Sixteen Years of Recurrent Selection of Ruzi Grass for Resistance to Spittlebugs (Hemiptera: Cercopidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location
2.2. Genetic Material
2.3. Obtaining the Plants
2.4. Obtaining Pest Insect Eggs
2.5. Conducting the Experiments
2.6. Experimental Design
2.7. Statistical Analysis
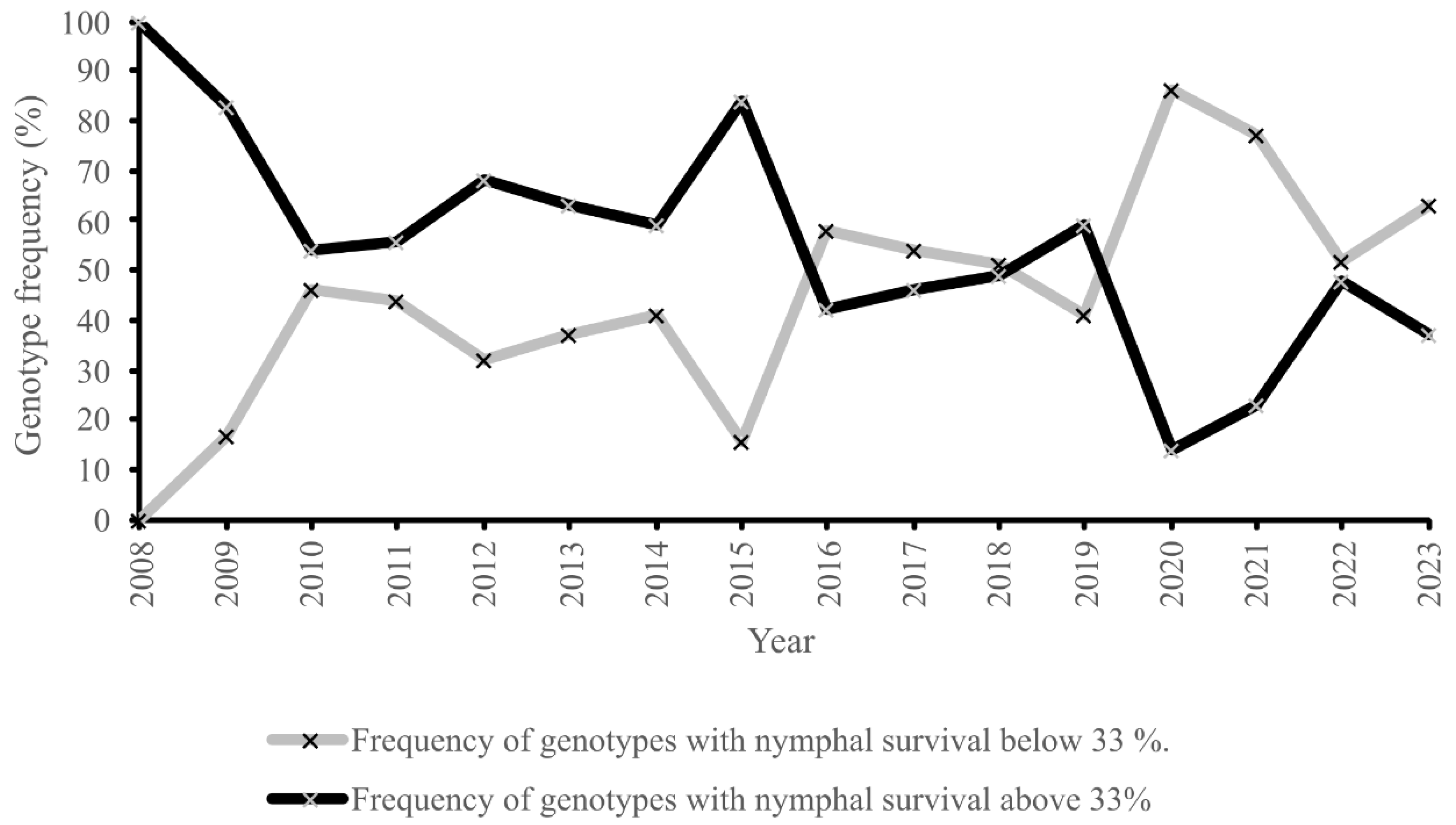
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simeão, R.; Silva, A.; Valle, C.; Resende, M.D.; Medeiros, S. Genetic Evaluation and Selection Index in Tetraploid Brachiaria ruziziensis. Plant Breed. 2016, 135, 246–253. [Google Scholar] [CrossRef]
- Martha, G.B., Jr.; Alves, E.; Contini, E. Land-Saving Approaches and Beef Production Growth in Brazil. Agric. Syst. 2012, 110, 173–177. [Google Scholar] [CrossRef]
- Wenzl, P.; Patiño, G.M.; Chaves, A.L.; Mayer, J.E.; Rao, I.M. The High Level of Aluminum Resistance in Signal-grass Is Not Associated with Known Mechanisms of External Aluminum Detoxification in Root Apices. Plant Physiol. 2001, 125, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, P.; Mancilla, L.I.; Mayer, J.E.; Albert, R.; Rao, I.M. Simulating Infertile Acid Soils with Nutrient Solutions. Soil Sci. Soc. Am. J. 2003, 67, 1457–1469. [Google Scholar] [CrossRef]
- Rao, I.; Miles, J.W.; García, R.; Ricaurte, J. Selección de Híbridos de Brachiaria Con Resistencia a Aluminio. Pasturas Trop. 2006, 28, 20–25. [Google Scholar]
- Souza Sobrinho, F.; Auad, A.M.; Lédo, J.F.S. Brazilian Society of Plant Breeding. Printed in Brazil Genetic Variability in Brachiaria ruziziensis for Resistance to Spittlebugs. Crop Breed. Appl. Biotechnol. 2010, 10, 83–88. [Google Scholar] [CrossRef]
- Serrão, E.A.S.; Neto, M.S. Informações Sobre Duas Espécies de Gramíneas Forrageiras Do Gênero Brachiaria Na Amazônia: B. Decumbens Stapf e B. ruziziensis Germain et Everard; Instituto de Pesquisa e Experimentação Agropecuária do Norte: Belém, Brazil, 1971.
- Pessoa-Filho, M.; Azevedo, A.L.S.; Sobrinho, F.S.; Gouvea, E.G.; Martins, A.M.; Ferreira, M.E. Genetic Diversity and Structure of Ruzigrass Germplasm Collected in Africa and Brazil. Crop Sci. 2015, 55, 2736–2745. [Google Scholar] [CrossRef]
