Simultaneous Biofortification: Interaction between Zinc and Selenium Regarding Their Accumulation in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description of Field Experiment
2.2. Experimental Design
2.3. Sample Collection and Analysis
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Zn and Se Contents in Grain, Husk, and Straw
3.2. Transfer Factors
3.3. Zn or Se Recovery
3.4. Effects of Application of Zn and Se on Other Elements in Wheat Grain
3.5. Estimation of Daily Intake
4. Discussion
4.1. Accumulation and Translocation of Zn in Different Parts of Wheat
4.2. Accumulation and Translocation of Se in Different Parts of Wheat
| Form | Dosage (g Zn hm−2) | Method | Application Time and Stage | Control (mg kg−1) | Grain Content (mg kg−1) | Increasing Rates (%) | Grain Recovery (%) | References |
|---|---|---|---|---|---|---|---|---|
| ZnSO4·7H2O | 2.71 | Foliar | Two times, at the end of anthesis and 1 week later | 29.01 | 58.30 | 100.97 | 5.93 | [44] |
| ZnSO4·7H2O | 2.71 | Foliar | Two times, at the end of anthesis and 1 week later | 24.10 | 77.90 | 223.24 | 7.00 | |
| ZnSO4·7H2O | 0.79 | Foliar | One time, at the end of anthesis | 15.90 | 20.50 | 28.93 | 4.36 | [45] |
| ZnSO4·7H2O | 0.79 | Foliar | One time, at the end of anthesis | 26.40 | 32.60 | 23.48 | 6.76 | |
| ZnSO4·7H2O | 11.30 | Soil | Before planting | 27.40 | 30.50 | 11.31 | 0.18 | [7] |
| ZnSO4·7H2O | 1.81 | Foliar | Two times, at heading and milk stages | 27.40 | 48.00 | 75.18 | 5.89 | |
| ZnSO4·7H2O | 1.45 | Foliar | Two times, at early milk stage, and the second spraying occurred a week later | 21.80 | 43.10 | 97.71 | 9.87 | [46] |
| ZnSO4·7H2O | 1.01 | Foliar | Three times, at tillering, booting and milking stages | 19.75 | 39.91 | 102.08 | 23.13 | [47] |
| ZnSO4·7H2O | 1.01 | Foliar | Three times, at tillering, booting and milking stages | 30.69 | 42.57 | 38.71 | 17.47 | |
| ZnSO4·7H2O | 1.01 | Foliar | Three times, at tillering, booting and milking stages | 28.75 | 45.41 | 57.95 | 21.90 | |
| ZnSO4·7H2O | 1.01 | Foliar | Three times, at tillering, booting and milking stages | 17.53 | 48.68 | 177.70 | 32.05 | |
| ZnSO4·7H2O | 9.60 | Foliar | Four times, at jointing, booting, flowering and grain filling stages | 24.19 | 36.86 | 52.38 | 0.96 | [48] |
| ZnSO4·7H2O | 11.40 | Soil | Before planting | 39.80 | 42.37 | 6.46 | 0.18 | [49] |
| ZnSO4·7H2O | 0.91 | Foliar | Two times, at the start of flowering and two weeks later | 34.45 | 46.89 | 36.11 | 6.33 | |
| ZnSO4·H2O | 11.00 | Soil | Before planting | 37.17 | 39.03 | 4.99 | 0.41 | This study |
| ZnSO4·H2O | 11.00 | Soil | Before planting | 37.41 | 38.30 | 2.38 | 0.06 | |
| ZnSO4·H2O | 1.74 | Foliar | Three times, at jointing, booting and filling stages | 39.03 | 41.66 | 6.74 | 6.70 | |
| ZnSO4·H2O | 1.74 | Foliar | Three times, at jointing, booting and filling stages | 38.30 | 46.02 | 20.17 | 3.94 | |
| ZnSO4·H2O | 2.61 | Foliar | Three times, at jointing, booting and filling stages | 39.03 | 42.44 | 8.75 | 2.13 | |
| ZnSO4·H2O | 2.61 | Foliar | Three times, at jointing, booting and filling stages | 38.30 | 56.98 | 48.77 | 4.49 | |
| ZnSO4·H2O | 3.48 | Foliar | Three times, at jointing, booting and filling stages | 39.03 | 44.24 | 13.35 | 3.78 | |
| ZnSO4·H2O | 3.48 | Foliar | Three times, at jointing, booting and filling stages | 38.30 | 64.30 | 67.89 | 4.71 | |
