Assessing the Evolution of Stability and Maturity in Co-Composting Sheep Manure with Green Waste Using Physico-Chemical and Biological Properties and Statistical Analyses: A Case Study of Botanique Garden in Rabat, Morocco
Abstract
:1. Introduction
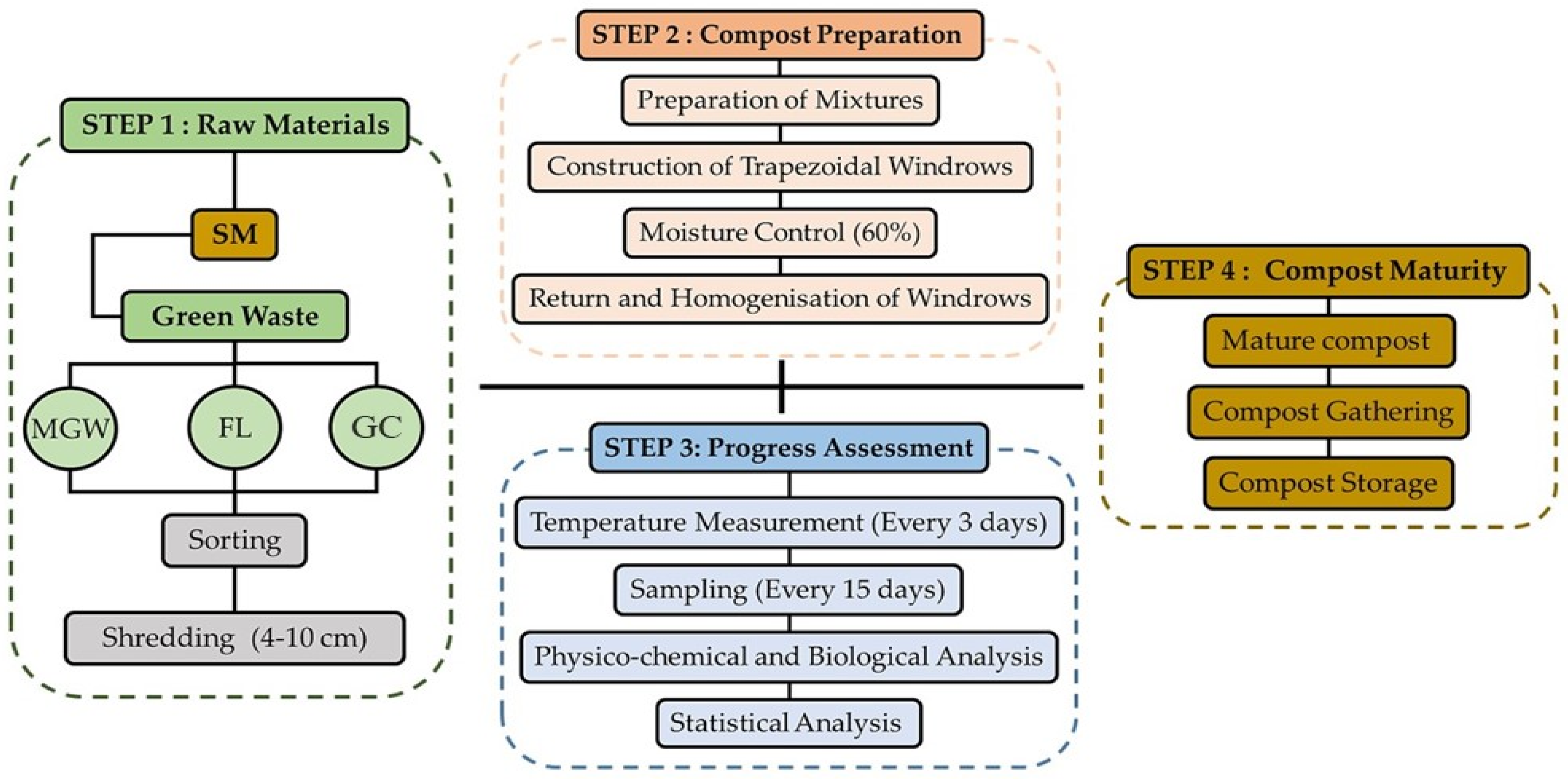
2. Materials and Methods
2.1. Study Area
2.2. Raw Material
2.3. Experimental Setup and Sampling
2.4. Physico-Chemical Parameters
2.5. Biological Analysis
2.6. Statistical Analysis
3. Results and Discussion
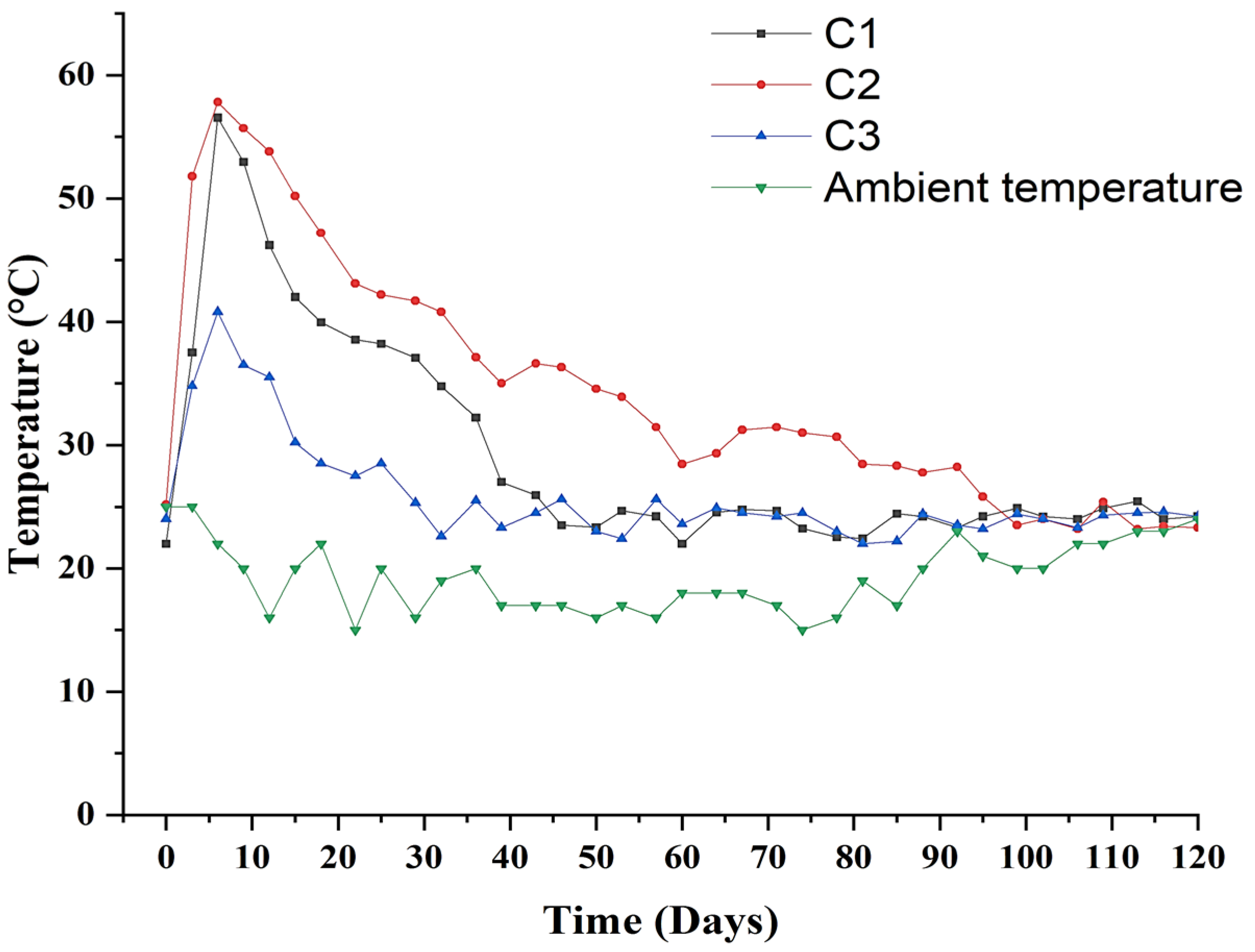
3.1. Temperature Profile
3.2. EC and pH Progress
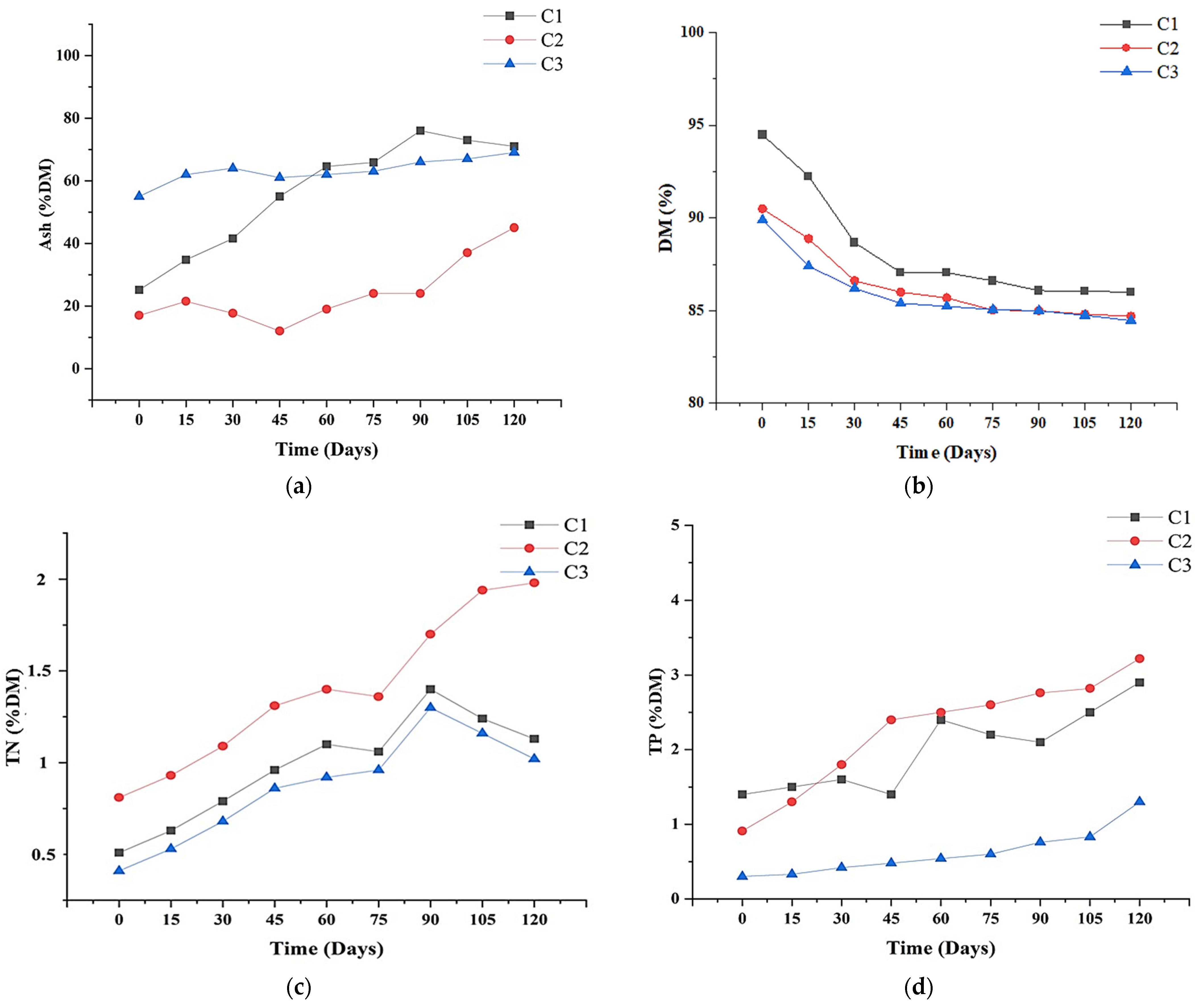
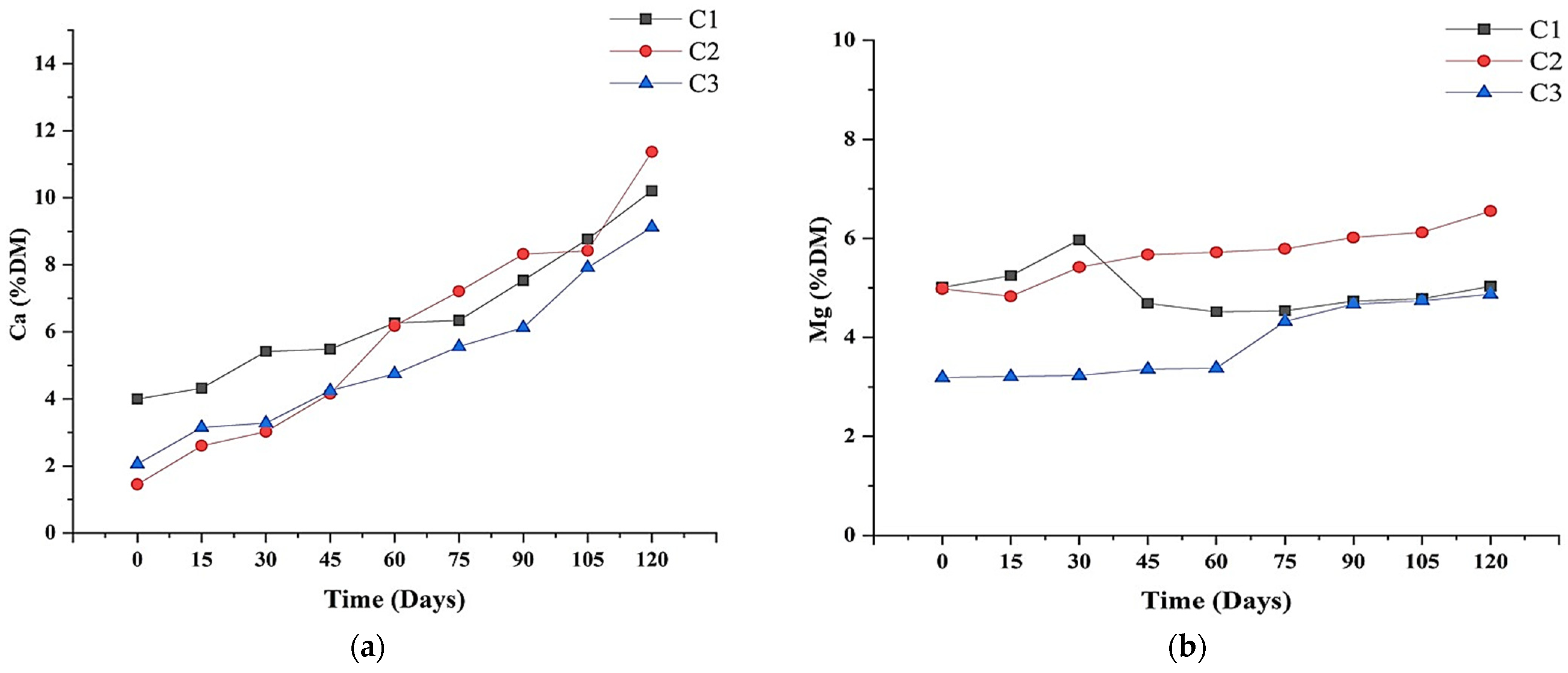
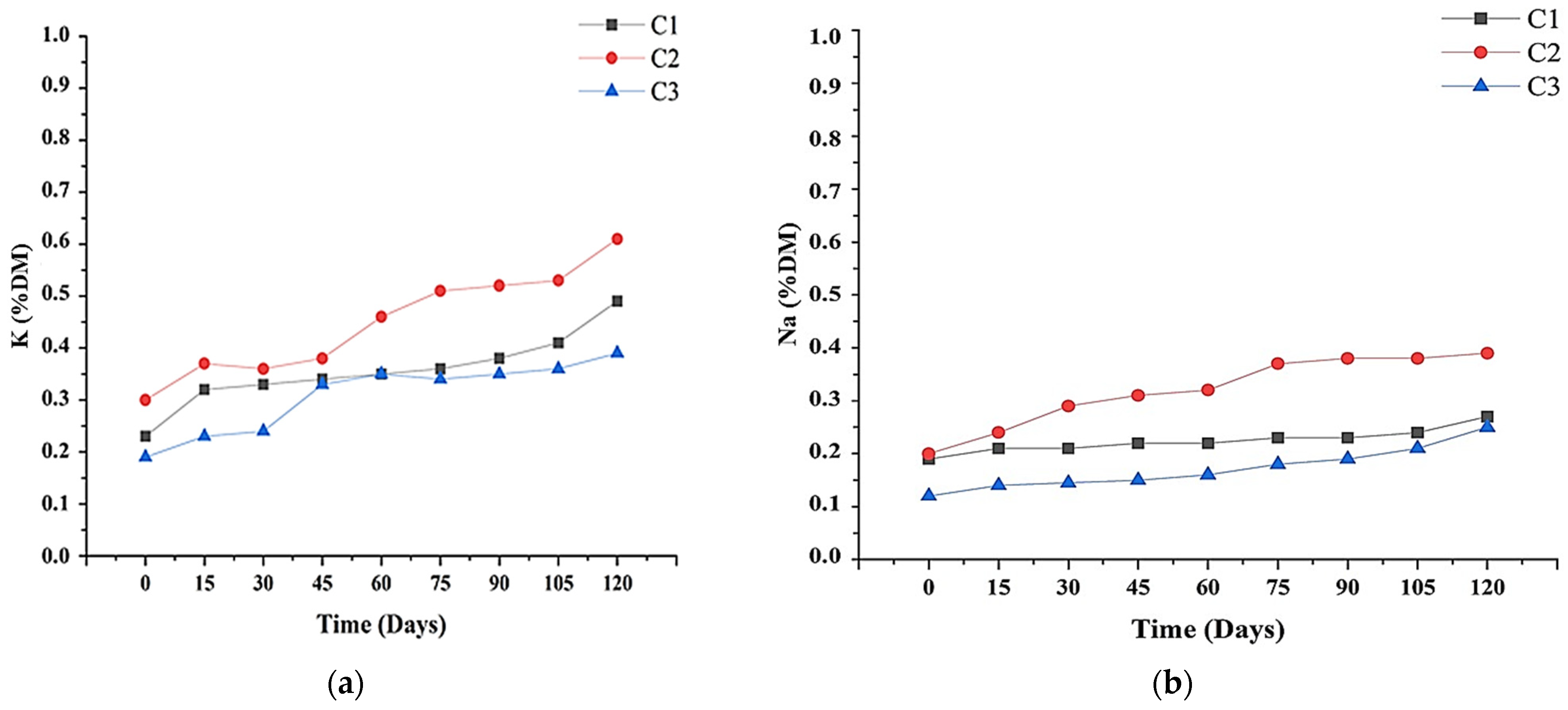
3.3. Elemental Analysis and Nutrient Levels
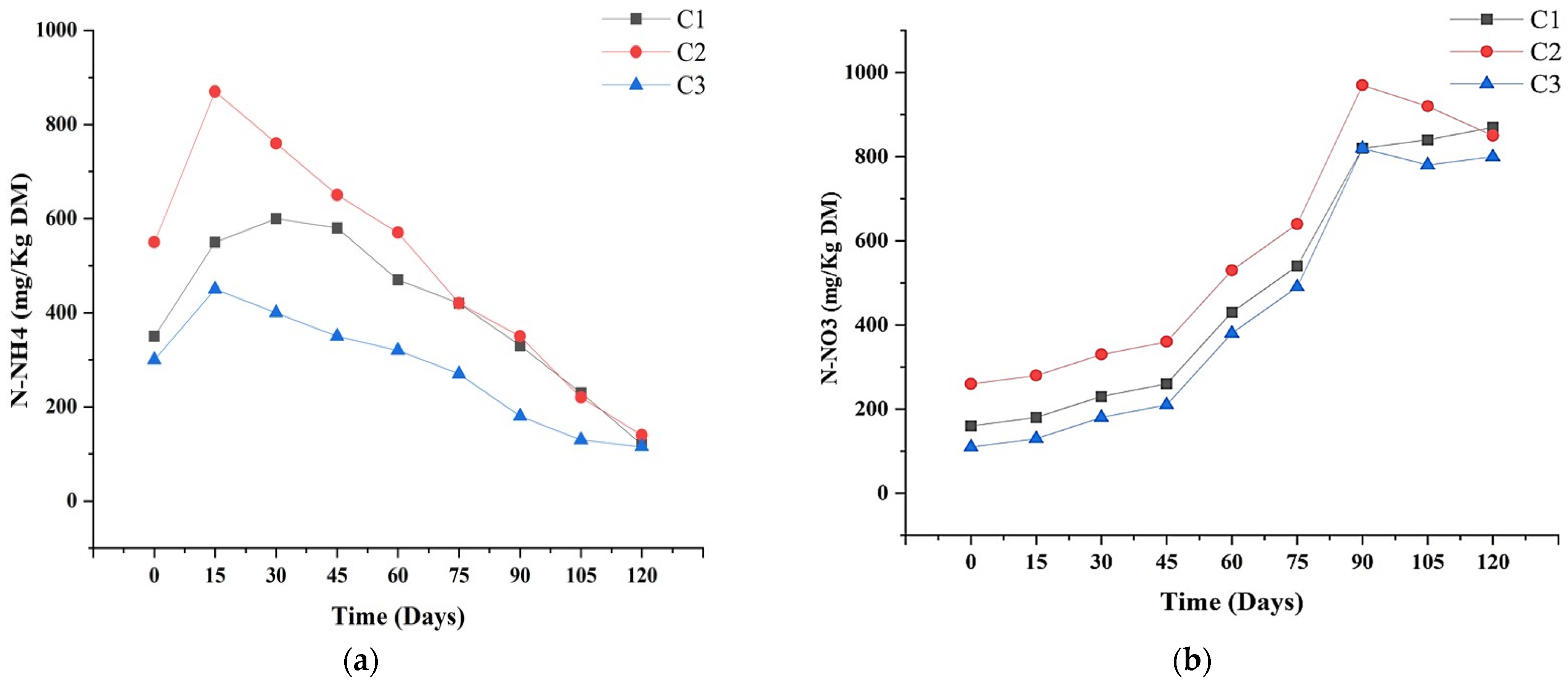
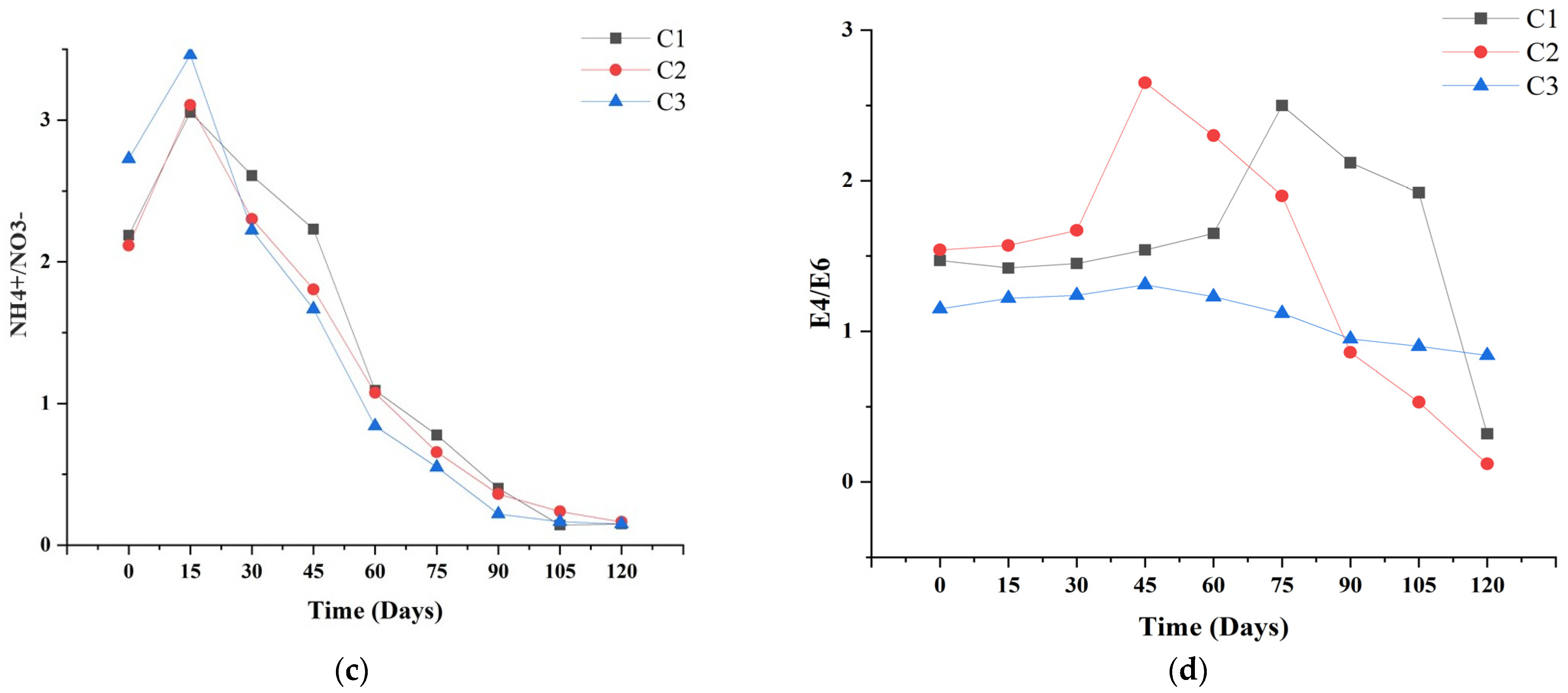
3.4. N-NH4 and N-NO3 Changes
3.5. Micronutrient Levels
3.6. Humification Index
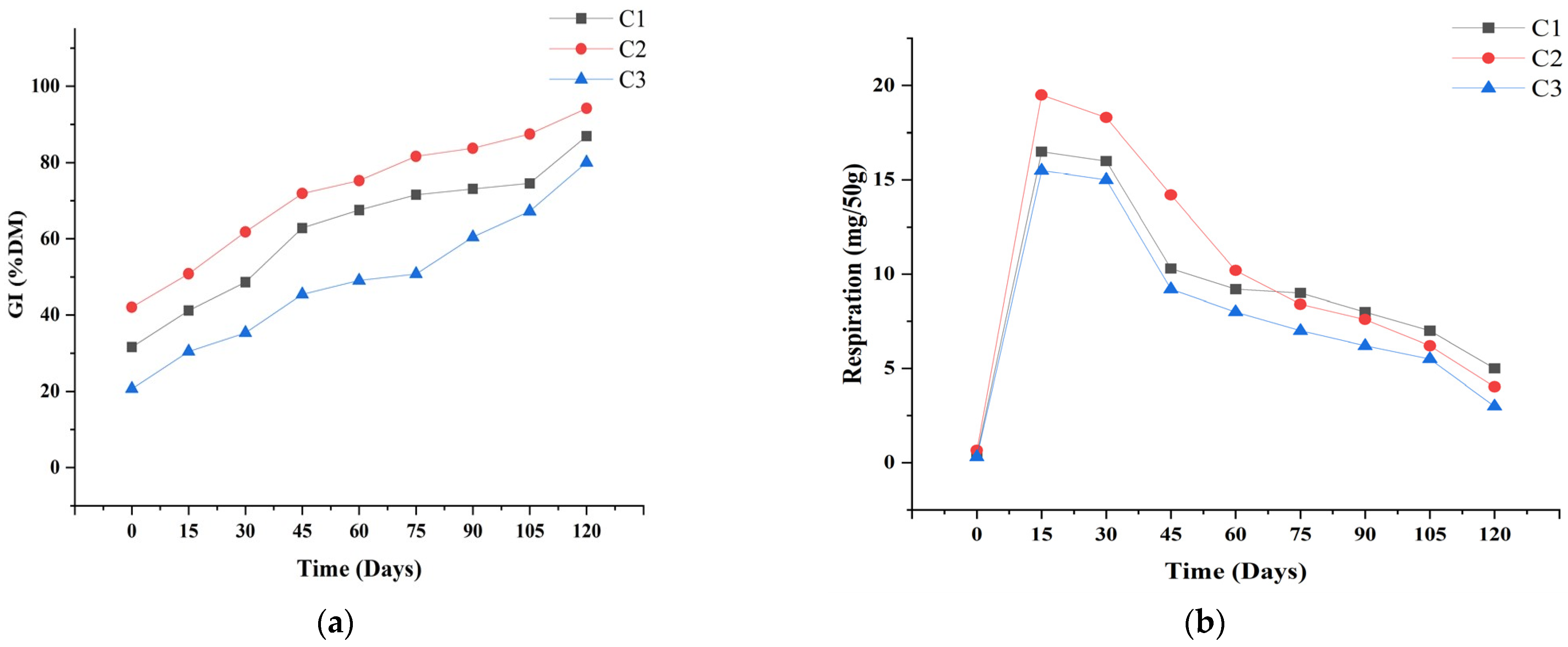
3.7. Biological Parameters
3.8. Statistical Analysis
3.8.1. The Correlation Studies between Various Physico-Chemical and Biological Parameters
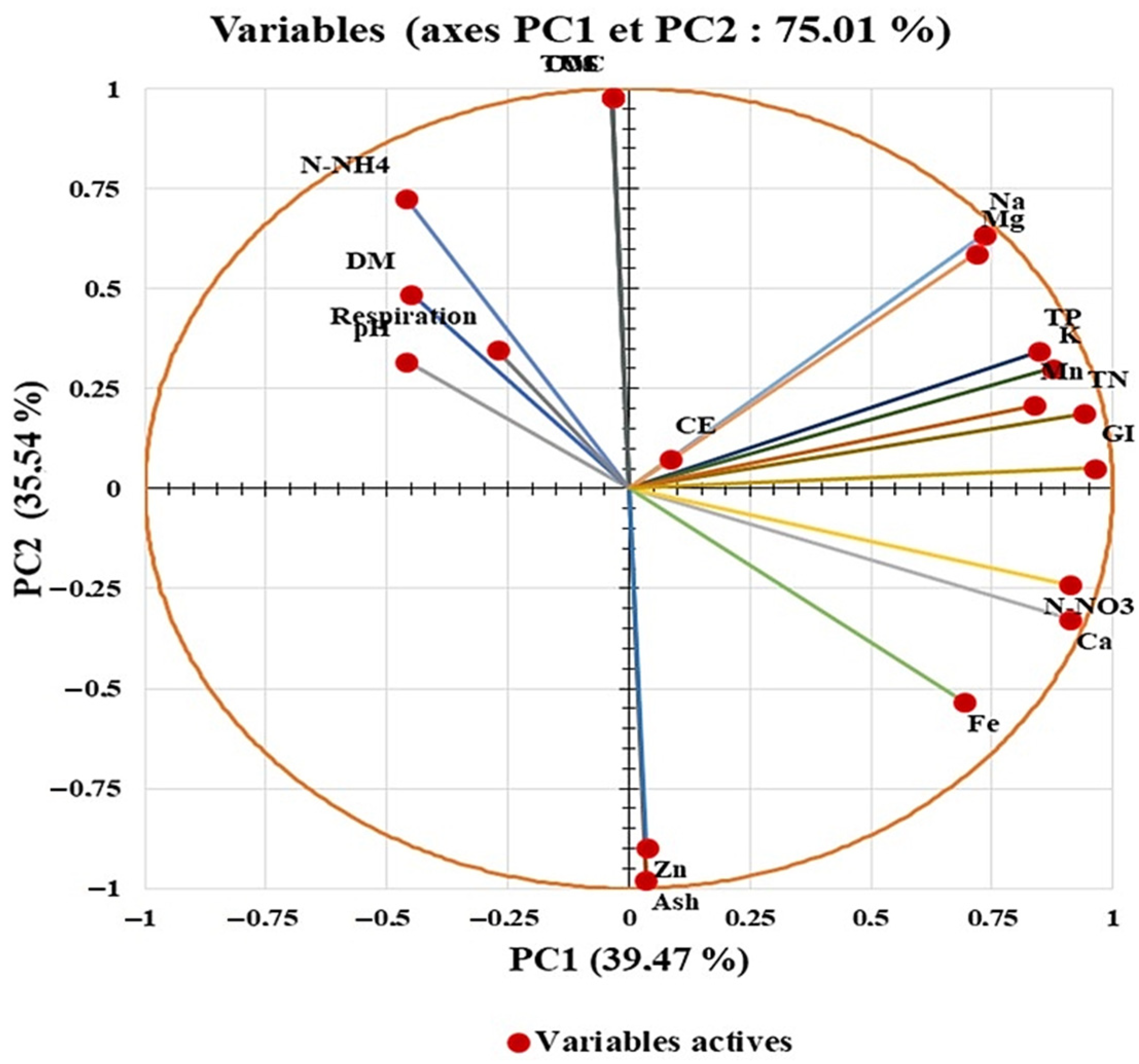
3.8.2. Principal Component Analysis
3.9. Practical Implications of This Study and Future Research