- Keller-Grein, G.; Maass, B.L.; Hanson, J. Natural Variation in Brachiaria and Existing Germplasm Collections. In Brachiaria: Biology, Agronomy, and Improvement; Miles, J.W., Maass, B.L., Valle, C.B., Eds.; Centro Internacional de Agricultura Tropical: Cali, Colombia, 1996; pp. 16–42. [Google Scholar]
- Lascano, V.P.B.; Euclides, C.E. Nutritional Quality and Animal Production of Brachiaria Pastures. In Brachiaria: Biology, Agronomy, and Improvement; Miles, J.W., Maass, B.L., Valle, C.B., Eds.; Centro Internacional de Agricultura Tropical: Cali, Colombia, 1996; pp. 106–123. [Google Scholar]
- Schöbel, C.; Carvalho, G.S. Niche Modeling of Economically Important Mahanarva (Hemiptera, Cercopidae) Species in South and Central America: Are Brazilian Spittlebug Sugarcane Pests Potential Invaders of South and Central America? J. Econ. Entomol. 2019, 113, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Souza Sobrinho, F. Melhoramento de Forrageiras No Brasil. In Forragicultura e Pastagens: Temas em Evidência; Evangelista, A.R., Amaral, P.N.C., Padovani, R.F., Tavares, V.B., Salvador, F.M., Peron, A.J., Eds.; UFLA: Lavras, Brazil, 2005; pp. 65–120. [Google Scholar]
- Sotelo; Paola, A.; María, F.; Miller, A.F.; Cardona, C.; Miles, J.W.; Sotelo, P.A.; Sotelo, G.; Montoya, J. Sublethal Effects of Antibiosis Resistance on the Reproductive Biology of Two Spittlebug (Hemiptera: Cercopidae) Species Affecting Brachiaria spp. J. Econ. Entomol. 2008, 101, 564–568. [Google Scholar] [CrossRef]
- Valério, J.; Cardona, C.; Peck, D.; Sotelo, G. Spittlebugs: Bioecology, Host Plant Resistance and Advances in IPM. In Proceedings of the International Grassland Congress, Sao Paulo, Brazil, 11–21 February 2001; FEALQ: Piracicaba, Brazil, 2001; pp. 217–221. [Google Scholar]
- Resende, T.T.; Auad, A.M.; Fonseca, M.G. How Many Adults of Mahanarva spectabilis (Hemiptera: Cercopidae) Should Be Used for Screening Brachiaria ruziziensis (Poales: Poaceae) Resistance? J. Econ. Entomol. 2014, 107, 396–402. [Google Scholar] [CrossRef]