| Soil | Coefficient of variation (CV) | 0.18 | 0.16 | 0.27 | 0.02 | |||
| Foliar | Coefficient of variation (CV) | 0.21 | 0.30 | 0.70 | 0.77 | |||
| Form | Dosage (g Se hm−2) | Method | Application Time and Stage | Control (mg kg−1) | Grain Content (mg kg−1) | Increasing Times | Grain Recovery (%) | References |
|---|---|---|---|---|---|---|---|---|
| Na2SeO3 | 27.41 | Foliar | Two times, at the end of anthesis and 1 week later | 0.03 | 0.74 | 23.7 | 12.98 | [44] |
| Na2SeO3 | 27.41 | Foliar | Two times, at the end of anthesis and 1 week later | 0.02 | 0.50 | 24.0 | 7.89 | |
| Nano-Se | 15 | Foliar | One time, at flowering stage | 0.05 | 0.24 | 3.6 | 9.20 | [50] |
| Organic-Se | 15 | Foliar | One time, at flowering stage | 0.05 | 0.28 | 4.4 | 11.00 | |
| Na2SeO3 | 30 | Foliar | One time, at flowering stage | 0.05 | 0.25 | 4.0 | 4.90 | |
| Na2SeO3 | 30 | Foliar | One time, at flowering stage | 0.07 | 0.38 | 4.4 | 6.65 | [51] |
| Na2SeO4 | 30 | Foliar | One time, at flowering stage | 0.07 | 0.29 | 3.1 | 4.77 | |
| SeMet | 30 | Foliar | One time, at flowering stage | 0.07 | 0.36 | 4.1 | 6.67 | |
| Nano-Se | 30 | Foliar | One time, at flowering stage | 0.07 | 0.16 | 1.3 | 2.28 | |
| Na2SeO4 | 10 | Foliar | One time, at the start of flowering | 0.03 | 0.10 | 2.4 | 3.00 | [49] |
| Na2SeO3 | 10 | Foliar | One time, at the end of tillering stage | 0.07 | 0.15 | 1.3 | 1.63 | [52] |
| Na2SeO3 | 20 | Foliar | One time, at the end of tillering stage | 0.07 | 0.25 | 2.8 | 1.69 | |
| Na2SeO3 | 40 | Foliar | One time, at the end of tillering stage | 0.07 | 0.43 | 5.5 | 1.68 | |
| Na2SeO4 | 10 | Foliar | One time, at the end of tillering stage | 0.07 | 0.27 | 3.0 | 3.53 | |
| Na2SeO4 | 20 | Foliar | One time, at the end of tillering stage | 0.07 | 0.82 | 11.3 | 6.87 | |
| Na2SeO4 | 40 | Foliar | One time, at the end of tillering stage | 0.07 | 1.38 | 19.8 | 5.90 | |
| Na2SeO3 | 15 | Foliar | One time, at filling stage | 0.05 | 0.18 | 2.5 | 6.20 | [53] |
| Na2SeO3 | 30 | Foliar | One time, at filling stage | 0.05 | 0.24 | 3.7 | 4.80 | |
| Na2SeO3 | 45 | Foliar | One time, at filling stage | 0.05 | 0.30 | 4.9 | 4.50 | |
| Na2SeO3 | 60 | Foliar | One time, at filling stage | 0.05 | 0.43 | 7.4 | 4.70 | |
| Na2SeO3 | 15 | Foliar | Three times, at jointing, booting and filling stages | 0.09 | 0.21 | 1.3 | 5.29 | This study |
| Na2SeO3 | 15 | Foliar | Three times, at jointing, booting and filling stages | 0.08 | 0.26 | 2.4 | 8.04 | |
| Na2SeO3 | 30 | Foliar | Three times, at jointing, booting and filling stages | 0.09 | 0.45 | 4.0 | 10.62 | |
| Na2SeO3 | 30 | Foliar | Three times, at jointing, booting and filling stages | 0.08 | 0.50 | 5.3 | 9.13 | |
| Coefficient of variation (CV) | 0.27 | 0.75 | 1.01 | 0.54 | ||||
4.3. Interactive Effects between Zn and Se in Wheat
4.4. Accumulation of Other Elements in Wheat Grain