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, S.K.; Yadav, K.D. Assessment of the Effect of Particle Size and Selected Physico-Chemical and Biological Parameters on the Efficiency and Quality of Composting of Garden Waste. J. Environ. Chem. Eng. 2022, 10, 107925. [Google Scholar] [CrossRef]
- Abid, W.; Mahmoud, I.B.; Masmoudi, S.; Triki, M.A.; Mounier, S.; Ammar, E. Physico-Chemical and Spectroscopic Quality Assessment of Compost from Date Palm (Phoenix dactylifera L.) Waste Valorization. J. Environ. Manag. 2020, 264, 110492. [Google Scholar] [CrossRef] [PubMed]
- Hemidat, S.; Jaar, M.; Nassour, A.; Nelles, M. Monitoring of Composting Process Parameters: A Case Study in Jordan. Waste Biomass Valor. 2018, 9, 2257–2274. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Sun, L.; Men, M.; Wang, B.; Deng, L.; Zhao, L.; Han, Y.; Jong, C.; Bi, R.; et al. Microecological Insight to Fungal Structure and Key Fungal Communities Regulating Nitrogen Transformation Based on Spatial Heterogeneity during Cow Manure Composting by Multi-Angle and Multi-Aspect Analyses. Waste Manag. 2022, 142, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Peña, H.; Mendoza, H.; Diánez, F.; Santos, M. Parameter Selection for the Evaluation of Compost Quality. Agronomy 2020, 10, 1567. [Google Scholar] [CrossRef]