- Aguiar, D.D.M.; Auad, A.M.; Fonseca, M.G.; Leite, M.V. Brachiaria ruziziensis Responses to Different Fertilization Doses and to the Attack of Mahanarva spectabilis (Hemiptera: Cercopidae) Nymphs and Adults. Sci. World J. 2014, 2014, 543813. [Google Scholar] [CrossRef] [PubMed]
- Thompson, V. Associative Nitrogen Fixation, C 4 Photosynthesis, and the Evolution of Spittlebugs (Hemiptera: Cercopidae) as Major Pests of Neotropical Sugarcane and Forage Grasses. Bull. Entomol. Res. 2004, 94, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Auad, A.M.; de Carvalho, C.A.; da Silva, D.M.; Deresz, F. Flutuação Populacional de Cigarrinhas-Das-Pastagens Em Braquiária e Capim-Elefante. Pesqui. Agropecu. Bras. 2009, 44, 1205–1208. [Google Scholar] [CrossRef]
- Buitrago, P.A.E.; Manzano, M.R.; Hernández, L.M. Spittlebugs (Hemiptera: Cercopidae): Integrated Pest Management on Gramineous Crops in the Neotropical Ecozone. Front. Sustain. Food Syst. 2022, 6, 891417. [Google Scholar] [CrossRef]
- Pereira, J.F.; Azevedo, A.L.S.; Pessoa-Filho, M.; Romanel, E.A.D.C.; Pereira, A.V.; Vigna, B.B.Z.; Souza, F.D.; Benites, F.R.G.; Ledo, F.J.D.S.; Brito, G.G.D.; et al. Research Priorities for Next-Generation Breeding of Tropical Forages in Brazil. Crop Breed. Appl. Biotechnol. 2018, 18, 314–319. [Google Scholar] [CrossRef]
- Cardona, C.; Sotelo, G. Mecanismos de Resistencia a Insectos: Naturaleza e Importancia En La Formulación de Estrategias de Mejoramiento Para Incorporar Resistencia a Salivazo En Brachiaria. Pasturas Trop. 2005, 27, 2. [Google Scholar]
- Barnes, D.K.; Goplen, B.P.; Baylor, J.E. Highlights in the USA and Canada. In Alfalfa and Alfalfa Improvement; Hanson, A.A., Barnes, D.K., Jr., Hill, R.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1988. [Google Scholar]
- Casler, M.D.; Pedersen, J.F.; Eizenga, G.C.; Stratton, S.D. Germplasm and Cultivar Development. In Cool-Season Forage Grasses; Moser, L.E., Buxton, D.R., Casler, M.D., Eds.; American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- Cardona, C.; Miles, J.W.; Sotelo, G. An Improved Methodology for Massive Screening of Brachiaria spp. Genotypes for Resistance to Aeneolamia varia (Homoptera: Cercopidae). J. Econ. Entomol. 1999, 92, 490–496. [Google Scholar] [CrossRef]