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Ntoupa, P.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phyto. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Leff, B.; Ramankutty, N.; Foley, J.A. Geographic distribution of major crops across the world. Glob. Biogeochem. Cycle 2004, 18, 1–27. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.H.; Li, F.; Li, K.; Yang, N.; Yang, Y.; Huang, D.; Liang, D.; Zhao, H.; Mao, H.; et al. Grain iron and zinc concentrations of wheat and their relationships to yield in major wheat production areas in China. Field Crop. Res. 2014, 156, 151–160. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.E.; Wang, Z.H.; Li, F.C.; Li, K.Y.; Yang, N.; Wang, S.; Wang, H.; Gang, H.E.; Dai, J. Selenium content of wheat grain and its regulation in different wheat production regions of China. Sci. Agric. Sin. 2016, 49, 1715–1728. (In Chinese) [Google Scholar]
- GH/T 1135-2017; Selenium-Enriched Agricultural Products. All China Federation of Supply and Marketing Cooperatives: Beijing, China, 2017. (In Chinese)
- Zhao, A.Q.; Tian, X.H.; Cao, Y.X.; Lu, X.C.; Liu, T. Comparison of soil and foliar zinc application for enhancing grain zinc content of wheat when grown on potentially zinc-deficient calcareous soils. J. Sci. Food Agric. 2014, 94, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Banuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.C.; Oliveira, K.; Ribeiro, M.M.; Pelica, J.; Pataco, I.; Ramalho, J.C.; Leitão, A.E.; Almeida, A.S.; Campos, P.S.; Ribeiro-Barros, A.I.; et al. Selenium biofortification of rice grains and implications on macronutrients quality. J. Cereal Sci. 2018, 81, 22–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Ye, Y.; Karim, M.R.; Xue, Y.; Yan, P.; Meng, Q.; Cui, Z.; Cakmak, I.; Zhang, F.; et al. Zinc biofortification of wheat through fertilizer applications in different locations of China. Field Crop. Res. 2012, 125, 1–7. [Google Scholar] [CrossRef]
- Germ, M.; Pongrac, P.; Regvar, M.; Vogel-Mikuš, K.; Stibilj, V.; Jaćimović, R.; Kreft, I. Impact of double Zn and Se biofortification of wheat plants on the element concentrations in the grain. Plant Soil Environ. 2013, 59, 316–321. [Google Scholar] [CrossRef]
- Reynolds-Marzal, M.D.; Rivera-Martín, A.M.; Rodrigo, S.M.; Santamaria, O.; Poblaciones, M.J. Biofortification of forage peas with combined application of selenium and zinc under Mediterranean conditions. J. Soil Sci. Plant Nutr. 2021, 21, 286–300. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, L.; Xin, Z.; Zhao, L.; An, X.; Hu, Q. Effect of foliar application of zinc, selenium, and iron fertilizers on nutrients concentration and yield of rice grain in China. J. Agric. Food Chem. 2008, 56, 2079–2084. [Google Scholar] [CrossRef]
- Mangueze, A.V.D.J.; Pessoa, M.F.G.; Silva, M.J.; Ndayiragije, A.; Magaia, H.E.; Cossa, V.S.I.; Reboredo, F.H.; Carvalho, M.L.; Santos, J.P.; Guerra, M.; et al. Simultaneous zinc and selenium biofortification in rice. Accumulation, localization and implications on the overall mineral content of the flour. J. Cereal Sci. 2018, 82, 34–41. [Google Scholar] [CrossRef]
- Guilherme, A.S.; Jonathan, J.H.; Janice, G.C.; Michael, A.R.; Júlio, C.A.; Luiz, R.G.G.; Leon, V.K.; Li, L. Genotypic variation of zinc and selenium concentration in grains of Brazilian wheat lines. Plant Sci. 2014, 224, 27–35. [Google Scholar]
- Li, J.F.; Hu, R.Q.; Song, Y.Q.; Shi, J.L.; Bhattacharya, S.C.; Abdul Salam, P. Assessment of sustainable energy potential of non-plantation biomass resources in China. Biomass Bioenerg. 2005, 29, 167–177. [Google Scholar]
- Food and Agriculture Organization of the United Nations. 2013. Available online: http://www.fao.org/faostat/en/#data/CC (accessed on 1 March 2023).
