Critical Leaf Magnesium Thresholds for Growth, Chlorophyll, Leaf Area, and Photosynthesis in Rice (Oryza sativa L.) and Cucumber (Cucumis sativus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Analyses of Gas Exchange
2.3. Determination of Chlorophyll, Mg Concentrations, and Biomass
2.4. Statistical Analysis
3. Results
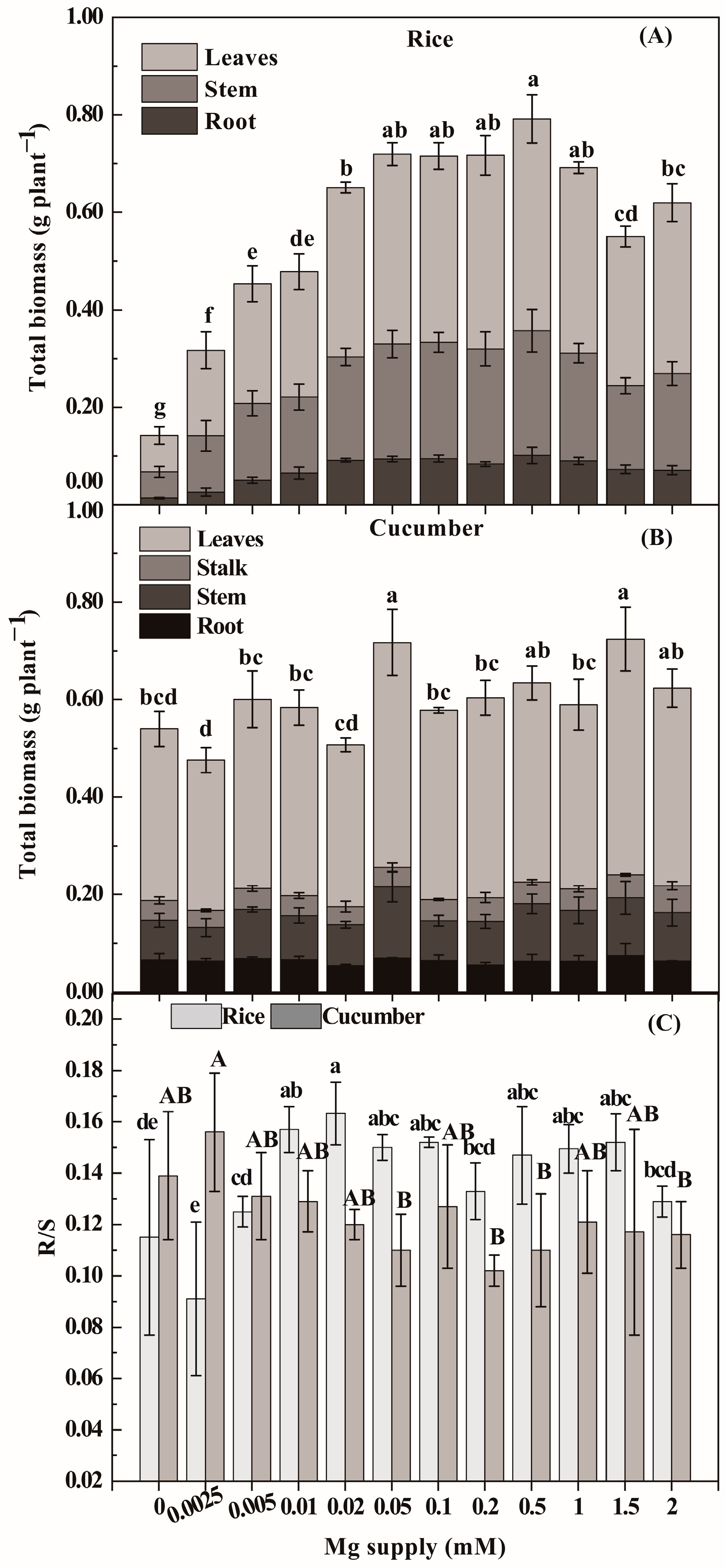
3.1. Phenotypic Changes and Plant Dry Matter Formation
3.2. Mg Concentration in Leaves at Different Leaf Positions
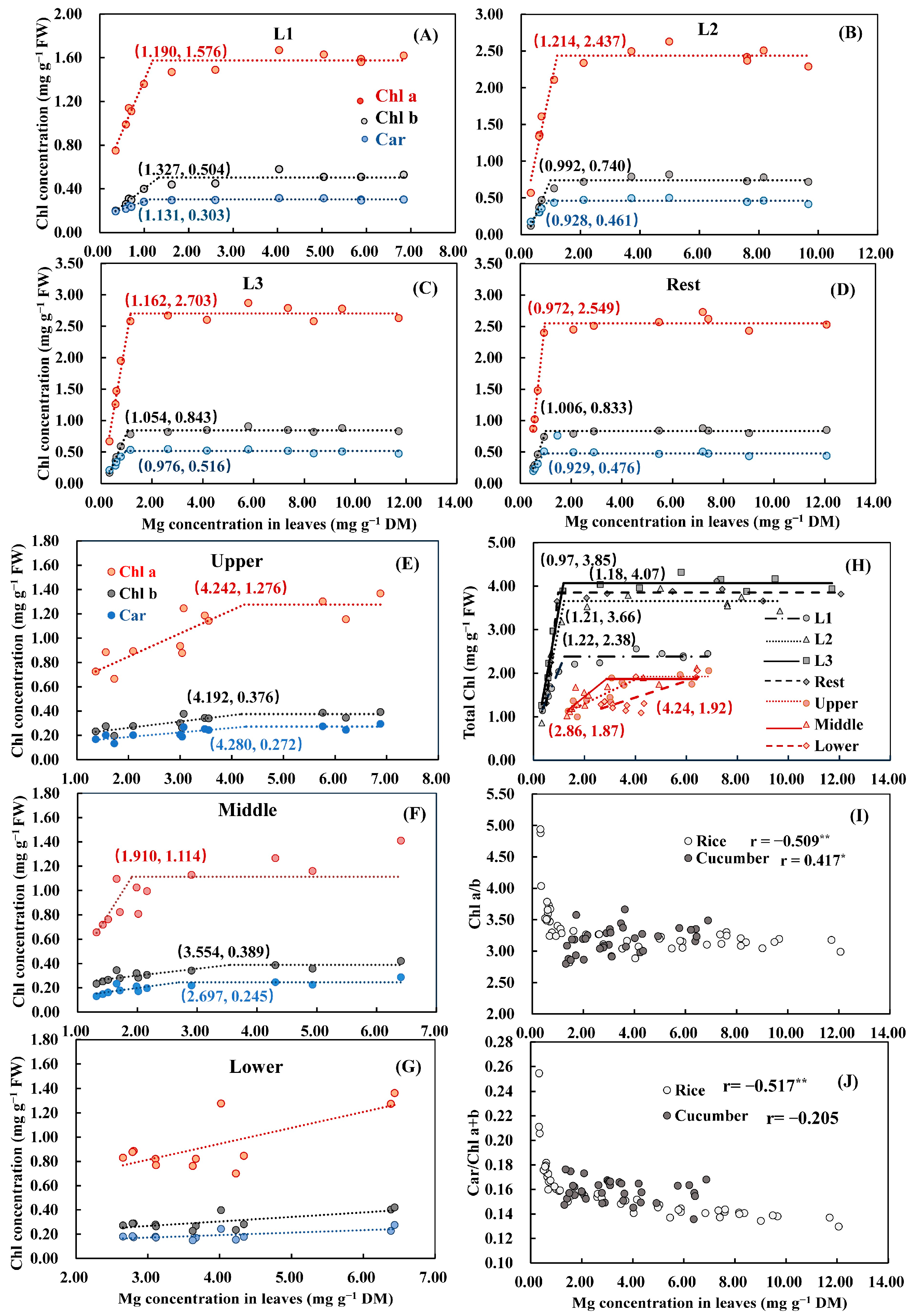
3.3. Chlorophyll Concentration at Different Leaf Positions
3.4. Leaf Area and Specific Leaf Mass
3.5. Gas Exchange Parameters
3.6. The Critical Mg Threshold for Biomass, Chl a+b, Pn, and LA
4. Discussion
4.1. Critical Mg Thresholds for Chlorophyll Were Higher Than Those for Photosynthesis, Leaf Area, and Biomass, Suggesting That Chlorophyll Is More Sensitive to Mg Deficiency Stress
4.2. The Upper Young Mature Leaves Had a Higher Chlorophyll Critical Mg Threshold, Whereas Visible Symptoms of Mg Deficiency Were Predominantly Found in Mid-Aged Leaves with a Higher Rate of Mg Remobilization
4.3. In Terms of Chl, LA, Pn, and Biomass, Cucumber Was More Sensitive to Mg Deficiency Stress Compared to Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of china. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Verbruggen, N. Physiological characterization of mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems i and ii. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J. Exp. Bot. 1994, 45, 1251–1257. [Google Scholar] [CrossRef]
- Farzadfar, S.; Zarinkamar, F.; Hojati, M. Magnesium and manganese affect photosynthesis, essential oil composition and phenolic compounds of Tanacetum parthenium. Plant Physiol. Biochem. 2017, 112, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Xu, H.; Wang, Y.; Ye, X.; Lai, N.; Huang, Z.; Yang, L.; Li, Y.; Chen, L.-S.; Guo, J. Differences in morphological and physiological features of citrus seedlings are related to Mg transport from the parent to branch organs. BMC Plant Biol. 2021, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.X.; Tibbitts, T.W. Growth, carbon dioxide exchange and mineral accumulation in potatoes grown at different magnesium concentrations. J. Plant Nutr. 1992, 15, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jákli, M.; Tränkner, M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: A systematic review and meta-analysis on 70 years of research. Front. Plant Sci. 2019, 10, 766. [Google Scholar] [CrossRef]
