Bakanae Disease Resistance in Rice: Current Status and Future Considerations
Abstract
:1. Background
2. Fungal Invasion and Colonization during F. fujikuroi—Rice Interactions
3. Screening of Rice Germplasms That Are Resistant to BD
4. Mapping of QTLs Related to BD Resistance in Rice
5. BD-Resistance-Related Genes Explored Using Omics Methods
6. Phytohormone-Related Genes and Endogenous Phytohormones during Rice—F. fujikuroi Interactions
7. Natural Fungicides for Controlling Bakanae
8. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, H.; Kim, T.-H.; Lee, G.-S.; Kang, H.-J.; Lee, S.-B.; Suh, S.C.; Kim, S.L.; Choi, I.; Baek, J.; Kim, K.-H. Mapping of a major quantitative trait locus for bakanae disease resistance in rice by genome resequencing. Mol. Genet. Genom. 2018, 293, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kimura, J. Studies on the ‘bakanae’ disease of the rice plant. Rep. Hokkaido. Natl. Agric. Exp. Stn. 1931, 27, 1–95. [Google Scholar]
- Lee, S.-B.; Kim, N.; Jo, S.; Hur, Y.-J.; Lee, J.-Y.; Cho, J.-H.; Lee, J.-H.; Kang, J.-W.; Song, Y.-C.; Bombay, M.; et al. Mapping of a major QTL, qBK1Z, for bakanae disease resistance in rice. Plants 2021, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, S.Y.; Liu, C.W.; Wu, D.H.; Kuo, C.C.; Lin, C.C.; Chou, H.P.; Wang, Y.Y.; Tsai, Y.C.; Lai, M.H.; et al. Invasion and colonization pattern of Fusarium fujikuroi in rice. Phytopathology 2020, 110, 1934–1945. [Google Scholar] [CrossRef]
- Volante, A.; Tondelli, A.; Aragona, M.; Valente, M.T.; Biselli, C.; Desiderio, F.; Bagnaresi, P.; Matic, S.; Gullino, M.L.; Infantino, A.; et al. Identification of bakanae disease resistance loci in japonica rice through genome wide association study. Rice 2017, 10, 29. [Google Scholar] [CrossRef]
- Mew, T.; Gonzales, P. A Handbook of Rice Seedborne Fungi; International Rice Research Institute: Los Baňos, Philippines; Science Publishers, Inc.: Enfield, UK, 2002. [Google Scholar]
- Ou, S. Rice Diseases; Commonwealth Mycological Institute: Kew, UK, 1985. [Google Scholar]
- Hwang, I.S.; Ahn, I.-P. Multi-homologous recombination-based gene manipulation in the rice pathogen Fusarium fujikuroi. Plant Pathol. J. 2016, 32, 173–181. [Google Scholar] [CrossRef]
- Gupta, A.; Solanki, I.; Bashyal, B.; Singh, Y.; Srivastava, K. Bakanae of rice- an emerging disease in Asia. J. Anim. Plant Sci. 2015, 25, 1499–1514. [Google Scholar]
- Wiemann, P.; Sieber, C.M.K.; von Bargen, K.W.; Studt, L.; Niehaus, E.M.; Espino, J.J.; Huß, K.; Michielse, C.B.; Albermann, S.; Wagner, D.; et al. Deciphering the cryptic genome: Genome-wide analyses of the rice pathogen Fusarium fujikuroi reveal complex regulation of secondary metabolism and novel metabolites. PLoS Pathog. 2013, 9, e1003475. [Google Scholar] [CrossRef]
- Wulff, E.G.; Sørensen, J.L.; Lübeck, M.; Nielsen, K.F.; Thrane, U.; Torp, J. Fusarium spp. associated with rice Bakanae: Ecology, genetic diversity, pathogenicity and toxigenicity. Environ. Microbiol. 2010, 12, 649–657. [Google Scholar] [CrossRef]