- WS/T 578.3-2017; Chinese Dietary Reference Intakes-Part 3: Trace Element. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2017. (In Chinese)
- Chinese Nutrition Society. Dietary Guidelines for Chinese Residents; People’s Medical Publishing House: Beijing, China, 2022; p. 343. ISBN 978-7-117-31404-6. [Google Scholar]
- Singh, S.; Kaur, J.; Ram, H.; Singh, J.; Kaur, S. Agronomic bio-fortification of wheat (Triticum aestivum L.) to alleviate zinc deficiency in human being. Rev. Environ. Sci. Bio-Technol. 2023, 22, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Alahmadi, T.A.; Ansari, M.J.; Khan, Z.; Khan, H. Agro-biofortification of maize with selenium for higher grain selenium contents and productivity. S. Afr. J. Bot. 2024, 168, 253–259. [Google Scholar] [CrossRef]
- Yin, H.J.; Gao, X.P.; Stomph, T.; Li, L.J.; Zhang, F.S.; Zou, C.Q. Zinc concentration in rice (Oryza sativa L.) grains and allocation in plants as affected by different zinc fertilization strategies. Commun. Soil Sci. Plant Anal. 2016, 47, 761–768. [Google Scholar] [CrossRef]
- Lian, J.P.; Cheng, L.P.; Wang, X.; Chen, Y.L.; Deng, C.Y.; Wang, Y.; Pan, J.Q.; Shohag, M.J.I.; Xin, X.P.; He, Z.L.; et al. Bespoke ZnO NPs synthesis platform to optimize their performance for improving the grain yield, zinc biofortification, and Cd mitigation in wheat. Acs Sustain. Chem. Eng. 2024, 12, 716–727. [Google Scholar] [CrossRef]
- Tan, J.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Li, H.; McGrath, S.P.; Zhao, F. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phyto. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- Renkema, H.; Koopmans, A.; Kersbergen, L.; Kikkert, J.; Hale, B.; Berkelaar, E. The effect of transpiration on selenium uptake and mobility in durum wheat and spring canola. Plant Soil 2012, 354, 239–250. [Google Scholar] [CrossRef]
- Yuan, Z.; Long, W.; Liang, T.; Zhu, M.; Zhu, A.; Luo, X.; Fu, L.; Hu, Z.; Zhu, R.; Wu, X. Effect of foliar spraying of organic and inorganic selenium fertilizers during different growth stages on selenium accumulation and speciation in rice. Plant Soil 2023, 486, 87–101. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Guo, A.; Qi, Z.; Chen, J.; Huang, T.; Yang, Z.; Gao, Z.; Sun, M.; Wang, J. Combined foliar and soil selenium fertilizer increased the grain yield, quality, total Se, and organic Se content in naked oats. J. Cereal Sci. 2021, 100, 103265. [Google Scholar] [CrossRef]
- Zou, C.; Zhai, R.; Huang, K.; Hua, T.; Debo, Z.; Aihua, H.; Xinxing, W.; Runxiu, M.O.; Faqian, X.; Hui, W.; et al. Effect of foliar application of selenium fertilizer on yield, selenium content and heavy metal contents of waxy maize. Asian Agric. Res. 2020, 12, 40–44+48. [Google Scholar]
- Xiao, T.; Qiang, J.; Sun, H.; Luo, F.; Li, X.; Yan, Y. Overexpression of wheat selenium-binding protein gene TaSBP-A enhances plant growth and grain selenium accumulation under spraying sodium selenite. Int. J. Mol. Sci. 2024, 25, 7007. [Google Scholar] [CrossRef]
- Galinha, C.; Sánchez-Martínez, M.; Pacheco, A.M.G.; Freitas, M.D.C.; Coutinho, J.; Maçãs, B.; Almeida, A.S.; Pérez-Corona, M.T.; Madrid, Y.; Wolterbeek, H.T. Characterization of selenium-enriched wheat by agronomic biofortification. J. Food Sci. Technol. 2015, 52, 4236–4245. [Google Scholar] [CrossRef]
- Qu, L.L.; Xu, J.Y.; Dai, Z.H.; Elyamine, A.M.; Huang, W.X.; Han, D.; Dang, B.J.; Xu, Z.C.; Jia, W. Selenium in soil-plant system: Transport, detoxification and bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]
- Di, X.; Qin, X.; Zhao, L.; Liang, X.; Xu, Y.; Sun, Y.; Huang, Q. Selenium distribution, translocation and speciation in wheat (Triticum aestivum L.) after foliar spraying selenite and selenate. Food Chem. 2022, 400, 134077. [Google Scholar] [CrossRef]
- World Health Organization and Food and Agriculture Organization of the United Nations. 2004. Available online: https://www.researchgate.net/publication/236035586_Vitamin_and_mineral_requirements_in_human_nutrition (accessed on 1 March 2023).