- Mrode, R.; Thompson, R. Linear Models for the Prediction of Animal Breeding Values; Mrode, R., Ed.; CABI: Oxfordshire, UK, 2014; ISBN 9781780643915. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Resende, M.D.V.; Duarte, J.B. Precisão e Controle de Qualidade Em Experimentos de Avaliação de Cultivares. Pesqui. Agropecuária Trop. 2007, 37, 182–194. [Google Scholar]
- Cullis, B.R.; Smith, A.B.; Coombes, N.E. On the Design of Early Generation Variety Trials with Correlated Data. J. Agric. Biol. Environ. Stat. 2006, 11, 381–393. [Google Scholar] [CrossRef]
- Vencovsky, R.; Moraes, A.; Garcia, J.; Teixeira, N. Progresso Genético Em Vinte Anos de Melhoramento Do Milho No Brasil. In Proceedings of the 9o Congresso de Milho e Sorgo, Anais; EMBRAPA-CNPMS: Sete Lagoas, Brazil, 1986; pp. 300–307. [Google Scholar]
- Souza Sobrinho, F.; Auad, A.M.; Santos, A.M.B.; Gomide, C.A.M.; Martins, C.E.; Castro, C.R.T.; Paciullo, D.S.C.; Benites, F.R.G.; Rocha, W.S.D. Boletim Técnico; Embrapa: Juiz de Fora, Brazil, 2022.
- Souza Sobrinho, F.; Benites, F.R.G. Melhoramento Genético de Brachiaria ruziziensis: Histórico e Estratégias. In Tópicos Especiais em Ciência Animal IV; Martins, C.B., Deminicis, B.B., Moreira, G.R., Mendonça, P.P., Eds.; CAUFES: Alegre, Brazil, 2016; pp. 309–329. [Google Scholar]
- Cardona, C.; Fory, P.; Sotelo, G.; Pabon, A.; Diaz, G.; Miles, J.W. Antibiosis and Tolerance to Five Species of Spittlebug (Homoptera: Cercopidae) in Brachiaria spp.: Implications for Breeding for Resistance. J. Econ. Entomol. 2004, 97, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.W.; Cardona, C.; Sotelo, G. Recurrent Selection in a Synthetic Brachiaria grass Population Improves Resistance to Three Spittlebug Species. Crop Sci. 2006, 46, 1088–1093. [Google Scholar] [CrossRef]
- Auad, A.M.; Resende, T.T.; Santos, D.R.; Souza Sobrinho, F.; Fonseca, M.G.; Madalena, I.S.P. Seleção de Clones de Brachiairia Ruziziensis resistentes à Deois flavopicta (Hemiptera: Cercopidae). In Proceedings of the 48a Reunião Anual da Sociedade Brasileira de Zootecnia, Belém, Brazil, 18–21 July 2011. [Google Scholar]