- Hwang, I.S.; Kang, W.-R.; Hwang, D.-J.; Bae, S.-C.; Yun, S.-H.; Ahn, I.-P. Evaluation of bakanae disease progression caused by Fusarium fujikuroi in Oryza sativa L. J. Microbiol. 2013, 51, 858–865. [Google Scholar] [CrossRef]
- Hayasaka, T.; Ishiguro, K.; Shibutani, K.; Namai, T. Seed disinfection using hot water immersion to control several seed-borne diseases of rice plants. Jpn. J. Phytopathol. 2001, 67, 26–32. [Google Scholar] [CrossRef]
- Shakeel, Q.; Mubeen, M.; Sohail, M.A.; Ali, S.; Iftikhar, Y.; Tahir Bajwa, R.; Aqueel, M.A.; Upadhyay, S.K.; Divvela, P.K.; Zhou, L. An explanation of the mystifying bakanae disease narrative for tomorrow’s rice. Front. Microbiol. 2023, 14, 1153437. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.A.; Uddin, M.B.; Rashid, M.M.; Hossain, M.; Akter, S.; Jahan, Q.S.A.; Hossain, M.S.; Ali, M.A.; Hossain, M.A. Rice bakanae disease: Yield loss and management issues in Bangladesh. Food Sci. Technol. 2021, 9, 7–16. [Google Scholar]
- Iqbal, M.; Javed, N.; Yasin, S.I.; Sahi, S.T.; Wakil, W. Studies on chemical control of bakanae disease (F. moniliforme) of rice in Pakistan. Pak. J. Phytopathol. 2013, 25, 146–154. [Google Scholar]
- Singh, R.; Kumar, P.; Laha, G.S. Present status of Bakanae of rice caused by F. fujikuroi Nirenberg. Indian Phytopathol. 2019, 72, 587–597. [Google Scholar] [CrossRef]
- Qu, X.P.; Li, J.S.; Wang, J.X.; Wu, L.Y.; Wang, Y.F.; Chen, C.J.; Zhou, M.G.; Hou, Y.P. Effects of the dinitroaniline fungicide fluazinam on F. fujikuroi and ric. Pestic. Biochem. Phys. 2018, 152, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Gu, C.-Y.; Pan, R.; Abid, M.; Zang, H.-Y.; Yang, X.; Tan, G.-J.; Chen, Y. Activity of A Novel Succinate Dehydrogenase Inhibitor Fungicide Pydiflumetofen Against Fusarium fujikuroi causing Rice Bakanae Disease. Plant Dis. 2021, 105, 3208–3217. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Park, J.K.; Lee, C.H.; Hahn, B.S.; Koo, J.C. Comparison of the antimicrobial properties of chitosan oligosaccharides (COS) and EDTA against F. fujikuroi causing rice bakanae disease. Curr. Microbiol. 2016, 72, 496–502. [Google Scholar] [CrossRef]
- Hossain, K.S.; Mia, M.T.; Bashar, M.A. Management of bakanae disease of rice. Bangladesh J. Bot. 2015, 44, 277–283. [Google Scholar] [CrossRef]
- Li, M.; Li, T.; Duan, Y.; Yang, Y.; Wu, J.; Zhao, D.; Xiao, X.; Pan, X.; Chen, W.; Wang, J.; et al. Evaluation of Phenamacril and Ipconazole for Control of Rice Bakanae Disease Caused by Fusarium fujikuroi. Plant Dis. 2018, 102, 1234–1239. [Google Scholar] [CrossRef]
- An, Y.N.; Murugesan, C.; Choi, H.; Kim, K.D.; Chun, S.C. Current Studies on Bakanae Disease in Rice: Host Range, Molecular Identification, and Disease Management. Mycobiology 2023, 51, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, B.M.; Aggarwal, R.; Sharma, S.; Gupta, S.; Rawat, K.; Singh, D.; Singh, A.K.; Krishnan, S.G. Occurrence, identification and pathogenicity of Fusarium species associated with bakanae disease of basmati rice in India. Eur. J. Plant Pathol. 2016, 144, 457–466. [Google Scholar] [CrossRef]
