Zinc Oxide Nanoparticles in the “Soil–Bacterial Community–Plant” System: Impact on the Stability of Soil Ecosystems
Abstract
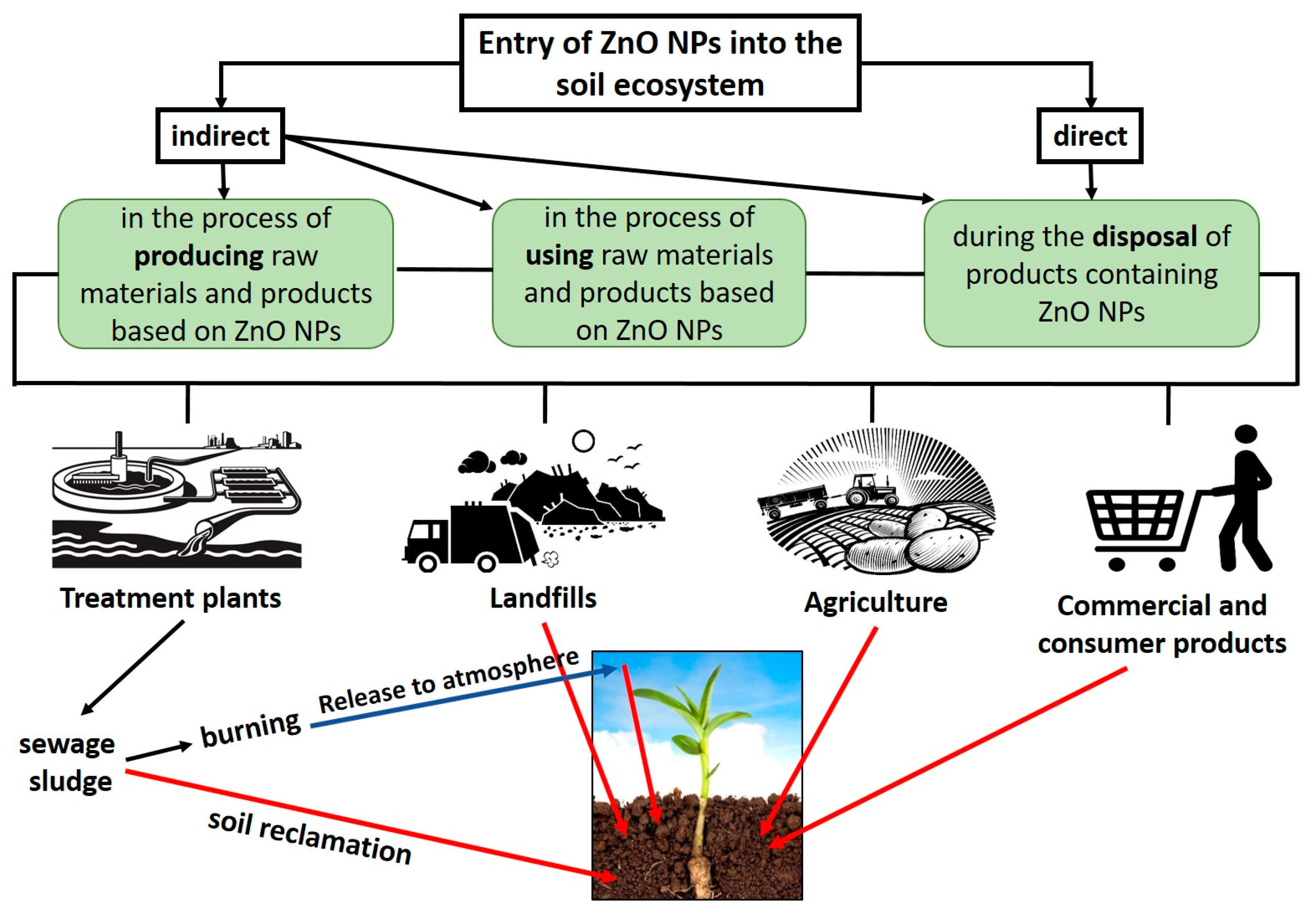
:1. Introduction
2. Main Effects of ZnO Nanoparticles on the Physical and Chemical Properties of Soil
3. Effects of ZnO NPs on Crops
3.1. Seed Priming
3.2. Foliar Treatment
3.3. Application to the Soil
| Size of ZnO NPs, nm | Plant | Concentration of ZnO NPs, mg/L | Main Effect | Reference |
|---|---|---|---|---|
| Positive Effect | ||||
| 37 | Zea mays L. | 100 | Improve seed germination parameters and plant growth processes. | [45] |
| n.d. 1 | Capsicum annuum L. | 750 | Increase seed germination and plant growth expressed in stimulating morphometric characteristics. | [49] |
| 20–30 | Triticum aestivum L. | 10 | Enhance seed germination and water uptake by seeds, increase α-amylase activity and the content of photosynthetic pigments. | [50] |
| 9–13 | Triticum aestivum L. | 10, 25, 50, 100 | Stimulate seed germination and development of morphometric characteristics in seedlings. | [53] |
| 40–50 | Triticum aestivum L. | 250–500 | Increase shoot and root growth and chlorophyll content. | [54] |
| 25 | Arachis hypogea L. | 1000 | Enhance seed germination, promote the appearance of early shoots and flowering and have high chlorophyll content in the leaves. | [56] |
| 25 | Zea mays L. | 50–1500 | Increase germination, root length and shoot growth. | [58] |
| 20 | Zea mays L. | 500 | Increase the percentage of germination of planting material, grain weight, potassium content and α-amylase activity, stimulate growth physiological processes in the plant and photosynthesis mechanisms. | [59] |
| 100 | Zea mays L. | 100 | [60] | |
| 35 | Oryza sativa L. | 25 | Promote plant growth, increase pigment content and accumulation of Zn and Fe. | [62] |
| 20–30 | Cicer arietinum L. | 2000 | Improve seed germination and root growth, increase synthesis of growth-stimulating hormones in shoots. | [63] |
| ≤50 | Portulaca oleracea L. | 10, 100 | Increase the percentage of seed germination, plant growth processes and amount of chlorophyll and carotenoids. | [65] |
| 20–60 | Allium cepa L. | 50–1600 | Stimulate seed germination | [66] |
| 50 | Oryza sativa L. | 500, 1000, 5000 | Increase in shoot length, root biomass, chlorophyll and Zn content in plants, yield indicators. | [76] |
| 20 | Vigna radiata L., Cicer arietinum L. | 20 | Stimulate plant development and root formation | [79] |
| 1.2–6.8 | Cyamopsis tetragonoloba L | 10 | A pronounced increase in plant biomass, shoot length, root length, root surface area, chlorophyll content and total soluble protein in leaves. | [80] |
| 51 | Triticum aestivum L., Oryza sativa L. | 7 | Increase in growth, chlorophyll, Zn content in shoots, roots and grains and plant resistance to abiotic stress (salinity). | [85] |
| 71 | Triticum aestivum L. | 0.1–5 | Promote seed germination and stimulate the development of morphometric characteristics. | [86] |
| n.d. | Triticum aestivum L. | 25, 50, 100, 200 | Stimulate the development of morphometric characteristics, increase the layer of cortical cells, the thickness of phloem and xylem and chlorophyll content. | [87] |