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Fei, P.W.; Wu, T.Q.; Li, Y.F.; Qu, C.Y.; Li, Y.N.; Shi, J.L.; Tian, X.H. Combined foliar application of zinc sulphate and selenite affects the magnitude of selenium biofortification in wheat (Triticum aestivum L.). Food Energy Secur. 2022, 11, 342. [Google Scholar] [CrossRef]
- Ivanović, D.; Dodig, D.; Đurić, N.; Kandić, V.; Tamindžić, G.; Nikolić, N.; Savić, J. Zinc biofortification of bread winter wheat grain by single zinc foliar application. Cereal Res. Commun. 2021, 49, 673–679. [Google Scholar] [CrossRef]
- Yu, B.; Liu, Y.; Chen, X.; Cao, W.; Ding, T.; Zou, C. Foliar zinc application to wheat may lessen the zinc deficiency burden in rural Quzhou, China. Front. Nutr. 2021, 8, 697817. [Google Scholar] [CrossRef] [PubMed]
- Kiran, A.; Wakeel, A.; Sultana, R.; Khalid, A.; Qurrat-ul-Ain; Mubaraka, R.; Shahzad, A.N.; Khan, S.J.; Noor, M. Concentration and localization of Fe and Zn in wheat grain as affected by its application to soil and foliage. Bull. Environ. Contam. Toxicol. 2021, 106, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, Y.; Wang, C.; Ma, G.; Lv, J.; Liu, J.; Guo, T. Distribution and accumulation of zinc and nitrogen in wheat grain pearling fractions in response to foliar zinc and soil nitrogen applications. J. Integr. Agric. 2021, 20, 3277–3288. [Google Scholar] [CrossRef]
- Reynolds-Marzal, D.; Rivera-Martin, A.; Santamaria, O.; Poblaciones, M.J. Combined selenium and zinc biofortification of bread-making wheat under Mediterranean conditions. Plants 2021, 10, 1209. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.F.; Gao, Z.Q.; Sun, M.; Dong, S.F.; Ren, A.X.; Hou, F.F.; Yin, X.B. Effects of exogenous selenium fertilizer on grain yield and selenium accumulation in wheat. J. Agric. Univ. Hebei 2020, 43, 17–22. (In Chinese) [Google Scholar]