- Karthik, C.; Shu, Q. Current insights on rice (Oryza sativa L.) bakanae disease and exploration of its management strategies. Zhejiang Univ. Sci. B 2023, 24, 755–778. [Google Scholar] [CrossRef] [PubMed]
- Sunani, S.K.; Bashyal, B.M.; Kharayat, B.S.; Prakash, G.; Krishnan, S.G.; Aggarwal, R. Identification of rice seed infection routes of Fusarium fujikuroi inciting bakanae disease of rice. J. Plant Pathol. 2020, 102, 113–121. [Google Scholar] [CrossRef]
- Lee, S.B.; Hur, Y.J.; Cho, J.H.; Lee, J.H.; Kim, T.H.; Cho, S.M.; Song, Y.C.; Seo, Y.S.; Lee, J.; Kim, T.S.; et al. Molecular mapping of qBK1 (WD), a major QTL for bakanae disease resistance in rice. Rice 2018, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Campos-Soriano, L.; Piombo, E.; Romano, E.; Segundo, B.S.; Spadaro, D.; Infantino, A. Imaging the invasion of rice roots by the bakanae agent Fusarium fujikuroi using a GFP-tagged isolate. Eur. J. Plant Pathol. 2021, 161, 25–36. [Google Scholar] [CrossRef]
- Carneiro, G.A.; Matić, S.; Ortu, G.; Garibaldi, A.; Spadaro, D.; Gullino, M.L. Development and validation of a TaqMan real time PCR assay for the specific detection and quantification of Fusarium fujikuroi in rice plants and seeds. Phytopathology 2017, 107, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Lai, M.-H.; Tung, C.-W.; Wu, D.-H.; Chang, F.-Y.; Lin, T.-C.; Chung, C.-L. Genome-wide association mapping of gene loci affecting disease resistance in the rice-Fusarium fujikuroi pathosystem. Rice 2019, 12, 85–96. [Google Scholar] [CrossRef]
- Elshafey, R.A.S.; Tahoon, A.M.; El-Emary, F.A. Analysis of varietal response to bakanae infection Fusarium fujikuroi and gibberellic acid through morphological, anatomical and hormonal changes in three rice varieties. J. Phytopathol. Pest Manag. 2018, 5, 63–87. [Google Scholar]
- Kumar, P.; Sunder, S.; Singh, R. Survival of Fusarium moniliforme causing foot rot and bakanae disease in different parts of rice grains. Indian Phytopathol. 2015, 68, 454–455. [Google Scholar]
- Ji, Z.J.; Ma, L.Y.; Li, X.M.; Yang, C.D. Identification of resistance to bakanae disease for rice germplasms. Zhejiang Nongye Kexue 2008, 5, 590–592. [Google Scholar]
- Li, D.J.; Luo, K.; Chen, Z. Studies on resistance of rice varieties to bakanae disease and pathogenicity of pathogen (Fusarium moniliforme). Acta. Phytopathol. Sin. 1993, 23, 315–319. [Google Scholar]
- Zheng, G.X.; Lu, B.; Wu, R.Z.; Nie, H. Study on screening methods for resistance of bakanae disease of rice. Acta Phytophylacica Sin. 1993, 20, 289–293. [Google Scholar]
- Ghazanfar, M.U.; Javed, N.; Wakil, W.; Iqbal, M. Screening of some fine and coarse rice varieties against bakanae disease. J. Agric. Res. 2013, 51, 41–49. [Google Scholar]
- Prashantha, S.T.; Gaurav Kumar, Y.; Gopala Krishnan, S.; Maya Bashyal, B. Identification of resistant sources against bakanae disease in short grained aromatic rice (Oryza sativa). Indian J. Agric. Sci. 2024, 94, 044–049. [Google Scholar]