- Chen, J.; Jin, C.; Sun, S.; Yang, D.; He, Y.; Gan, P.; Nalume, W.G.; Ma, Y.; He, W.; Li, G. Recognizing the Challenges of Composting: Critical Strategies for Control, Recycling, and Valorization of Nitrogen Loss. Resour. Conserv. Recycl. 2023, 198, 107172. [Google Scholar] [CrossRef]
- Meng, X.; Liu, B.; Zhang, H.; Wu, J.; Yuan, X.; Cui, Z. Co-Composting of the Biogas Residues and Spent Mushroom Substrate: Physicochemical Properties and Maturity Assessment. Bioresour. Technol. 2019, 276, 281–287. [Google Scholar] [CrossRef]
- Chand, S.; Devi, S.; Devi, D.; Arya, P.; Manorma, K.; Kesta, K.; Sharma, M.; Bishist, R.; Tomar, M. Microbial and Physico-Chemical Dynamics Associated with Chicken Feather Compost Preparation Vis-à-Vis Its Impact on the Growth Performance of Tomato Crop. Biocatal. Agric. Biotechnol. 2023, 54, 102885. [Google Scholar] [CrossRef]
- Moubareck, C.A.; Alawlaqi, B.; Alhajeri, S. Characterization of Physicochemical Parameters and Bacterial Diversity of Composted Organic Food Wastes in Dubai. Heliyon 2023, 9, e16426. [Google Scholar] [CrossRef]
- El-mrini, S.; Aboutayeb, R.; Zouhri, A. Effect of Initial C/N Ratio and Turning Frequency on Quality of Final Compost of Turkey Manure and Olive Pomace. J. Eng. Appl. Sci. 2022, 69, 37. [Google Scholar] [CrossRef]