- Auad, A.M.; Souza Sobrinho, F.; Resende, T.T.; Benites, F.R.G. Seleção de Clones de Brachiaria ruziziensis Quanto a Resistência à Mahanarva Spectabilis. In Proceedings of the XXII Congresso de Pós graduação da UFLA, Lavras, Brazil, 14–18 October 2013; p. 4. [Google Scholar]
- Auad, A.M.; Resende, T.T.; Souza Sobrinho, F.; Toledo, A.M.O.; Lucindo, T.S. Identificação De Brachiaria ruziziensis Resistentes à Mahanarva spectabilis (Hemiptera: Cercopidae): Quarto Ciclo de Seleção. In Proceedings of the XXXVII Semana de Biologia da UFJF, Juiz de Fora, Brazil, 10–14 November 2014. [Google Scholar]
- Auad, A.M.; Souza Sobrinho, F.; Fonseca, M.G.; Resende, T.T.; Parchen, H.A.; Rodrigues, B.S.; Lucindo, T.S. Seleção de Populações de Brachiaria ruziziensis (Germain & Edvard) Quanto à Resistência a Deois schach (Fabricius, 1787) (Hemiptera: Cercopidae). In Proceedings of the Semana De Biologia Da UFJF, Juiz de Fora, Brazil, 19–25 October 2015. [Google Scholar]
- Auad, A.M.; Resende, T.T.; Souza Sobrinho, F.; Silva, S.E.B.; Claudino, S.S.; Rodrigues, B.S. Seleção de Genótipos de Brachiaria ruziziensis (Germain & Evrard) Resistentes à Mahanarva spectabilis (Distant, 1909) (Hemiptera: Cercopidae). In Proceedings of the XXV Congresso de Pós Graduação da UFLA, Lavras, Brazil, 22–27 November 2016. [Google Scholar]
- Toledo, A.M.O.; Auad, A.M.; Fonseca, C.S.; Souza Sobrinho, F.; Resende, T.T.; Rodrigues, B.S.; Borges, R.A. Seleção de Genótipos de Brachiaria ruziziensis (Germain & Edvard) Resistentes à Deois schach (Fabricius, 1787) (Hemiptera:Cercopidae). In Proceedings of the Semana de Biologia da UFJF, Juiz de Fora, Brazil, 19–25 October 2015; pp. 1–3. [Google Scholar]
- Berilli, A.P.C.G.; Pereira, M.G.; dos Trindade, R.S.; da Costa, F.R.; Cunha, K.S.D. Resposta a Seleção No 11o Ciclo de Seleção Recorrente Recíproca Entre Famílias de Irmãos completos de Milho. Acta Sci. Agron. 2013, 35, 435–441. [Google Scholar] [CrossRef]
- Ramalho, M.A.P.; Santos, J.B.; Pinto, C.A.B.P. Genética Na Agropecuária, 2nd ed.; UFLA: Lavras, Brazil, 2000. [Google Scholar]