- Ji, Z.J. Identification of the Resistance–Related Gene to Bakanae Disease and Pyramiding of Multiple Resistance Genes in Rice Breeding. Ph.D. Thesis, Shengyang Agricultural University, Shenyang, China, 2016. [Google Scholar]
- Lee, S.-B.; Lee, J.-Y.; Kang, J.-W.; Mang, H.; Kabange, N.R.; Seong, G.-U.; Kwon, Y.; Lee, S.-M.; Shin, D.; Lee, J.-H.; et al. A Novel Locus for Bakanae Disease Resistance, qBK4T, Identified in Rice. Agronomy 2022, 12, 2567. [Google Scholar] [CrossRef]
- Ma, L.; Ji, Z.; Bao, J.; Zhu, X.; Li, X.; Zhuang, J.; Yang, C.; Xia, Y. Response of rice genotypes carrying different dwarf genes to Fusarium moniliforme and gibberellic acid. Plant Prod. Sci. 2008, 11, 134–138. [Google Scholar] [CrossRef]
- Kim, M.-H.; Hur, Y.-J.; Lee, S.B.; Kwon, T.; Hwang, U.-H.; Park, S.-K.; Yoon, Y.-N.; Lee, J.-H.; Cho, J.-H.; Shin, D.; et al. Large-scale screening of rice accessions to evaluate resistance to bakanae disease. J. Gen. Plant Pathol. 2014, 80, 408–414. [Google Scholar] [CrossRef]
- Hossain, K.S.; Mia, M.A.T.; Bashar, M.A. New method for screening rice varieties against bakanae disease. Bangladesh J. Bot. 2014, 42, 315–320. [Google Scholar] [CrossRef]
- Yang, C.D.; Guo, L.B.; Li, X.M.; Ji, Z.J.; Ma, L.Y.; Qian, Q. Analysis of QTLs for resistance to rice bakanae disease. Chin. J. Rice Sci. 2006, 6, 657–659. [Google Scholar]
- Lee, S.-B.; Kim, N.; Hur, Y.-J.; Cho, S.-M.; Kim, T.-H.; Lee, J.-Y.; Cho, J.-H.; Lee, J.-H.; Song, Y.-C.; Seo, Y.-S.; et al. Fine mapping of qBK1, a major QTL for bakanae disease resistance in rice. Rice 2019, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Fiyaz, R.A.; Yadav, A.K.; Krishnan, S.G.; Ellur, R.K.; Bashyal, B.M.; Grover, N.; Bhowmick, P.K.; Nagarajan, M.; Vinod, K.K.; Singh, N.K.; et al. Mapping quantitative trait loci responsible for resistance to Bakanae disease in rice. Rice 2016, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-Y.; Cheon, K.-S.; Oh, J.; Oh, H.; Kim, S.L.; Kim, N.; Lee, E.; Choi, I.; Baek, J.; Kim, K.-H.; et al. Rice genome resequencing reveals a major quantitative trait locus for resistance to bakanae disease caused by Fusarium fujikuroi. Int. J. Mol. Sci. 2019, 20, 2598. [Google Scholar] [CrossRef] [PubMed]
- Cheon, K.-S.; Jeong, Y.-M.; Lee, Y.-Y.; Oh, J.; Kang, D.-Y.; Oh, H.; Kim, S.L.; Kim, N.; Lee, E.; Baek, J.; et al. Kompetitive allele-specific PCR marker development and quantitative trait locus mapping for bakanae disease resistance in Korean japonica rice varieties. Plant Breed. Biotechnol. 2019, 7, 208–219. [Google Scholar] [CrossRef]
- Ji, Z.; Zeng, Y.; Liang, Y.; Qian, Q.; Yang, C. Transcriptomic dissection of the rice–Fusarium fujikuroi interaction by RNA-Seq. Euphytica 2016, 211, 123–137. [Google Scholar] [CrossRef]
- Hur, Y.-J.; Lee, S.B.; Kim, T.H.; Kwon, T.; Lee, J.-H.; Shin, D.-J.; Park, S.-K.; Hwang, U.-H.; Cho, J.H.; Yoon, Y.-N.; et al. Mapping of qBK1, a major QTL for bakanae disease resistance in rice. Mol. Breed. 2015, 35, 78. [Google Scholar] [CrossRef]
- Cheng, A.-P.; Chen, S.-Y.; Lai, M.-H.; Wu, D.-H.; Lin, S.-S.; Chen, C.-Y.; Chung, C.-L. Transcriptome analysis of early defenses in rice against Fusarium fujikuroi. Rice 2020, 13, 65. [Google Scholar] [CrossRef]