- Ishfaq, M.; Zhong, Y.; Wang, Y.; Li, X. Magnesium limitation leads to transcriptional down-tuning of auxin synthesis, transport, and signaling in the tomato root. Front. Plant Sci. 2021, 12, 802399. [Google Scholar] [CrossRef]
- Britz, S.J.; Adamse, P. Uv-b induced increase in specific leaf weight of cucumber as a consequence of increased starch content. Photochem. Photobiol. 1994, 60, 116–119. [Google Scholar] [CrossRef]
- Zhang, M.; Geng, Y.; Cao, G.; Wang, L.; Wang, M.; Stephano, M.F. Magnesium accumulation, partitioning and remobilization in spring maize (Zea mays L.) under magnesium supply with straw return in northeast china. J. Sci. Food Agric. 2020, 100, 2568–2578. [Google Scholar] [CrossRef] [PubMed]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Hermans, C.; Bourgis, F.; Faucher, M.; Strasser, R.J.; Delrot, S.; Verbruggen, N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Jiang, J.; Xie, H.; Bai, M.; Xu, Q.; Wang, X.; Yu, X.; Chen, Z.; Guan, Y. Metabolomics reveals distinct carbon and nitrogen metabolic responses to magnesium deficiency in leaves and roots of soybean [Glycine max (linn.) merr.]. Front. Plant Sci. 2017, 8, 2091. [Google Scholar] [CrossRef] [PubMed]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2012; Volume 24. [Google Scholar]
- Ding, Y.; Luo, W.; Xu, G. Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Prado, R.d.M.; Rozane, D.E. Leaf analysis as diagnostic tool for balanced fertilization in tropical fruits. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–141. [Google Scholar]
- Veloso, C.A.C.; Araujo, S.M.B.; Viégas, I.d.J.M.; Rodrigues, J.E.L.F. Amostragem e diagnose foliar. In Recomendac ¸~oes de Calagem e Adubac ¸~ao Para o Estado do Para; Brasil, M.S.C., Viegas, I.J.M., Eds.; Embrapa: Brasília, Brazil, 2020; Volume 2, pp. 247–250. [Google Scholar]
- Trankner, M.; Jaghdani, S.J. Minimum magnesium concentrations for photosynthetic efficiency in wheat and sunflower seedlings. Plant Physiol. Biochem. 2019, 144, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Ulrich, A. Effects of magnesium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1974, 54, 379–381. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Welch, R.M.; Mayland, H.F.; Grunes, D.L. Magnesium in plants: Uptake, distribution, function and utilization by man and animals. Met. Ions Biol. Syst. 1990, 26, 33–56. [Google Scholar]
