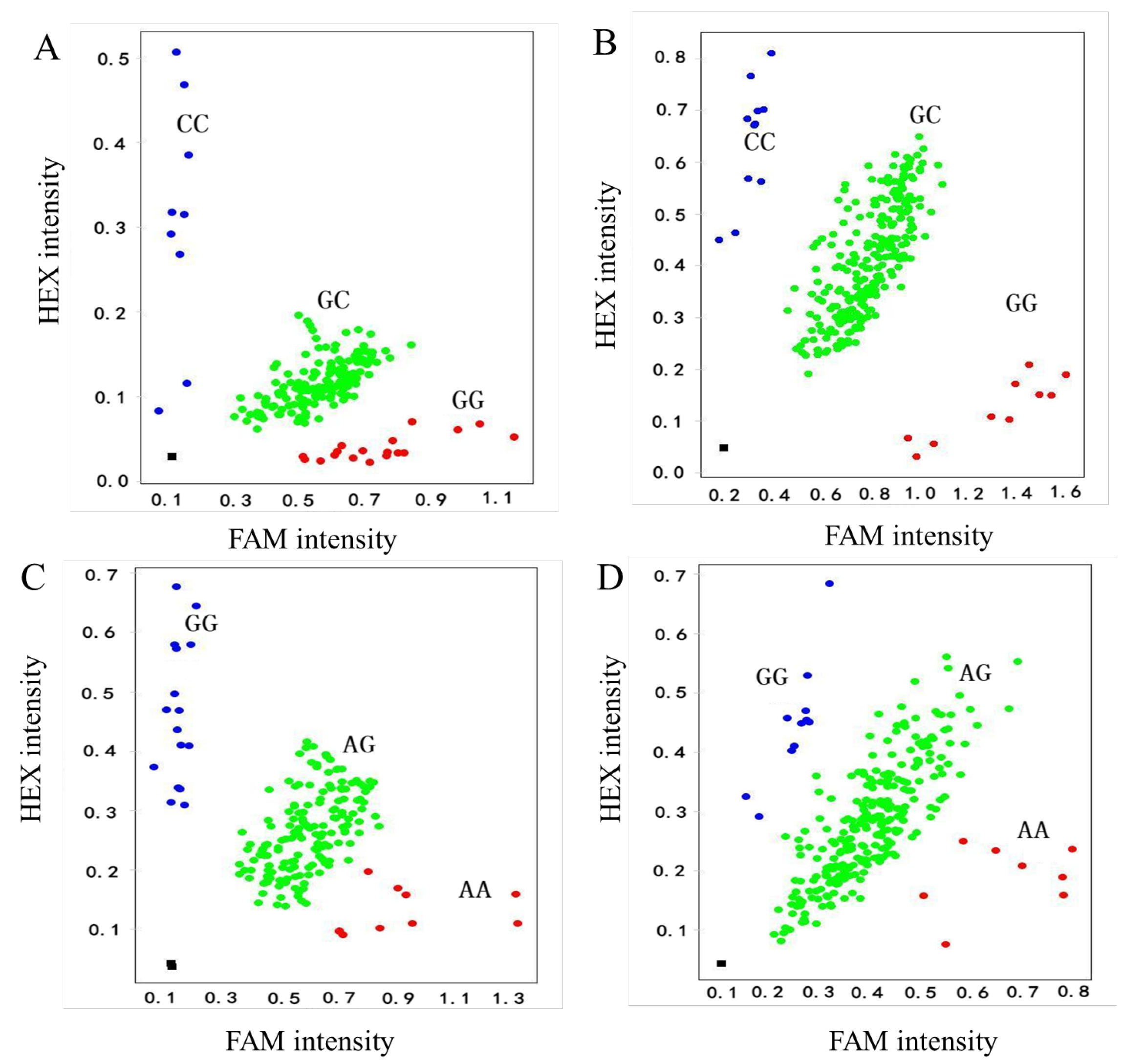

| Hybrid Combination (♀ × ♂) | Total Number of F1 (Plants) | SNP4010 Typing Statistics (Plants) | SNP22550 Typing Statistics (Plants) | True Hybrids (Plants) | Pseudohybrids (Plants) | True Hybrid Rate (%) | ||
|---|---|---|---|---|---|---|---|---|
| ‘MD2’ × ‘Josapine’ | 451 | GG | 28 | AA | 17 | 395 | 56 | 87.58 |
| GC | 404 | AG | 407 | |||||
| CC | 19 | GG | 27 | |||||
| Statistical Information | Maximum Value | Corresponds to Marker | Minimum Value | Corresponds to Marker | Average Value |
|---|---|---|---|---|---|
| Frequency of secondary effector loci | 0.500 | SNP4010 | 0.164 | SNP28156 | 0.334 |
| Gene diversity | 0.500 | SNP4010 | 0.274 | SNP28156 | 0.422 |
| Heterozygosity | 0.994 | SNP4010 | 0.191 | SNP30909 | 0.429 |
| Polymorphic information content | 0.375 | SNP5172, P20855 SNP4010 | 0.236 | SNP28156 | 0.331 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, P.; Liu, S.; Lin, W.; Yu, H.; Zhang, X.; Xiao, X.; Sun, W.; Lu, X.; Wu, Q. Authenticity Identification of F1 Hybrid Offspring and Analysis of Genetic Diversity in Pineapple. Agronomy 2024, 14, 1490. https://doi.org/10.3390/agronomy14071490
Jia P, Liu S, Lin W, Yu H, Zhang X, Xiao X, Sun W, Lu X, Wu Q. Authenticity Identification of F1 Hybrid Offspring and Analysis of Genetic Diversity in Pineapple. Agronomy. 2024; 14(7):1490. https://doi.org/10.3390/agronomy14071490
Chicago/Turabian StyleJia, Panpan, Shenghui Liu, Wenqiu Lin, Honglin Yu, Xiumei Zhang, Xiou Xiao, Weisheng Sun, Xinhua Lu, and Qingsong Wu. 2024. "Authenticity Identification of F1 Hybrid Offspring and Analysis of Genetic Diversity in Pineapple" Agronomy 14, no. 7: 1490. https://doi.org/10.3390/agronomy14071490