- Ji, Z.; Zeng, Y.; Liang, Y.; Qian, Q.; Yang, C. Proteomic dissection of the rice-Fusarium fujikuroi interaction and the correlation between the proteome and transcriptome under disease stress. BMC Genom. 2019, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Bagnaresi, P.; Biselli, C.; Orru, L.; Carneiro, G.A.; Siciliano, I.; Valé, G.; Gullino, M.L.; Spadaro, D. Comparative transcriptome profiling of resistant and susceptible rice genotypes in response to the seedborne pathogen Fusarium fujikuroi. BMC Genom. 2016, 17, 608. [Google Scholar] [CrossRef]
- Pedley, K.F.; Martin, G.B. Role of mitogen-activated protein kinases in plant immunity. Curr. Opin. Plant Biol. 2005, 8, 541–547. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, K.P.; Gaur, R.K.; Gupta, V.K. Role of chitinase in plant defense. Asian J. Biochem. 2011, 6, 29–37. [Google Scholar] [CrossRef]
- Kawahara, Y.; Oono, Y.; Kanamori, H.; Matsumoto, T.; Itoh, T.; Minami, E. Simultaneous RNA-seq analysis of a mixed transcriptome of rice and blast fungus interaction. PLoS ONE 2012, 7, e49423. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.P. The molecular mechanism and evolution of the GA–GID1–DELLA signaling module in plants. Curr. Biol. 2011, 21, R338–R345. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D.G. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2011, 18, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Son, S.; Nam, S.; Suh, E.-J.; Lee, S.I.; Park, S.R. OsWRKY114 Is a Player in Rice Immunity against Fusarium fujikuroi. Int. J. Mol. Sci. 2023, 24, 6604. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, I.; Carneiro, G.A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Jasmonic acid, abscisic acid, and salicylic acid are involved in the phytoalexin responses of rice to Fusarium fujikuroi, a high gibberellin producer pathogen. J. Agric. Food Chem. 2015, 63, 8134–8142. [Google Scholar] [CrossRef] [PubMed]
- Hernani; Yuliani, S.; Rahmini. Natural biopesticide from liquid rice hull smoke to control brown planthopper. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012067. [Google Scholar] [CrossRef]
- Sarwar, A.; Hassan, M.N.; Imran, M.; Iqbal, M.; Majeed, S.; Brader, G.; Sessitsch, A.; Hafeez, F.Y. Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol. Res. 2018, 209, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saraf, M.; Pandya, U.; Thakkar, A. Role of allelochemicals in plant growth-promoting rhizobacteria for biocontrol of phytopathogens. Microbiol. Res. 2014, 169, 18–29. [Google Scholar] [CrossRef]
- Quach, N.T.; Vu, T.H.N.; Nguyen, T.T.A.; Le, P.C.; Do, H.G.; Nguyen, T.D.; Thao, P.T.H.; Nguyen, T.T.L.; Chu, H.H.; Phi, Q.-T. Metabolic and genomic analysis deciphering biocontrol potential of endophytic Streptomyces albus RC2 against crop pathogenic fungi. Braz. J. Microbiol. 2023, 54, 2617–2626. [Google Scholar] [CrossRef]