- Pereira de Castro, A.; Breseghello, F.; Furtini, I. Population Improvement via Recurrent Selection Drives Genetic Gain in Upland Rice Breeding. Heredity 2023, 131, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Fritsche-Neto, R.; Sabadin, F.; DoVale, J.C.; Borges, K.L.R.; de Souza, P.H.; Crossa, J.; Garbuglio, D.D. Realized Genetic Gains via Recurrent Selection in a Tropical Maize Haploid Inducer Population and Optimizing Simultaneous Selection for the next Cycles. Crop Sci. 2023, 63, 2865–2876. [Google Scholar] [CrossRef]
- Resende, M.D.V. Software Selegen-Reml/Blup; Embrapa Floresta: Colombo, Brazil, 2002; p. 67.
- Missio, R.F.; Dias, L.A.S.; Moraes, M.L.T.; Resende, M.D.V. Selection of Pinus caribaea Var. Bahamensis Progenies Based on the Predicted Genetic Value. Crop Breed. Appl. Biotechnol. 2004, 4, 399–407. [Google Scholar] [CrossRef]
- Silva, D.M.; de Moraes, J.C.; Auad, A.M.; das Fonseca, M.G.; Souza Sobrinho, F. Genetic Variability of Brachiaria ruziziensis Clones to Collaria oleosa (Hemiptera: Miridae) Based on Leaf Injuries. Am. J. Plant Sci. 2013, 4, 2418–2424. [Google Scholar] [CrossRef]
- Juhász, A.C.P.; de Morais, D.L.B.; Soares, B.O.; Pimenta, S.; de Rabello, H.O.; de Resende, M.D.V. Parâmetros Genéticos e Ganho Com a Seleção Para Populações de Pinhão Manso (Jatropha curcas). Pesqui. Florest. Bras. 2010, 30, 25–35. [Google Scholar] [CrossRef]
- Vencovsky, R.; Barriga, P. Genética Biométrica No Fito Melhoramento; Sociedade Brasileira de Genética: Ribeirão Preto, Brazil, 1992. [Google Scholar]
- Borém, A. Melhoramento de Plantas, 20th ed.; UFV: Viçosa, Brazil, 1997. [Google Scholar]
- Cruz, C.D.; Carneiro, P.C. Modelos Biométricos Aplicados Ao Melhoramento de Plantas; Ceres: Viçosa, Brazil, 2003. [Google Scholar]
- Fradgley, N.; Gardner, K.A.; Bentley, A.R.; Howell, P.; Mackay, I.J.; Scott, M.F.; Mott, R.; Cockram, J. Multi-Trait Ensemble Genomic Prediction and Simulations of Recurrent Selection Highlight Importance of Complex Trait Genetic Architecture for Long-Term Genetic Gains in Wheat. Silico Plants 2023, 5, diad002. [Google Scholar] [CrossRef]
- Benites, F.R.G.; Pinto, C.A.B.P. Genetic Gains for Heat Tolerance in Potato in Three Cycles of Recurrent Selection. Crop Breed. Appl. Biotechnol. 2011, 11, 133–140. [Google Scholar] [CrossRef]