- Aboutayeb, R.; El-Mrini, S.; Zouhri, A.; Azim, K. Effect of Carbon to Nitrogen Ratio and Aeration Rate on Phosphorus and Exchangeable Cation Contents and Their Leaching in the Soil during Olive Pomace and Turkey Manure Co-Composting. J. Eng. Appl. Sci. 2023, 70, 12. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, L.; Li, R. Effects of Additives on Physical, Chemical, and Microbiological Properties during Green Waste Composting. Bioresour. Technol. 2021, 340, 125719. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, J.; Yang, Y.; Liu, Y.; Zhang, L.; Wang, G.; Liu, G.; Dang, R.; Li, G.; Yuan, J. Determining the Extraction Conditions and Phytotoxicity Threshold for Compost Maturity Evaluation Using the Seed Germination Index Method. Waste Manag. 2023, 171, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Moral, R.; Paredes, C.; Bustamante, M.A.; Marhuenda-Egea, F.; Bernal, M.P. Utilisation of Manure Composts by High-Value Crops: Safety and Environmental Challenges. Bioresour. Technol. 2009, 100, 5454–5460. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.P.; Sommer, S.G.; Chadwick, D.; Qing, C.; Guoxue, L.; Michel, F.C. Chapter Three-Current Approaches and Future Trends in Compost Quality Criteria for Agronomic, Environmental, and Human Health Benefits. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 144, pp. 143–233. [Google Scholar]
- Hameed, M.; Bhat, R.A.; Pandit, B.A.; Ramzan, S.; Dijoo, Z.K.; Wani, M.A. Qualitative Assessment of Compost Engendered from Municipal Solid Waste and Green Waste by Indexing Method. J. Air Waste Manag. Assoc. 2022, 72, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Rocha, P.; Antelo, J.; Valderrama, P.; López, R.; Geraldo, D.; Proença, M.F.; Pinheiro, J.P.; Fiol, S.; Bento, F. Comparison of a Variety of Physico-Chemical Techniques in the Chronological Characterization of a Compost from Municipal Wastes. Process Saf. Environ. Prot. 2022, 164, 781–793. [Google Scholar] [CrossRef]
- Wichuk, K.M.; McCartney, D. Compost Stability and Maturity Evaluation—A Literature Review. J. Environ. Eng. Sci. 2013, 8, 601–620. [Google Scholar] [CrossRef]
- Tiquia, S.M. Microbiological Parameters as Indicators of Compost Maturity. J. Appl. Microbiol. 2005, 99, 816–828. [Google Scholar] [CrossRef]
- European Standard 13037 Determination of pH. In Soil Improvers and Growing Media; European Committee for Standardization: Brussels, Belgium, 1999.