- Hossain, M.T.; Khan, A.; Chung, E.J.; Rashid, H.-O.; Chung, Y.R. Biological Control of Rice Bakanae by an Endophytic Bacillus oryzicola YC7007. Plant Pathol. J. 2016, 32, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.-E.N.; Malik, K.; Hassan, M.N. Rice-associated antagonistic bacteria suppress the Fusarium fujikoroi causing rice bakanae disease. BioControl 2022, 67, 101–109. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, Y.B.; Chen, M.; Lu, F.; Sun, K.; Tang, M.-J.; Zhang, W.; Bu, Y.-Q.; Dai, C.-C. Preinoculation with endophytic fungus Phomopsis liquidambaris reduced rice bakanae disease caused by Fusarium proliferatum via enhanced plant resistance. J. Appl. Microbiol. 2022, 133, 1566–1580. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Sasaki, M.; Nonaka, Y.; Tanaka, J.; Tokunaga, T.; Kato, A.; Thuy, T.T.T.; Vang, L.V.; Tuong, L.M.; Kanematsu, S.; et al. Spray Application of Nonpathogenic Fusaria onto Rice Flowers Controls Bakanae Disease (Caused by Fusarium fujikuroi) in the Next Plant Generation. Appl. Environ. Microbiol. 2021, 87, e01959-20. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gao, J.; Chen, X.; Zhang, M.; Yang, F.; Du, Y.; Moe, T.S.; Munir, I.; Xue, J.; Zhang, X. Isolation and Characterization of Plant Growth-Promoting Endophytic Bacteria Paenibacillus polymyxa SK1 from Lilium lancifolium. BioMed Res. Int. 2020, 2020, 8650957. [Google Scholar] [CrossRef] [PubMed]
- Dongmo, A.N.; Nguefack, J.; Dongmo, J.B.L.; Fouelefack, F.R.; Azah, R.U.; Nkengfack, E.A.; Stefani, E. Chemical characterization of an aqueous extract and the essential oil of Tithonia diversifolia and their biocontrol activity against seed-borne pathogens of rice. J. Plant Dis. Prot. 2021, 128, 703–713. [Google Scholar] [CrossRef]
- Kalboush, Z.; Hassan, A.A. Antifungal potential and characterization of plant extracts against F. fujikuroi on rice. J. Plant Prot. Path. 2019, 10, 369–376. [Google Scholar] [CrossRef]
- Baria, T.T.; Rakholiya, K. Environment friendly way to management of Fusarium fruit rot disease of banana in vivo by essential oils. Int. J. Genet. 2020, 12, 798–800. [Google Scholar]
- Gupta, A.; Kumar, R. Integrated management of bakanae disease in basmati rice. Environ. Crossroads Chall. Green Solut. 2020, 55, 337. [Google Scholar]
- Akhila, A. Essential Oil-Bearing Grasses: The Genus Cymbopogon; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Habibi, A.; Mansouri, S.M.; Sadeghi, B. Fusarium species associated with medicinal plants of Lamiaceae and Asteraceae. Mycol. Iran. 2018, 5, 91–101. [Google Scholar]
- Elamawi, R.M.; Tahoon, A.M.; Elsharnoby, D.E.; El-Shafey, R.A. Bio-production of silica nanoparticles from rice husk and their impact on rice bakanae disease and grain yield. Arch. Phytopathol. Plant Prot. 2020, 53, 459–478. [Google Scholar] [CrossRef]
- Reglinski, T.; Dann, E.; Deverall, B. Implementation of Induced Resistance for Crop Protection. In Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection; Walters, D., Newton, A., Lyon, G., Eds.; Blackwell Publishing: Oxford, UK, 2014. [Google Scholar]