- Wang, Q.; Yu, Y.; Li, J.; Wan, Y.; Huang, Q.; Guo, Y.; Li, H. Effects of different forms of selenium fertilizers on Se accumulation, distribution, and residual effect in winter wheat-summer maize rotation system. J. Agric. Food Chem. 2017, 65, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Poblaciones, M.J.; Rodrigo, S.; Santamaría, O.; Chen, Y.; McGrath, S.P. Agronomic selenium biofortification in Triticum durum under Mediterranean conditions: From grain to cooked pasta. Food Chem. 2014, 146, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Jia, L.L.; Jia, Y.H.; Erken, D.L.X.T.; Liu, J.; Shi, S.B. Effect of selenium application on yield and selenium content of spring wheat under different planting density. J. Triticeae Crops. 2018, 38, 834–840. (In Chinese) [Google Scholar]
- Poblaciones, M.J.; Rengel, Z. Combined foliar selenium and zinc biofortification in field pea (Pisum sativum): Accumulation and bioavailability in raw and cooked grains. Crop Pasture Sci. 2017, 68, 265. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Malik, J.A.; Goel, S.; Kaur, N.; Sharma, S.; Singh, I.; Nayyar, H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012, 77, 242–248. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.J.; Minlan, L.; Guoqing, W. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotox. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Ma, J.F. Node-controlled allocation of mineral elements in Poaceae. Curr. Opin. Plant Biol. 2017, 39, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Na, G.; Salt, D.E. The role of sulfur assimilation and sulfur-containing compounds in trace element homeostasis in plants. Environ. Exp. Bot. 2011, 72, 18–25. [Google Scholar] [CrossRef]
- Sors, T.G.; Ellis, D.R.; Na, G.N.; Lahner, B.; Lee, S.; Leustek, T.; Pickering, I.J.; Salt, D.E. Analysis of sulfur and selenium assimilation in astragalus plants with varying capacities to accumulate selenium. Plant J. 2005, 42, 785–797. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium metabolism in plants. BBA—Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Asp. Med. 2005, 26, 235–244. [Google Scholar] [CrossRef]