- European Standard 13038 Determination of Electrical Conductivity. In Soil Improvers and Growing Media; European Committee for Standardization: Brussels, Belgium, 1999.
- Qian, X.; Shen, G.; Wang, Z.; Guo, C.; Liu, Y.; Lei, Z.; Zhang, Z. Co-Composting of Livestock Manure with Rice Straw: Characterization and Establishment of Maturity Evaluation System. Waste Manag. 2014, 34, 530–535. [Google Scholar] [CrossRef]
- Hachicha, S.; Sellami, F.; Cegarra, J.; Hachicha, R.; Drira, N.; Medhioub, K.; Ammar, E. Biological Activity during Co-Composting of Sludge Issued from the OMW Evaporation Ponds with Poultry Manure—Physico-Chemical Characterization of the Processed Organic Matter. J. Hazard. Mater. 2009, 162, 402–409. [Google Scholar] [CrossRef]
- Thompson, W.; Millner, P.; Watson, M.; Leege, P.B. Test Methods for the Examination of Composting and Compost; US Composting Council: Raleigh, NC, USA, 2001. [Google Scholar]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis. Part (3). Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; SSSA, ASA: Madison, WI, USA, 1996; pp. 1085–1122. [Google Scholar]
- Xu, Z.; Qi, C.; Zhang, L.; Ma, Y.; Li, J.; Li, G.; Luo, W. Bacterial Dynamics and Functions for Gaseous Emissions and Humification in Response to Aeration Intensities during Kitchen Waste Composting. Bioresour. Technol. 2021, 337, 125369. [Google Scholar] [CrossRef] [PubMed]
- AL-Jaboobi, M.; Zouahri, A.; Tijane, M.; Housni, A.E.; Mennane, Z.; Yachou, H.; Bouksaim, M. Evaluation of Heavy Metals Pollution in Groundwater, Soil and Some Vegetables Irrigated with Wastewater in the Skhirat Region “Morocco”. J. Mater. Environ. Sci. 2014, 5, 961–966. [Google Scholar]
- Zucconi, F.; Forte, M.; Monaco, A.; De Bertoldi, M. Biological Evaluation of Compost Maturity. Biocycle 1981, 22, 27–29. [Google Scholar]
- Sanad, H.; Mouhir, L.; Zouahri, A.; Moussadek, R.; El Azhari, H.; Yachou, H.; Ghanimi, A.; Oueld Lhaj, M.; Dakak, H. Assessment of Groundwater Quality Using the Pollution Index of Groundwater (PIG), Nitrate Pollution Index (NPI), Water Quality Index (WQI), Multivariate Statistical Analysis (MSA), and GIS Approaches: A Case Study of the Mnasra Region, Gharb Plain, Morocco. Water 2024, 16, 1263. [Google Scholar] [CrossRef]
- Wang, T.-T.; Wang, S.-P.; Zhong, X.-Z.; Sun, Z.-Y.; Huang, Y.-L.; Tan, L.; Tang, Y.-Q.; Kida, K. Converting Digested Residue Eluted from Dry Anaerobic Digestion of Distilled Grain Waste into Value-Added Fertilizer by Aerobic Composting. J. Clean. Prod. 2017, 166, 530–536. [Google Scholar] [CrossRef]
- Aboutayeb, R.; Elgharous, M.; Abail, Z.; Elhari, M.; Koulali, Y. Stabilization and Sanitation of Chicken Litter by Heap Composting. Int. J. Eng. Res. 2013, 2, 529–534. [Google Scholar]
- Methods Book for the Analysis of Compost; FCQAO (Ed.) Kompost-Information; Abfall Now e.V. Publ. House: Stuttgart, Germany, 1994; ISBN 978-3-928179-33-1. [Google Scholar]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of Animal Manures and Chemical Criteria for Compost Maturity Assessment. A Review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Ben Ayed, L.; Hassen, A.; Jedidi, N.; Saidi, N.; Bouzaiane, O.; Murano, F. Caractérisation des paramètres physico-chimiques et microbiologiques au cours d’un cycle de compostage d’ordures ménagères. Environ. Ingénierie Déve. 2005, 40, 7989. [Google Scholar] [CrossRef]
- El Fels, L.; Zamama, M.; Asli, A.E.; Hafidi, M. Assessment of Biotransformation of Organic Matter during Co-Composting of Sewage Sludge-Lignocelullosic Waste by Chemical, FTIR Analyses, and Phytotoxicity Tests. Int. Biodeterior. Biodegrad. 2014, 87, 128–137. [Google Scholar] [CrossRef]
- Majbar, Z.; Lahlou, K.; Ben Abbou, M.; Ammar, E.; Triki, A.; Abid, W.; Nawdali, M.; Bouka, H.; Taleb, M.; El Haji, M.; et al. Co-Composting of Olive Mill Waste and Wine-Processing Waste: An Application of Compost as Soil Amendment. J. Chem. 2018, 2018, 7918583. [Google Scholar] [CrossRef]
- Vico, A.; Pérez-Murcia, M.D.; Bustamante, M.A.; Agulló, E.; Marhuenda-Egea, F.C.; Sáez, J.A.; Paredes, C.; Pérez-Espinosa, A.; Moral, R. Valorization of Date Palm (Phoenix Dactylifera L.) Pruning Biomass by Co-Composting with Urban and Agri-Food Sludge. J. Environ. Manag. 2018, 226, 408–415. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, R.; Yang, H.; Zhou, B.; Wang, X.; Xie, Y. Comparison of Chlorination Behaviors between Norfloxacin and Ofloxacin: Reaction Kinetics, Oxidation Products and Reaction Pathways. Chemosphere 2019, 215, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Shen, L.; Wu, X.; Amanze, C.; Zeng, W. Effect of Bamboo Sphere Amendment on the Organic Matter Decomposition and Humification of Food Waste Composting. Waste Manag. 2021, 133, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Amanze, C.; Yu, R.; Li, J.; Wu, X.; Shen, L.; Liu, Y.; Yu, Z.; Wang, J.; Zeng, W. Insight into the Microbial Mechanisms for the Improvement of Composting Efficiency Driven by Aneurinibacillus sp. LD3. Bioresour. Technol. 2022, 359, 127487. [Google Scholar] [CrossRef] [PubMed]
- Zaman, B.; Hardyanti, N.; Purwono; Ramadan, B.S. An Innovative Thermal Composter to Accelerate Food Waste Decomposition at the Household Level. Bioresour. Technol. Rep. 2022, 19, 101203. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Pandey, A.K.; Khan, J.; Bundela, P.S.; Wong, J.W.C.; Selvam, A. Evaluation of Thermophilic Fungal Consortium for Organic Municipal Solid Waste Composting. Bioresour. Technol. 2014, 168, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Abdool-Ghany, A.A.; Pollier, C.G.L.; Oehlert, A.M.; Swart, P.K.; Blare, T.; Moore, K.; Solo-Gabriele, H.M. Assessing Quality and Beneficial Uses of Sargassum Compost. Waste Manag. 2023, 171, 545–556. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Guo, X.; Zhao, T.; Zhang, B. Succession and Diversity of Microorganisms and Their Association with Physicochemical Properties during Green Waste Thermophilic Composting. Waste Manag. 2018, 73, 101–112. [Google Scholar] [CrossRef]