- El-Hendawy, S.; Shaban, W.; Sakagami, J. Does treating faba bean seeds with chemical inducers simultaneously increase chocolate spot disease resistance and yield under field conditions? Turk. J. Agric. For. 2010, 34, 475–485. [Google Scholar]
- Katz, O.; Puppe, D.; Kaczorek, D.; Prakash, N.B.; Schaller, J. Silicon in the Soil–Plant Continuum: Intricate Feedback Mechanisms within Ecosystems. Plants 2021, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, Y.; Li, P.; Wen, L.; Hou, M. Silicon amendment to rice plants impairs sucking behaviors and population growth in the phloem feeder Nilaparvata lugens (Hemiptera: Delphacidae). Sci. Rep. 2017, 7, 1101. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, R.; Jeevan, B.; Baite, M.S.; Prabhukarthikeyan, S.R.; Keerthana, U.; Annamalai, M.; Pati, P.; Mohapatra, S.D.; Govindharaj, G.-P.-P. Dual Role of Potassium Silicate and Salicylic Acid: Plant Growth Promotor and Plant Immunity Booster against Bakanae Disease of Rice. Silicon 2024, 16, 1173–1182. [Google Scholar] [CrossRef]
- Ptaszek, M.; Canfora, L.; Pugliese, M.; Pinzari, F.; Gilardi, G.; Trzciński, P.; Malusà, E. Microbial based products to control soil-borne pathogens: Methods to improve efficacy and to assess impacts on microbiome. Microorganisms 2023, 11, 224. [Google Scholar] [CrossRef]

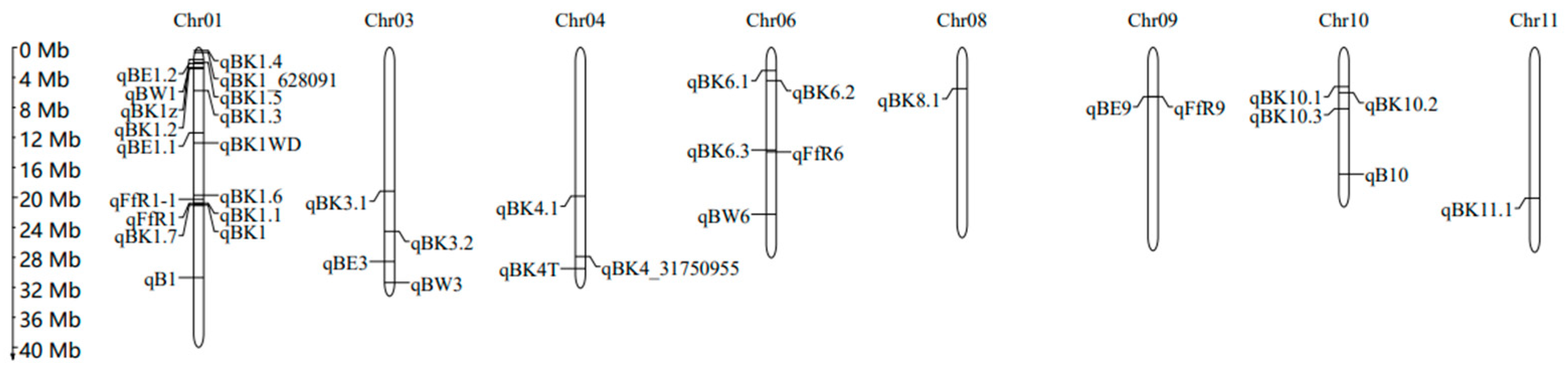
| QTL | Chromosome | QTL Region (Mb) | PVE (%) a | Mapping Population/Method | Mapping Population Size | Parents or Type of Population | References |
|---|---|---|---|---|---|---|---|
| qB1 | 1 | 34.10–34.95 | 13.4 | DH/QTL mapping | 120 | Chunjiang 06 (indica)/TN1 (japonica) | [43] |
| qB10 | 10 | 18.72–19.23 | 13.3 | DH/QTL mapping | 120 | Chunjiang 06 (indica)/TN1 (japonica) | [43] |
| qBK1 | 1 | 23.21–23.72 | 65.0 | NIL/QTL mapping | 168 | YR24982-9-1 (indica)/Ilpum (japonica) | [49] |
| 1 | 23.64–23.67 | - | NIL/QTL mapping | 168 | YR24982-9-1 (indica)/Ilpum (japonica) | [44] | |
| qBK1.1 | 1 | 23.32–23.34 | 4.8 | RIL/QTL mapping | 168 | Pusa 1342 (indica)/Pusa Basmati 1121 (indica) | [45] |
| qBK1.2 | 1 | 3.10–3.36 | 24.7 | RIL/QTL mapping | 168 | Pusa 1342 (indica)/Pusa Basmati 1121 (indica) | [45] |
| qBK1.3 | 1 | 4.65–8.41 | 6.5 | RIL/QTL mapping | 168 | Pusa 1342 (indica)/Pusa Basmati 1121 (indica) | [45] |
| qBK3.1 | 3 | 21.43–21.78 | 9.1 | RIL/QTL mapping | 168 | Pusa 1342 (indica)/Pusa Basmati 1121 (indica) | [45] |
| qBE1.1 | 1 | 11.91–13.71 | 15.3 | RIL/QTL mapping | 132 | Peiai 64S (indica)/9311 (indica) | [38] |
| qBE9 | 9 | 6.38–8.28 | 11.0 | RIL/QTL mapping | 132 | Peiai 64S (indica)/9311 (indica) | [38] |
| qBW1 | 1 | 0.56–5.62 | 12.8 | RIL/QTL mapping | 132 | Peiai 64S (indica)/9311 (indica) | [38] |
| qBW3 | 3 | 34.95–35.60 | 12.8 | RIL/QTL mapping | 132 | Peiai 64S (indica)/9311 (indica) | [38] |
| qBW6 | 6 | 24.40–25.88 | 11.5 | RIL/QTL mapping | 132 | Peiai 64S (indica)/9311 (indica) | [38] |
| qBE1.2 | 1 | 0.30–4.56 | 18.7 | RIL/QTL mapping | 159 | Nipponbare (japonica)/9311 (indica) | [38] |
| qBE3 | 3 | 28.68–35.77 | 22.3 | RIL/QTL mapping | 159 | Nipponbare (japonica)/9311 (indica) | [38] |