| 20 | Salvia miltiorrhiza (Bge.) | 100, 700 | increase in above- and under-ground biomass, root diameter and Zn content in roots. | [88] |
| <50 | Oryza sativa L. | 25, 50, 100 | Improve the absorption capacity of roots and increase Zn in them. | [89] |
| 16–31 | Solanum lycopersicum L. | 500 | Increase in plant height, fruit weight, activity of antioxidant enzymes and decrease in the ROS content. | [90] |
| Negative effect | ||||
| n.d. | Capsicum annuum L. | 250, 500 | Reduce seed germination and plant growth processes. | [49] |
| 3–5 | Triticum aestivum L. | 1000–2000 | [55] | |
| n.d. | Oryza sativa L. | 100–500 | Decrease in morphometric characteristics of roots. | [61] |
| ≤50 | Portulaca oleracea L. | 500 | Rupture of cell membranes, deformation of chloroplasts and a decrease in their number in plants. | [65] |
| n.d. | Cicer arietinum L var. HC-1 | 10 | Adverse effect on root growth. | [77] |
| 19 | Lolium perenne L. | 1, 5, 10 | Reduce biomass, shrink root tips and vacuolate epidermal and cortical root cells. | [78] |
| 20 | Vigna radiata L., Cicer arietinum L. | 2000 | Slow down the growth of roots and shoots. | [79] |
| 50 | Arabidopsis thaliana L. | 200, 300 | Inhibit root and shoot growth, reduce chlorophyll content, photosynthesis intensity, leaf stomatal conductivity, intercellular CO2 concentration and transpiration rate. | [84] |
| <50 | Oryza sativa L. | 500 | Suppress seedling growth by reducing their biomass, reduce root elongation and chlorophyll content. | [89] |
4. Effects of ZnO NPs on the Microbiota of Soil and Rhizosphere
5. Effects of ZnO NPs on Soil Invertebrates
6. Mechanisms of Toxicity of ZnO NPs to Plants, Microorganisms and Invertebrates of Soil and Rhizosphere
6.1. Effect of Soil on Toxicity of ZnO NPs to Plants and Bacteria
6.1.1. Soil Acidity
6.1.2. Soil Organic Matter
6.2. Toxic Effects of ZnO NPs on Plants
6.3. Mechanism of ZnO NPs Toxicity on Bacteria
6.4. Mechanism of ZnO NPs Toxicity on Invertebrates
7. Conclusions
- Nanopriming of seeds with ZnO NPs at a concentration not exceeding 500 mg/L showed good results on the germination and viability of plant seeds, and, therefore, NPs can be used as a potential fertilizer to increase crop yields. These results indicate that relatively low concentrations of ZnO NPs can have a stimulating effect on the germination and growth parameters of various crop plants.
- Soil acidity affected the solubility of NPs and their toxicity. Acidic soil promotes the dissolution of ZnO NPs with the release of free ions and a decrease in the aggregation of NPs, which manifests itself in an increased toxic effect on soil microorganisms.
- Smaller NPs (up to ~35–40 nm) tended to have a stronger inhibitory effect; with increasing size, the inhibitory effect decreased (>50 nm). Smaller NPs are characterized by a larger specific surface area, which determines their surface charge density and is critical for attachment to the cell membrane and subsequent penetration into the cell, high free surface energy, promoting the formation of free radicals and ROS. All this contributes to causing significant damage to microbial cells.