| Experiment | Species of Spittlebug | Experimental Design | Number of Plants | Number of Plants/Year |
|---|---|---|---|---|
| POP01-2008 | M. spectabilis | RCBD | 270 | 270 |
| POP02-2009 | M. spectabilis | RCBD | 148 | |
| POP03-2009 | D. schach | RCBD | 84 | |
| POP04-2009 | M. spectabilis | FABD | 552 | 784 |
| POP05-2010 | D. schach | RCBD | 252 | |
| POP06-2010 | M. spectabilis | RCBD | 430 | |
| POP07-2010 | D. schach | RCBD | 216 | |
| POP08-2010 | M. spectabilis | FABD | 297 | |
| POP09-2010 | M. spectabilis | RCBD | 450 | 1645 |
| POP10-2011 | M. spectabilis | RCBD | 180 | 180 |
| POP11-2012 | M. spectabilis | RCBD | 102 | |
| POP12-2012 | M. spectabilis | RCBD | 216 | |
| POP13-2012 | D. schach | RCBD | 78 | |
| POP14-2012 | M. spectabilis | FABD | 240 | 636 |
| POP15-2013 | M. spectabilis | FABD | 640 | |
| POP16-2013 | M. spectabilis | RCBD | 128 | |
| POP17-2013 | M. spectabilis | RCBD | 240 | |
| POP18-2013 | M. spectabilis | RCBD | 120 | |
| POP19-2013 | M. spectabilis | RCBD | 408 | 1536 |
| POP20-2014 | M. spectabilis | RCBD | 414 | |
| POP21-2014 | M. spectabilis | RCBD | 240 | |
| POP22-2014 | M. spectabilis | FABD | 24 | |
| POP23-2014 | D. schach | FABD | 132 | 810 |
| POP24-2015 | D. schach | RCBD | 315 | |
| POP25-2015 | D. schach | RCBD | 428 | |
| POP26-2015 | D. schach | RCBD | 368 | 1111 |
| POP27-2016 | M. spectabilis | FABD | 200 | |
| POP28-2016 | M. spectabilis | RCBD | 750 | 950 |
| POP29-2017 | M. spectabilis | RCBD | 315 | 315 |
| POP30-2018 | M. spectabilis | RCBD | 300 | 300 |
| POP31-2019 | M. spectabilis | FABD | 539 | |
| POP32-2019 | M. spectabilis | RCBD | 90 | 629 |
| POP33-2020 | D. schach | FABD | 1673 | 1673 |
| POP34-2021 | D. schach | FABD | 781 | 781 |
| POP35-2022 | D. schach | FABD | 357 | 357 |
| POP36-2023 | M. spectabilis | FABD | 1137 | 1137 |
| Total plants evaluated | 13,114 | |||
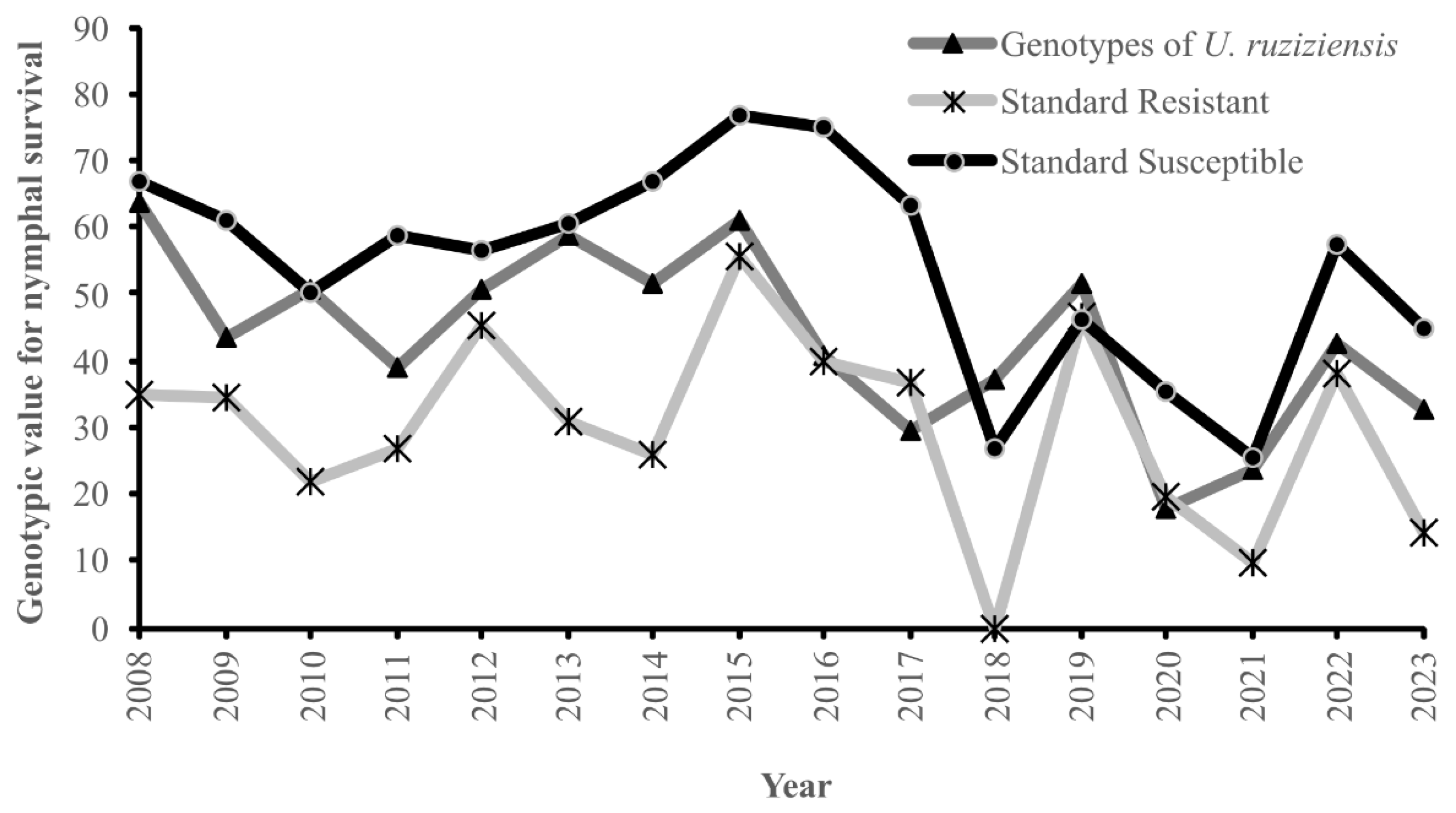
| Year | Average Genotypic Value ± SE | Genetic Gain (%) | |||
|---|---|---|---|---|---|
| Genotypes of U. ruziziensis | Standard | Year/Year | Year/First Year | ||
| Resistant | Susceptible | ||||
| 2008 | 63.73 ± 2.4 | 35.00 ± 8.03 | 66.67 ± 10.54 | * | * |