| qBK1_628091 | 1 | 0.62–1.04 | - | japonica rice accessions/GWAS | 138 | tropical and temperate japonica | [5] |
| qBK4_31750955 | 4 | 31.16–31.75 | - | japonica rice accessions/GWAS | 138 | tropical and temperate japonica | [5] |
| qBK1WD | 1 | 13.54–15.13 | 20.2 | RIL/QTL mapping | 200 | Wonseadaesoo (japonica)/Junam (japonica) | [27] |
| qFfR1 | 1 | 22.56–24.10 | - | F2:F3/Genome resequencing | 180 | Nampyeong (japonica)/DongjinAD (japonica) | [1] |
| qFfR9 | 9 | 7.24–7.56 | - | F2:F3/Genome resequencing | 188 | Samgwang (japonica)/Junam (japonica) | [46] |
| qBK1.4 | 1 | 0.4–0.43 | - | Parts of RDP 1 b/GWAS | 76 | indica subgroup from RDP 1 | [30] |
| qBK1.5 | 1 | 2.25–2.33 | - | RDP 1/GWAS | 231 | accessions from RDP 1 | [30] |
| qBK1.6 | 1 | 22.09–22.25 | - | RDP 1/GWAS | 231 | accessions from RDP 1 | [30] |
| qBK1.7 | 1 | 23.63–23.64 | - | Parts of RDP 1/GWAS | 76 | indica subgroup from RDP 1 | [30] |
| qBK3.2 | 3 | 27.48–27.64 | - | RDP 1/GWAS | 231 | accessions from RDP 1 | [30] |
| qBK4.1 | 4 | 22.37–22.43 | - | RDP 1/GWAS | 231 | accessions from RDP 1 | [30] |
| qBK6.1 | 6 | 3.28–3.64 | - | RDP 1/GWAS | 231 | accessions from RDP 1 | [30] |
| qBK6.2 | 6 | 4.87–5.06 | - | RDP 1/GWAS | 231 | accessions from RDP 1 | [30] |
| qBK6.3 | 6 | 25.30–25.64 | - | Parts of RDP 1/GWAS | 76 | indica subgroup from RDP 1 | [30] |
| qBK8.1 | 8 | 6.14–6.24 | - | RDP 1/GWAS | 231 | accessions from RDP 1 | [30] |
| qBK10.1 | 10 | 5.68–6.02 | - | Parts of RDP 1/GWAS | 76 | indica subgroup from RDP 1 | [30] |
| qBK10.2 | 10 | 6.85–6.86 | - | RDP 1/GWAS | 231 | accessions from RDP 1 | [30] |
| qBK10.3 | 10 | 9.09–9.34 | - | Parts of RDP 1/GWAS | 76 | indica subgroup from RDP 1 | [30] |
| qBK11.1 | 11 | 22.577–22.583 | - | RDP 1/GWAS | 231 | accessions from RDP 1 | [30] |
| qFfR1-1 | 1 | 21.36–24.37 | - | F2:F3/QTL mapping | 205 | Junam (japonica)/Nampyeong (japonica) | [47] |
| qFfR6 | 6 | 15.20–16.22 | - | F2:F3/QTL mapping | 188 | Saenuri (japonica)/Nampyeong (japonica) | [47] |
| qBK1z | 1 | 1.43–2.16 | 30.9 | RIL/QTL mapping | 180 | Zenith (indica)/Ilpum (japonica) | [3] |
| qBK4T | 4 | 33.12–33.44 | 34.4 | RIL/QTL mapping and GWAS | 143 | Ilpum/Tung Ting Wan Hien1 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, L.; Chen, L.; Hou, Y.; Zeng, Y.; Ji, Z. Bakanae Disease Resistance in Rice: Current Status and Future Considerations. Agronomy 2024, 14, 1507. https://doi.org/10.3390/agronomy14071507
Zhan L, Chen L, Hou Y, Zeng Y, Ji Z. Bakanae Disease Resistance in Rice: Current Status and Future Considerations. Agronomy. 2024; 14(7):1507. https://doi.org/10.3390/agronomy14071507
Chicago/Turabian StyleZhan, Liwei, Ling Chen, Yuxuan Hou, Yuxiang Zeng, and Zhijuan Ji. 2024. "Bakanae Disease Resistance in Rice: Current Status and Future Considerations" Agronomy 14, no. 7: 1507. https://doi.org/10.3390/agronomy14071507
APA StyleZhan, L., Chen, L., Hou, Y., Zeng, Y., & Ji, Z. (2024). Bakanae Disease Resistance in Rice: Current Status and Future Considerations. Agronomy, 14(7), 1507. https://doi.org/10.3390/agronomy14071507