- Lončarić, Z.; Ivezić, V.; Kerovec, D.; Rebekić, A. Foliar zinc-selenium and nitrogen fertilization affects content of Zn, Fe, Se, P, and Cd in wheat grain. Plants 2021, 10, 1549. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C.; Luo, Z.; Zhu, H.; Wang, S.; Zhu, Q.; Huang, D.; Zhang, Y.; Xiong, J.; He, Y. Foliar application of Zn can reduce Cd concentrations in rice (Oryza sativa L.) under field conditions. Environ. Sci. Pollut. Res. 2018, 25, 29287–29294. [Google Scholar] [CrossRef]
- Imtiaz, M.; Alloway, B.J.; Shah, K.H.; Siddiqui, S.H.; Memon, M.Y.; Aslam, M.; Khan, P. Zinc nutrition of wheat: II: Interaction of zinc with other trace elements. Asian J. Plant Sci. 2003, 2, 156–160. [Google Scholar] [CrossRef]
- Shahriaripour, R.; Tajabadipour, A. Zinc nutrition of pistachio: Interaction of zinc with other trace elements. Commun. Soil Sci. Plant Anal. 2010, 41, 1885–1888. [Google Scholar] [CrossRef]
- Zembala, M.; Filek, M.; Walas, S.; Mrowiec, H.; Kornaś, A.; Miszalski, Z.; Hartikainen, H. Effect of selenium on macro- and microelement distribution and physiological parameters of rape and wheat seedlings exposed to cadmium stress. Plant Soil 2010, 329, 457–468. [Google Scholar] [CrossRef]

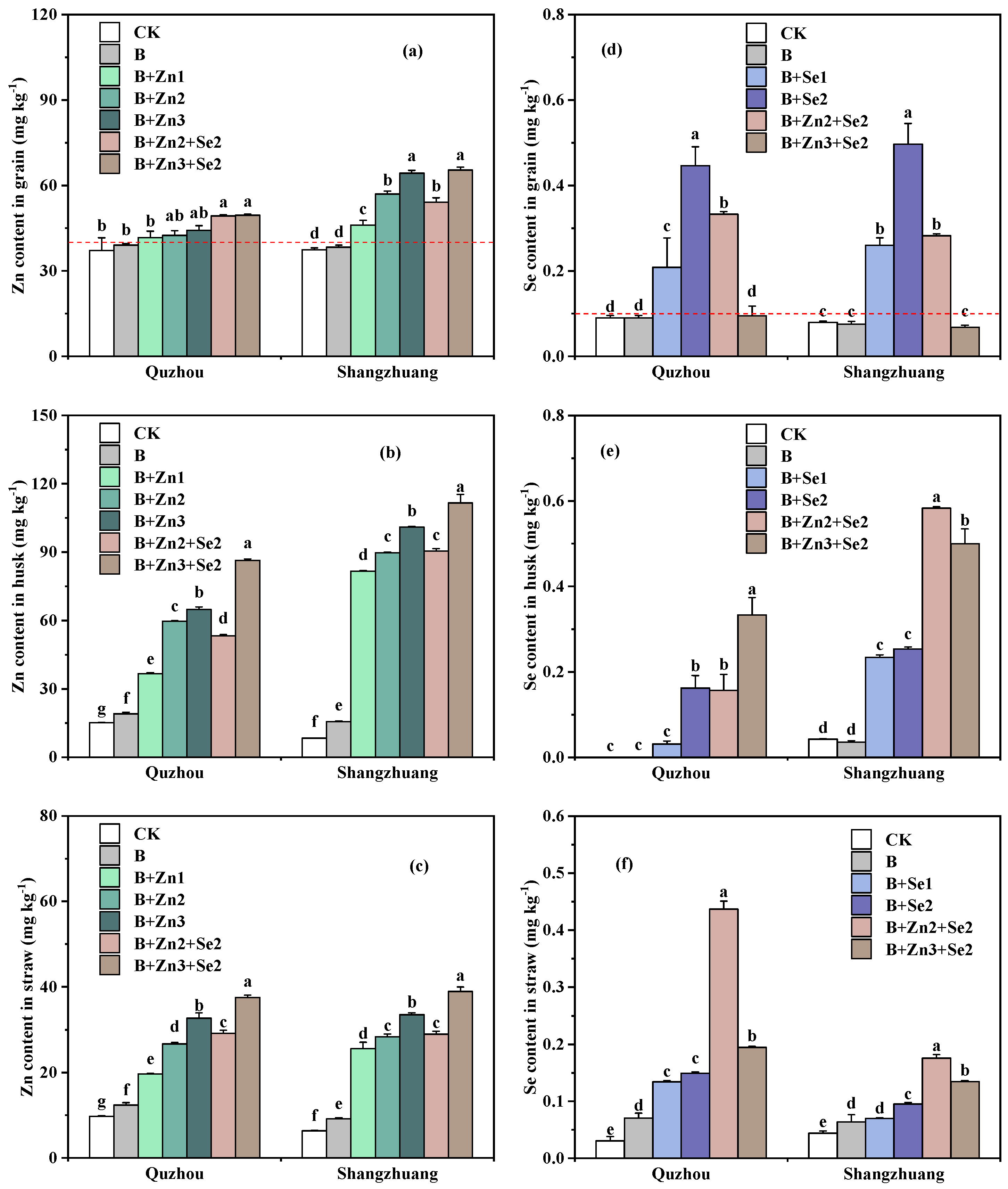
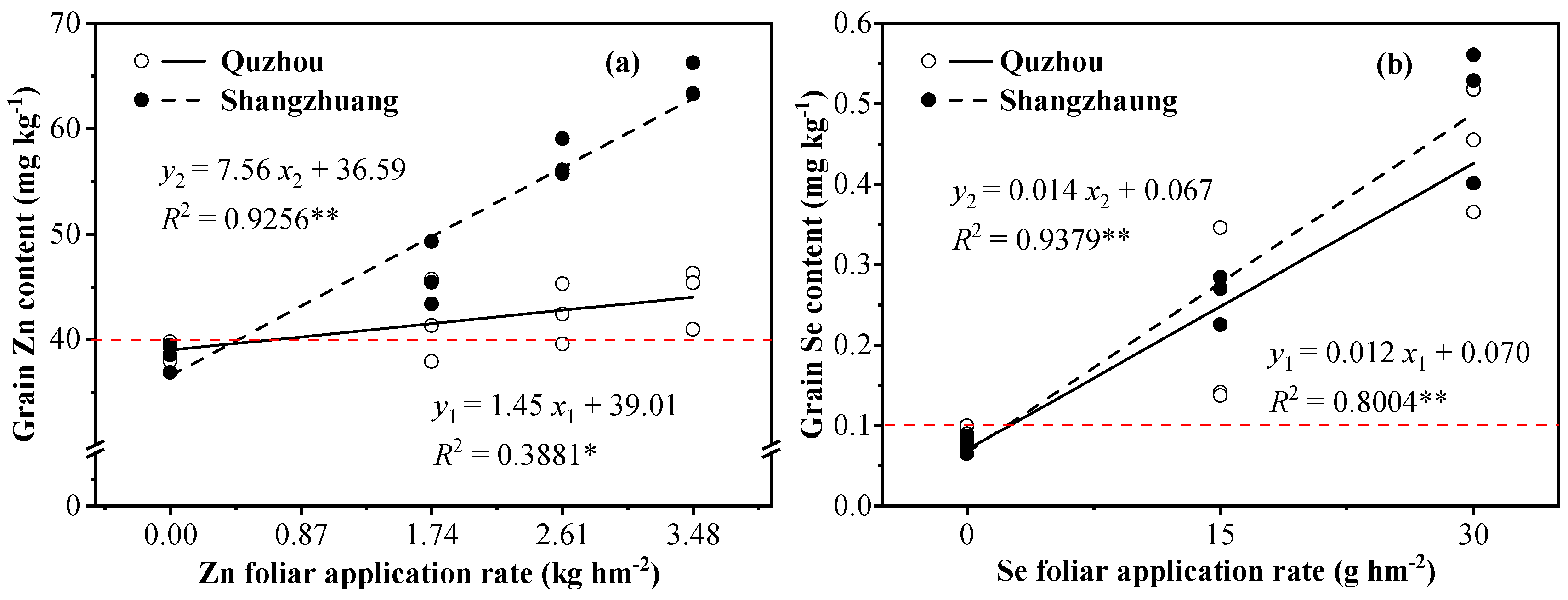


| Treatment | Quzhou | Shangzhuang | |||
|---|---|---|---|---|---|
| Grain/Husk | Husk/Straw | Grain/Husk | Husk/Straw | ||
| Zn | CK | 2.45 ± 0.3 a | 1.56 ± 0.03 d | 4.49 ± 0.10 a | 1.32 ± 0.03 d |
| B | 2.06 ± 0.07 b | 1.54 ± 0.06 d | 2.45 ± 0.08 b | 1.70 ± 0.02 c | |
| B + Zn1 | 1.14 ± 0.08 c | 1.86 ± 0.02 c | 0.56 ± 0.02 c | 3.21 ± 0.18 a | |
| B + Zn2 | 0.82 ± 0.11 cd | 2.23 ± 0.03 a | 0.63 ± 0.01 c | 3.17 ± 0.08 a | |
| B + Zn3 | 0.68 ± 0.03 d | 1.98 ± 0.04 b | 0.64 ± 0.01 c | 3.01 ± 0.03 ab | |
| B + Zn2 + Se2 | 0.93 ± 0.00 cd | 1.83 ± 0.06 c | 0.60 ± 0.01 c | 3.13 ± 0.08 ab | |
| B + Zn3 + Se2 | 0.57 ± 0.01 d | 2.31 ± 0.04 a | 0.59 ± 0.03 c | 2.87 ± 0.07 b | |
| Se | CK | - | - | 1.86 ± 0.09 a | 0.98 ± 0.08 d |
| B | - | - | 2.12 ± 0.02 a | 0.58 ± 0.07 e | |
| B + Se1 | 8.36 ± 4.49 a | 0.23 ± 0.05 c | 1.11 ± 0.05 b | 3.34 ± 0.04 b | |
| B + Se2 | 3.00 ± 0.68 ab | 1.08 ± 0.18 b | 1.96 ± 0.19 a | 2.67 ± 0.03 c | |
| B + Zn2 + Se2 | 2.48 ± 0.77 ab | 0.35 ± 0.08 c | 0.48 ± 0.01 c | 3.33 ± 0.12 b | |
| B + Zn3 + Se2 | 0.30 ± 0.08 b | 1.72 ± 0.21 a | 0.14 ± 0.02 d | 3.71 ± 0.21 a | |