- Li, C.; Qi, Y.; Zhang, J.; Yang, L.; Wang, D.; Ye, X.; Lai, N.; Tan, L.; Lin, D.; Chen, L. Magnesium-deficiency-induced alterations of gas exchange, major metabolites and key enzymes differ among roots, and lower and upper leaves of Citrus sinensis seedlings. Tree Physiol. 2017, 37, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, X.F.; Deng, C.L.; Yang, L.T.; Lai, N.W.; Guo, J.X.; Chen, L.S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Kobayashi, N.I.; Saito, T.; Iwata, N.; Ohmae, Y.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Leaf senescence in rice due to magnesium deficiency-mediated defect in transpiration rate before sugar accumulation and chlorosis. Physiol. Plant. 2012, 148, 490–501. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, W.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2017, 74, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cakmak, I.; Feng, J.; Yu, C.; Chen, X.; Xie, D.; Wu, L.; Song, Z.; Cao, J.; He, Y. Magnesium deficiency reduced the yield and seed germination in wax gourd by affecting the carbohydrate translocation. Front. Plant Sci. 2020, 11, 797. [Google Scholar] [CrossRef]
- Wang, Z.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2019, 10, 1727. [Google Scholar] [CrossRef]
- Meng, X.S.; Bai, S.; Wang, S.Y.; Pan, Y.H.; Chen, K.H.; Xie, K.L.; Wang, M.; Guo, S.W. The sensitivity of photosynthesis to magnesium deficiency differs between rice (Oryza sativa L.) and cucumber (Cucumis sativus L.). Front. Plant Sci. 2023, 14, 1164866. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Hansen, T.H.; Laursen, K.H.; Persson, D.P.; Pedas, P.; Husted, S.; Schjoerring, J.K. Micro-scaled high-throughput digestion of plant tissue samples for multi-elemental analysis. Plant Methods 2009, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.L.; Liu, D.Y.; Liu, B.; Wu, C.C.; Liu, C.Y.; Wang, X.Z.; Zou, C.Q.; Chen, X.P. Critical leaf magnesium concentrations for adequate photosynthate production of soilless cultured cherry tomato-interaction with potassium. Agronomy 2020, 10, 1863. [Google Scholar] [CrossRef]
- Yang, G.-H.; Yang, L.-T.; Jiang, H.-X.; Li, Y.; Wang, P.; Chen, L.-S. Physiological impacts of magnesium-deficiency in citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Tränkner, M.; Jákli, B.; Tavakol, E.; Geilfus, C.M.; Cakmak, I.; Dittert, K.; Senbayram, M. Magnesium deficiency decreases biomass water-use efficiency and increases leaf water-use efficiency and oxidative stress in barley plants. Plant Soil 2016, 406, 409–423. [Google Scholar] [CrossRef]