- ZnO NPs had a significant negative impact on the diversity, biomass, activity and functions of the soil microbiome. It is noteworthy that the decrease in microbial biomass and soil enzyme activity was more pronounced than the decrease in microbial diversity.
- The toxicity of NPs towards soil microbiota had a dose-dependent nature, also known as the hormesis effect: low doses (up to 250 mg/kg) promoted stimulation, high doses (>500 mg/kg)—inhibition.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parada, J.; Rubilar, O.; Fernández-Baldo, M.A.; Bertolino, F.A.; Durán, N.; Seabra, A.B.; Tortella, G.R. The nanotechnology among US: Are metal and metal oxides nanoparticles a nano or mega risk for soil microbial communities? Crit. Rev. Biotechnol. 2019, 39, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Keswani, C.; Abhilash, P.C.; Fraceto, L.F.; Singh, H.B. Integrated approach of agri-nanotechnology: Challenges and future trends. Front. Plant Sci. 2017, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, A.; Kolesnikov, S.; Rajput, V.D.; Minkina, T. Effects of zinc-oxide nanoparticles on soil microbial community and their functionality. In Zinc-Based Nanostructures for Environmental and Agricultural Applications; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 14; pp. 267–284. [Google Scholar] [CrossRef]
- Solanki, M. The Zn as a vital micronutrient in plants. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e4026. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Meetei, T.T.; Devi, Y.B.; Panda, S.K.; Upadhyaya, H. Zinc oxide nanoparticles (ZnO-NPs): A promising nanoparticle in renovating plant science. Acta Physiol. Plant. 2021, 43, 136. [Google Scholar] [CrossRef]
- Khan, S.T.; Malik, A.; Alwarthan, A.; Shaik, M.R. The enormity of the zinc deficiency problem and available solutions; an overview. Arabian J. Chem. 2022, 15, 103668. [Google Scholar] [CrossRef]
- Praharaj, S.; Skalicky, M.; Maitra, S.; Bhadra, P.; Shankar, T.; Brestic, M.; Hossain, A. Zinc biofortification in food crops could alleviate the zinc malnutrition in human health. Molecules 2021, 26, 3509. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Khan, S.T. Interaction of Engineered Nanomaterials with Soil Microbiome and Plants: Their impact on plant and soil health. In Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development; Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 181–199. [Google Scholar] [CrossRef]
- Feng, J.N.; Guo, X.P.; Chen, Y.R.; Lu, D.P.; Niu, Z.S.; Tou, F.Y.; Yang, Y. Time-dependent effects of ZnO nanoparticles on bacteria in an estuarine aquatic environment. Sci. Total Environ. 2020, 698, 134298. [Google Scholar] [CrossRef]
- Chen, C.; Unrine, J.M.; Hu, Y.; Guo, L.; Tsyusko, O.V.; Fan, Z.; Wei, G. Responses of soil bacteria and fungal communities to pristine and sulfidized zinc oxide nanoparticles relative to Zn ions. J. Hazard. Mater. 2021, 405, 124258. [Google Scholar] [CrossRef]
- Verma, A.; Prasher, P.; Sharma, M.; Kumar, A.; Mudila, H. Zinc oxide nanoparticles: Physiological and molecular responses in plants. In Zinc-Based Nanostructures for Environmental and Agricultural Applications; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 17; pp. 339–365. [Google Scholar] [CrossRef]