- Jara-Samaniego, J.; Pérez-Murcia, M.D.; Bustamante, M.A.; Pérez-Espinosa, A.; Paredes, C.; López, M.; López-Lluch, D.B.; Gavilanes-Terán, I.; Moral, R. Composting as Sustainable Strategy for Municipal Solid Waste Management in the Chimborazo Region, Ecuador: Suitability of the Obtained Composts for Seedling Production. J. Clean. Prod. 2017, 141, 1349–1358. [Google Scholar] [CrossRef]
- Muscolo, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Jeske-Kaczanowska, A. Are Raw Materials or Composting Conditions and Time That Most Influence the Maturity and/or Quality of Composts? Comparison of Obtained Composts on Soil Properties. J. Clean. Prod. 2018, 195, 93–101. [Google Scholar] [CrossRef]
- Meng, X.; Yan, J.; Zuo, B.; Wang, Y.; Yuan, X.; Cui, Z. Full-Scale of Composting Process of Biogas Residues from Corn Stover Anaerobic Digestion: Physical-Chemical, Biology Parameters and Maturity Indexes during Whole Process. Bioresour. Technol. 2020, 302, 122742. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, X.; Kahn, B.A.; Stoffella, P.J.; Calvert, D.V. Plant Nutrition Benefits of Phosphorus, Potassium, Calcium, Magnesium, and Micronutrients from Compost Utilization. Compos. Util. Hortic. Crop. Syst. 2001, 307–320. [Google Scholar]
- Takahashi, S. Phosphorus Fractionation of Composted Crop Residues and Forms of Soil Phosphorus after 22 Years of Compost Application to Andosols. Commun. Soil Sci. Plant Anal. 2014, 45, 1003–1010. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Sun, H.; Zhou, S.; Zou, G. Straw Biochar Hastens Organic Matter Degradation and Produces Nutrient-Rich Compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Bohacz, J. Lignocellulose-Degrading Enzymes, Free-Radical Transformations during Composting of Lignocellulosic Waste and Biothermal Phases in Small-Scale Reactors. Sci. Total Environ. 2017, 580, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Rashad, F.M.; Saleh, W.D.; Moselhy, M.A. Bioconversion of Rice Straw and Certain Agro-Industrial Wastes to Amendments for Organic Farming Systems: 1. Composting, Quality, Stability and Maturity Indices. Bioresour. Technol. 2010, 101, 5952–5960. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.P.; Paredes, C.; Sánchez-Monedero, M.A.; Cegarra, J. Maturity and Stability Parameters of Composts Prepared with a Wide Range of Organic Wastes. Bioresour. Technol. 1998, 63, 91–99. [Google Scholar] [CrossRef]
- Wang, G.; Kong, Y.; Liu, Y.; Li, D.; Zhang, X.; Yuan, J.; Li, G. Evolution of Phytotoxicity during the Active Phase of Co-Composting of Chicken Manure, Tobacco Powder and Mushroom Substrate. Waste Manag. 2020, 114, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.; Noguera, P.; Burés, S. National Inventory of Organic Wastes for Use as Growing Media for Ornamental Potted Plant Production: Case Study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Fuchs, J.; Galli, U.; Schleiss, K.; Wellinger, A. ASCP Guidelines 2001: Quality Criteria for Composts and Digestates from Biodegradable Waste Management; Association of Swiss Compost Plants (ASCP) in collaboration with the Swiss Biogas Forum: Zurich, Switzerland, 2001. [Google Scholar]
- Zbytniewski, R.; Buszewski, B. Characterization of Natural Organic Matter (NOM) Derived from Sewage Sludge Compost. Part 1: Chemical and Spectroscopic Properties. Bioresour. Technol. 2005, 96, 471–478. [Google Scholar] [CrossRef]
- Abaker, M.G. Suivi de Maturation de Composts Mixtes par Spectrométrie D’absorption et de Fluorescence UV-Visible. PhD Thesis, Université de Toulon, La Valette-du-Var, France, 2016. [Google Scholar]
- Rigobello, E.S.; Campos, S.X.; Azevedo, E.R.D.; Dantas, A.D.B.; Vieira, E.M. Comparative Characterization of Humic Substances Extracted from Freshwater and Peat of Different Apparent Molecular Sizes. Rev. Ambiente Água 2017, 12, 774. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, Z.; Ma, T.; Zhang, X.; Wang, R.; Liu, Y.; Sun, B.; Xu, T.; Ding, G.; Wei, Y.; et al. Phosphorus Excess Changes Rock Phosphate Solubilization Level and Bacterial Community Mediating Phosphorus Fractions Mobilization during Composting. Bioresour. Technol. 2021, 337, 125433. [Google Scholar] [CrossRef]
- Gómez, R.B.; Lima, F.V.; Ferrer, A.S. The Use of Respiration Indices in the Composting Process: A Review. Waste Manag. Res. 2006, 24, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, W.; Czekała, W.; Nowak, M.; Dach, J. Production of Compost from Logging Residues. Bioresour. Technol. 2023, 376, 128878. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of Aeration Rate, C/N Ratio and Moisture Content on the Stability and Maturity of Compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Sanad, H.; Moussadek, R.; Mouhir, L.; Oueld Lhaj, M.; Dakak, H.; El Azhari, H.; Yachou, H.; Ghanimi, A.; Zouahri, A. Assessment of Soil Spatial Variability in Agricultural Ecosystems Using Multivariate Analysis, Soil Quality Index (SQI), and Geostatistical Approach: A Case Study of the Mnasra Region, Gharb Plain, Morocco. Agronomy 2024, 14, 1112. [Google Scholar] [CrossRef]


| Parameters | Units | MGW | FL | GC | WS | SM |
|---|---|---|---|---|---|---|
| TOC | %DM | 14.18 | 42.77 | 20.11 | 35.12 | 29.45 |
| OM | %DM | 24 | 78.98 | 35.40 | 60.40 | 53.36 |
| Ash | %DM | 76 | 21.02 | 64.60 | 39.60 | 46.64 |
| TN | %DM | 0.40 | 1.12 | 0.60 | 0.95 | 1.01 |
| pH | - | 7.16 | 7.05 | 7.10 | 6.5 | 8.05 |
| EC | mS/cm | 3.09 | 3.95 | 4.87 | 0.6 | 4.66 |
| Treatment | Mixture Content |
|---|---|
| C1 | 30% SM + 10% WS + 60% MGW |
| C2 | 30% SM + 10% WS + 60% FL |
| C3 | 30% SM + 10% WS + 60% GC |