- Hermans, C.; Conn, S.J.; Chen, J.; Xiao, Q.; Verbruggen, N. An update on magnesium homeostasis mechanisms in plants. Metallomics 2013, 5, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Craciun, A.; Inzé, D.; Verbruggen, N. Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol. 2010, 187, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Tanoi, K.; Kobayashi, I.N. Leaf senescence by magnesium deficiency. Plants 2015, 4, 756–772. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Liao, L.L.; Liu, S.; Nie, M.M.; Li, J.; Zhang, L.D.; Ma, J.F.; Chen, Z.C. Magnesium deficiency triggers SGR-mediated chlorophyll degradation for magnesium remobilization. Plant Physiol. 2019, 181, 262–275. [Google Scholar] [CrossRef]
- Billard, V.; Maillard, A.; Coquet, L.; Jouenne, T.; Cruz, F.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A.; Etienne, P. Mg deficiency affects leaf mg remobilization and the proteome in brassica napus. Plant Physiol. Biochem. 2016, 107, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; International Potash Institute: Zug, Switzerland, 1987. [Google Scholar]
- Dow, A.I.; Roberts, S. Proposal: Critical nutrient ranges for crop diagnosis1. Agron. J. 1982, 74, 401–403. [Google Scholar] [CrossRef]
- Bielczynski, L.W.; Łącki, M.K.; Hoefnagels, I.; Gambin, A.; Croce, R. Leaf and plant age affects photosynthetic performance and photoprotective capacity. Plant Physiol. 2017, 175, 1634–1648. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.; Pan, Y.; Meng, X.; Wang, M.; Guo, S. Critical Leaf Magnesium Thresholds for Growth, Chlorophyll, Leaf Area, and Photosynthesis in Rice (Oryza sativa L.) and Cucumber (Cucumis sativus L.). Agronomy 2024, 14, 1508. https://doi.org/10.3390/agronomy14071508
Xie K, Pan Y, Meng X, Wang M, Guo S. Critical Leaf Magnesium Thresholds for Growth, Chlorophyll, Leaf Area, and Photosynthesis in Rice (Oryza sativa L.) and Cucumber (Cucumis sativus L.). Agronomy. 2024; 14(7):1508. https://doi.org/10.3390/agronomy14071508
Chicago/Turabian StyleXie, Kailiu, Yonghui Pan, Xusheng Meng, Min Wang, and Shiwei Guo. 2024. "Critical Leaf Magnesium Thresholds for Growth, Chlorophyll, Leaf Area, and Photosynthesis in Rice (Oryza sativa L.) and Cucumber (Cucumis sativus L.)" Agronomy 14, no. 7: 1508. https://doi.org/10.3390/agronomy14071508
APA StyleXie, K., Pan, Y., Meng, X., Wang, M., & Guo, S. (2024). Critical Leaf Magnesium Thresholds for Growth, Chlorophyll, Leaf Area, and Photosynthesis in Rice (Oryza sativa L.) and Cucumber (Cucumis sativus L.). Agronomy, 14(7), 1508. https://doi.org/10.3390/agronomy14071508