- Guerra, C.A.; Bardgett, R.D.; Caon, L.; Crowther, T.W.; Delgado-Baquerizo, M.; Montanarella, L. Tracking, targeting, and conserving soil biodiversity. Science 2021, 371, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Upadhyay, S.K.; Kumari, A.; Ranjan, A.; Mandzhieva, S.; Verma, K.K. Nanotechnology in the restoration of polluted soil. Nanomaterials 2022, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Thies, J.E.; Grossman, J.M. The Soil Habitat and Soil Ecology. In Biological Approaches to Regenerative Soil Systems, 2nd ed.; Uphoff, N., Thies., J., Eds.; CRC Press: Boca Raton, FL, USA, 2023; Chapter 7; pp. 69–84. [Google Scholar] [CrossRef]
- Singh, S.; Chhabra, R.; Sharma, A.; Bisht, A. Harnessing the Power of Zinc-Solubilizing Bacteria: A Catalyst for a Sustainable Agrosystem. Bacteria 2024, 3, 15–29. [Google Scholar] [CrossRef]
- Hafeez, F.Y.; Abaid-Ullah, M.; Hassan, M.N. Plant growth-promoting Rhizobacteria as zinc mobilizers: A promising approach for cereals biofortification. In Bacteria in Agrobiology: Crop Productivity; Maheshwari, D., Saraf, M., Aeron, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 217–235. [Google Scholar] [CrossRef]
- Clunes, J.; Valle, S.; Dörner, J.; Martínez, O.; Pinochet, D.; Zúñiga, F.; Blum, W.E. Soil fragility: A concept to ensure a sustainable use of soils. Ecol. End. 2022, 139, 108969. [Google Scholar] [CrossRef]
- He, J.; Wang, D.; Zhou, D. Transport and retention of silver nanoparticles in soil: Effects of input concentration, particle size and surface coating. Sci. Total Environ. 2019, 648, 102–108. [Google Scholar] [CrossRef]
- He, X.; Fu, P.; Aker, W.G.; Hwang, H.M. Toxicity of engineered nanomaterials mediated by nano–bio–eco interactions. J. Environ. Sci. Health 2018, 36, 21–42. [Google Scholar] [CrossRef]
- Joo, S.H.; Zhao, D. Environmental dynamics of metal oxide nanoparticles in heterogeneous systems: A review. J. Hazard. Mater. 2017, 322, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Zaidi, S.J. Recent developments in the application of nanomaterials in agroecosystems. Nanomaterials 2020, 10, 2411. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, M.; Bhalla, V. Aggregates of the pentacenequinone derivative as reactors for the preparation of Ag@Cu2O core–shell NPs: An active photocatalyst for Suzuki and Suzuki type coupling reactions. Chem. Commun. 2015, 51, 12529–12532. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Su-shkova, S.N.; Mandzhieva, S.; Singh, R.; Movsesyan, H.S. Effects of zinc-oxide nanopar-ticles on soil, plants, animals and soil organisms: A review. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Feng, X.; Yan, Y.; Wan, B.; Li, W.; Jaisi, D.P.; Zheng, L.; Liu, F. Enhanced dissolution and transformation of ZnO nanoparticles: The role of inositol hexakisphosphate. Environ. Sci. Technol. 2016, 50, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.H.; Tsai, Y.C.; Hsiung, C.E.; Lin, Y.H.; Shih, Y.H. Influence of water chemistry on the environmental behaviors of commercial ZnO nanoparticles in various water and wastewater samples. J. Hazard. Mater. 2017, 322, 348–356. [Google Scholar] [CrossRef]