| Micronutrient | Units | Limit Values | Reference | C1 | C2 | C3 | |||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | ||||
| Zn | mg/Kg DM | <100 | [55] | 119 | 132 | 52 | 83 | 98 | 112 |
| Cu | mg/Kg DM | 431–600 | ND | ND | ND | ND | ND | ND | |
| Fe | mg/Kg DM | <9300 | 478 | 580 | 211 | 321 | 324 | 357 | |
| Mn | mg/Kg DM | <200 | 266 | 315 | 223 | 230 | 130 | 232 | |
| Variables | pH | CE | DM | OM | VS | Ash | TOC | TN | TP | K | Na | Mg | Ca | N-NO3 | N-NH4 | Fe | Zn | Mn | Respiration | GI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | |||||||||||||||||||
| CE | −0.207 | 1 | ||||||||||||||||||
| DM | 0.550 | 0.072 | 1 | |||||||||||||||||
| OM | 0.311 | 0.014 | 0.400 | 1 | ||||||||||||||||
| VS | 0.311 | 0.014 | 0.400 | 1.000 | 1 | |||||||||||||||
| Ash | −0.311 | −0.014 | −0.400 | −1.000 | −1.000 | 1 | ||||||||||||||
| TOC | 0.311 | 0.014 | 0.400 | 1.000 | 1.000 | −1.000 | 1 | |||||||||||||
| TN | −0.383 | 0.133 | −0.345 | 0.140 | 0.140 | −0.140 | 0.140 | 1 | ||||||||||||
| TP | −0.231 | 0.132 | 0.058 | 0.264 | 0.264 | −0.264 | 0.264 | 0.843 | 1 | |||||||||||
| K | −0.319 | 0.001 | −0.343 | 0.256 | 0.256 | −0.256 | 0.256 | 0.884 | 0.776 | 1 | ||||||||||
| Na | −0.136 | 0.021 | −0.092 | 0.597 | 0.597 | −0.597 | 0.597 | 0.794 | 0.794 | 0.871 | 1 | |||||||||
| Mg | 0.030 | 0.125 | −0.020 | 0.558 | 0.558 | −0.558 | 0.558 | 0.764 | 0.797 | 0.749 | 0.903 | 1 | ||||||||
| Ca | −0.402 | −0.043 | −0.554 | −0.349 | −0.349 | 0.349 | −0.349 | 0.772 | 0.674 | 0.691 | 0.463 | 0.498 | 1 | |||||||
| N-NO3 | −0.572 | 0.046 | −0.668 | −0.244 | −0.244 | 0.244 | −0.244 | 0.811 | 0.611 | 0.736 | 0.530 | 0.491 | 0.879 | 1 | ||||||
| N-NH4 | 0.388 | 0.359 | 0.705 | 0.647 | 0.647 | −0.647 | 0.647 | −0.284 | −0.062 | −0.209 | 0.070 | 0.093 | −0.671 | −0.628 | 1 | |||||
| Fe | −0.297 | 0.123 | −0.358 | −0.562 | −0.562 | 0.562 | −0.562 | 0.530 | 0.511 | 0.322 | 0.111 | 0.280 | 0.855 | 0.683 | −0.611 | 1 | ||||
| Zn | −0.104 | 0.031 | −0.290 | −0.886 | −0.886 | 0.886 | −0.886 | −0.161 | −0.206 | −0.295 | −0.585 | −0.390 | 0.358 | 0.185 | −0.604 | 0.670 | 1 | |||
| Mn | −0.159 | 0.101 | 0.076 | 0.120 | 0.120 | −0.120 | 0.120 | 0.808 | 0.953 | 0.741 | 0.690 | 0.732 | 0.707 | 0.630 | −0.140 | 0.612 | −0.036 | 1 | ||
| Respiration | 0.129 | 0.201 | 0.412 | 0.236 | 0.236 | −0.236 | 0.236 | −0.167 | −0.022 | −0.175 | −0.026 | 0.021 | −0.322 | −0.362 | 0.705 | −0.211 | −0.266 | −0.070 | 1 | |
| GI | −0.523 | 0.239 | −0.422 | −0.005 | −0.005 | 0.005 | −0.005 | 0.909 | 0.836 | 0.863 | 0.724 | 0.681 | 0.853 | 0.859 | −0.310 | 0.633 | −0.038 | 0.801 | −0.123 | 1 |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| pH | −0.459 | 0.318 | 0.091 | −0.679 |
| CE | 0.085 | 0.075 | 0.610 | 0.560 |
| DM | −0.451 | 0.486 | 0.516 | −0.393 |
| OM | −0.034 | 0.978 | −0.146 | 0.001 |
| VS | −0.034 | 0.978 | −0.146 | 0.001 |
| Ash | 0.034 | −0.978 | 0.146 | −0.001 |
| TOC | −0.034 | 0.978 | −0.146 | 0.001 |
| TN | 0.939 | 0.187 | 0.011 | 0.052 |
| TP | 0.846 | 0.343 | 0.269 | −0.167 |
| K | 0.876 | 0.300 | −0.152 | 0.049 |
| Na | 0.734 | 0.634 | −0.128 | 0.003 |
| Mg | 0.717 | 0.588 | 0.066 | −0.149 |
| Ca | 0.912 | −0.326 | 0.010 | −0.138 |
| N-NO3 | 0.912 | −0.241 | −0.143 | 0.135 |
| N-NH4 | −0.460 | 0.725 | 0.445 | 0.175 |
| Fe | 0.691 | −0.533 | 0.337 | −0.222 |
| Zn | 0.037 | −0.898 | 0.241 | −0.218 |
| Mn | 0.837 | 0.208 | 0.330 | −0.293 |
| Respiration | −0.272 | 0.347 | 0.593 | 0.223 |
| GI | 0.962 | 0.051 | 0.117 | 0.175 |
| Eigenvalue | 7.895 | 7.107 | 1.713 | 1.315 |
| Variability (%) | 39.474 | 35.537 | 8.564 | 6.576 |
| Cumulative (%) | 39.474 | 75.011 | 83.575 | 90.151 |
| Parameters | Units | C1 | C2 | C3 |
|---|---|---|---|---|
| pH | - | 6.61 | 6.80 | 5.90 |
| EC | mS/cm | 2.92 | 2.45 | 3.37 |
| OM | %DM | 29.00 | 55.00 | 31.00 |
| TOC | %DM | 16.86 | 31.98 | 18.02 |
| C/N | - | 12.49 | 16.15 | 17.67 |
| VS | %DM | 29.00 | 55.00 | 31.00 |
| DM | %DM | 83.00 | 75.30 | 63.46 |
| Ash | %DM | 71.00 | 45.00 | 69.00 |
| TN | %DM | 1.35 | 1.98 | 1.02 |
| TP | %DM | 2.90 | 3.22 | 1.30 |
| Ca | %DM | 10.21 | 11.37 | 9.12 |
| Mg | %DM | 5.03 | 6.55 | 4.87 |
| K | %DM | 0.49 | 0.61 | 0.39 |
| Na | %DM | 0.25 | 0.39 | 0.25 |
| N-NH4 | mg/Kg DM | 130.00 | 140.00 | 120.00 |
| N-NH3 | mg/Kg DM | 870.00 | 850.00 | 800.00 |
| Zn | mg/Kg DM | 132.00 | 83.00 | 112.00 |
| Fe | mg/Kg DM | 580.00 | 321.00 | 357.00 |
| Mn | mg/Kg DM | 315.00 | 230.00 | 232.00 |
| E4/E6 | - | 0.32 | 0.12 | 0.84 |
| GI | %DM | 86.93 | 94.20 | 80.03 |
| Respiration | mg/50 g | 5.00 | 4.02 | 3.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oueld Lhaj, M.; Moussadek, R.; Mouhir, L.; Mdarhri Alaoui, M.; Sanad, H.; Iben Halima, O.; Zouahri, A. Assessing the Evolution of Stability and Maturity in Co-Composting Sheep Manure with Green Waste Using Physico-Chemical and Biological Properties and Statistical Analyses: A Case Study of Botanique Garden in Rabat, Morocco. Agronomy 2024, 14, 1573. https://doi.org/10.3390/agronomy14071573
Oueld Lhaj M, Moussadek R, Mouhir L, Mdarhri Alaoui M, Sanad H, Iben Halima O, Zouahri A. Assessing the Evolution of Stability and Maturity in Co-Composting Sheep Manure with Green Waste Using Physico-Chemical and Biological Properties and Statistical Analyses: A Case Study of Botanique Garden in Rabat, Morocco. Agronomy. 2024; 14(7):1573. https://doi.org/10.3390/agronomy14071573
Chicago/Turabian StyleOueld Lhaj, Majda, Rachid Moussadek, Latifa Mouhir, Meriem Mdarhri Alaoui, Hatim Sanad, Oumaima Iben Halima, and Abdelmjid Zouahri. 2024. "Assessing the Evolution of Stability and Maturity in Co-Composting Sheep Manure with Green Waste Using Physico-Chemical and Biological Properties and Statistical Analyses: A Case Study of Botanique Garden in Rabat, Morocco" Agronomy 14, no. 7: 1573. https://doi.org/10.3390/agronomy14071573