| Treatment | Quzhou | Shangzhuang | |||
|---|---|---|---|---|---|
| Grain | Straw | Grain | Straw | ||
| RBZn | B | 0.41 ± 0.25 | 0.40 ± 0.15 | 0.06 ± 0.02 | 0.21 ± 0.03 |
| RFZn | Zn1 | 6.70 ± 0.57 a | 8.59 ± 1.39 a | 3.94 ± 1.44 a | 9.64 ± 1.53 a |
| Zn2 | 2.13 ± 0.69 bc | 4.80 ± 1.16 a | 4.49 ± 0.69 a | 6.68 ± 0.82 a | |
| Zn3 | 3.78 ± 0.95 b | 8.46 ± 1.93 a | 4.71 ± 1.44 a | 6.29 ± 1.04 a | |
| Zn2 + Se2 | 1.14 ± 0.20 c | 5.26 ± 0.53 a | 5.36 ± 0.07 a | 6.60 ± 1.28 a | |
| Zn3 + Se2 | 4.49 ± 0.96 ab | 8.93 ± 2.03 a | 6.88 ± 0.37 a | 9.30 ± 0.71 a | |
| RFSe | Se1 | 5.29 ± 4.55 ab | 3.01 ± 0.99 b | 8.04 ± 1.90 ab | 0.61 ± 0.09 b |
| Se2 | 10.62 ± 1.17 a | 3.47 ± 1.30 b | 9.13 ± 0.86 a | 0.82 ± 0.13 b | |
| Zn2 + Se2 | 5.34 ± 0.46 ab | 11.39 ± 0.81 a | 4.42 ± 0.81 b | 3.23 ± 0.77 a | |
| Zn3 + Se2 | 0.63 ± 0.12 b | 5.14 ± 1.27 b | 0.12 ± 0.02 c | 0.16 ± 0.03 b | |
| Site | Se | Zn | Mn | Cu | Mo | |
|---|---|---|---|---|---|---|
| Quzhou | Se | 1 | ns | ns | ns | ns |
| Zn | 1 | −0.443 * | 0.407 * | ns | ||
| Mn | 1 | −0.496 ** | ns | |||
| Cu | 1 | ns | ||||
| Mo | 1 | |||||
| Shangzhuang | Se | 1 | ns | ns | ns | ns |
| Zn | 1 | ns | 0.471 * | ns | ||
| Mn | 1 | 0.730 ** | ns | |||
| Cu | 1 | ns | ||||
| Mo | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Tao, Y.; Xu, Y.; Zhou, X.; Fu, G.; Zhao, L.; Wang, Q.; Li, H.; Wan, Y. Simultaneous Biofortification: Interaction between Zinc and Selenium Regarding Their Accumulation in Wheat. Agronomy 2024, 14, 1513. https://doi.org/10.3390/agronomy14071513
Kong L, Tao Y, Xu Y, Zhou X, Fu G, Zhao L, Wang Q, Li H, Wan Y. Simultaneous Biofortification: Interaction between Zinc and Selenium Regarding Their Accumulation in Wheat. Agronomy. 2024; 14(7):1513. https://doi.org/10.3390/agronomy14071513
Chicago/Turabian StyleKong, Lingxuan, Yanjin Tao, Yang Xu, Xuan Zhou, Guohai Fu, Lijie Zhao, Qi Wang, Huafen Li, and Yanan Wan. 2024. "Simultaneous Biofortification: Interaction between Zinc and Selenium Regarding Their Accumulation in Wheat" Agronomy 14, no. 7: 1513. https://doi.org/10.3390/agronomy14071513