| 2009 | 43.45 ± 3.0 | 34.38 ± 6.57 | 60.90 ± 4.60 | −31.81 | −31.81 |
| 2010 | 50.80 ± 4.0 | 22.04 ± 5.68 | 50.10 ± 4.99 | 16.91 | 11.53 |
| 2011 | 39.05 ± 6.5 | 26.67 ± 9.87 | 58.62 ± 11.48 | −23.14 | −18.45 |
| 2012 | 50.75 ± 6.4 | 45.21 ± 6.34 | 56.71 ± 5.44 | 29.96 | 18.36 |
| 2013 | 58.91 ± 4.1 | 30.93 ± 4.49 | 60.43 ± 4.74 | 16.09 | 12.81 |
| 2014 | 51.42 ± 6.5 | 25.83 ± 2.69 | 66.76 ± 3.41 | −12.72 | −11.76 |
| 2015 | 60.84 ± 2.5 | 55.79 ± 7.48 | 76.60 ± 5.83 | 18.32 | 14.78 |
| 2016 | 40.55 ± 3.9 | 40.00 ± 7.18 | 75.00 ± 9.69 | −33.36 | −31.84 |
| 2017 | 29.67 ± 5.2 | 36.67 ± 13.33 | 63.33 ± 6.24 | −26.81 | −17.06 |
| 2018 | 37.00 ± 7.6 | 0.00 ± 0.00 | 26.67 ± 19.44 | 24.68 | 11.49 |
| 2019 | 51.55 ± 8.4 | 46.69 ± 5.08 | 46.28 ± 5.01 | 39.32 | 22.83 |
| 2020 | 18.02 ± 1.2 | 19.57 ± 4.10 | 35.51 ± 4.64 | −65.05 | −52.62 |
| 2021 | 23.67 ± 1.4 | 9.77 ± 2.93 | 25.29 ± 4.58 | 31.41 | 8.88 |
| 2022 | 42.40 ± 2.5 | 37.88 ± 8.44 | 57.58 ± 11.32 | 79.11 | 29.39 |
| 2023 | 32.83 ± 2.2 | 14.35 ± 5.79 | 44.91 ± 7.04 | −22.59 | −15.03 |
| Average | 43.41 ± 4.24 | 30.05 ± 6.13 | 54.46 ± 7.44 | ||
| Genetic gain | −1.00 | −15.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, T.T.; Sobrinho, F.S.; Campagnani, M.O.; Veríssimo, B.A.; Calsavara, L.A.; Gonçalves, F.M.A.; Nunes, J.A.R.; Auad, A.M. Sixteen Years of Recurrent Selection of Ruzi Grass for Resistance to Spittlebugs (Hemiptera: Cercopidae). Agronomy 2024, 14, 1516. https://doi.org/10.3390/agronomy14071516
Resende TT, Sobrinho FS, Campagnani MO, Veríssimo BA, Calsavara LA, Gonçalves FMA, Nunes JAR, Auad AM. Sixteen Years of Recurrent Selection of Ruzi Grass for Resistance to Spittlebugs (Hemiptera: Cercopidae). Agronomy. 2024; 14(7):1516. https://doi.org/10.3390/agronomy14071516
Chicago/Turabian StyleResende, Tiago Teixeira, Fausto Souza Sobrinho, Michelle Oliveira Campagnani, Bruno Antônio Veríssimo, Luís Augusto Calsavara, Flávia Maria Avelar Gonçalves, José Airton Rodrigues Nunes, and Alexander Machado Auad. 2024. "Sixteen Years of Recurrent Selection of Ruzi Grass for Resistance to Spittlebugs (Hemiptera: Cercopidae)" Agronomy 14, no. 7: 1516. https://doi.org/10.3390/agronomy14071516