- Darlington, T.K.; Neigh, A.M.; Spencer, M.T.; Guyen, O.T.N.; Oldenburg, S.J. Nanoparticle characteristics affecting environmental fate and transport through soil. Environ. Toxicol. Chem. 2009, 28, 1191–1199. [Google Scholar] [CrossRef]
- Fang, J.; Shan, X.Q.; Wen, B.; Lin, J.M.; Owens, G. Stability of titania nanoparticles in soil sus-pensions and transport in saturated homogeneous soil columns. Environ. Pollut. 2009, 157, 1101–1109. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shaghaleh, H.; Hamoud, Y.A.; Holford, P.; Shao, H.; Qi, W.; Wu, T. Zinc oxide nano-particles: Potential effects on soil properties, crop production, food processing, and food quality. Environ. Sci. Pollut. Res. 2021, 28, 36942–36966. [Google Scholar] [CrossRef]
- Taha, M.R.; Taha, O.M.E. Influence of nano-material on the expansive and shrinkage soil behavior. J. Nanopart. Res. 2012, 14, 190. [Google Scholar] [CrossRef]
- Bayat, H.; Kolahchi, Z.; Valaey, S.; Rastgou, M.; Mahdavi, S. Novel impacts of nanoparticles on soil properties: Tensile strength of aggregates and compression characteristics of soil. Arch. Agron. Soil Sci. 2017, 64, 776–789. [Google Scholar] [CrossRef]
- Komendová, R.; Žídek, J.; Berka, M.; Jemelková, M.; Řezáčová, V.; Conte, P.; Kučerík, J. Small-Sized Platinum Nanoparticles in Soil Organic Matter: Influence on Water Holding Capacity, Evaporation and Structural Rigidity. Sci. Total Environ. 2019, 694, 133822. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Arancibia-Miranda, N.; Mlih, R.; Cáceres-Jensen, L.; Bolan, N.; Mora, M.D.L.L. Impact on some soil physical and chemi-cal properties caused by metal and metallic oxide engineered nanoparticles: A review. Nanomaterials 2023, 13, 572. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Sci. Total Environ. 2018, 644, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.; Klaessig, F.C.; Landry, T.D.; Moudgil, B.; Pauluhn, J.; Thomas, K.; Trottier, R.; Wood, S. Research strategies for safety evaluation of nanomaterials, part V: Role of dissolution in biological fate and effects of nanoscale particles. Toxicol. Sci. 2006, 90, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, G.E.; Philippe, A.; Bundschuh, M.; Metreveli, G.; Klitzke, S.; Rakcheev, D.; Grün, A.; Kumahor, S.K.; Kühn, M.; Baumann, T.; et al. Understanding the fate and biological effects of Ag and TiO2-nanoparticles in the environment: The quest for advanced analytics and interdisciplinary concepts. Sci. Total Environ. 2015, 535, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, X.; Wei, L.; Chen, L.; Song, B.; Shao, L. The toxicology of ion-shedding zinc oxide nanoparticles. Crit. Rev. Toxicol. 2016, 46, 348–384. [Google Scholar] [CrossRef] [PubMed]
- Domingos, R.F.; Baalousha, M.A.; Ju-Nam, Y.; Reid, M.M.; Tufenkji, N.; Lead, J.R.; Wilkinson, K.J. Characterizing manufactured nanoparticles in the environment: Multimethod determination of particle sizes. Environ. Sci. Technol. 2009, 43, 7277–7284. [Google Scholar] [CrossRef] [PubMed]
- Domingos, R.F.; Rafiei, Z.; Monteiro, C.E.; Khan, M.A.; Wilkinson, K.J. Agglomeration and dissolution of zinc oxide nanoparticles: Role of pH, ionic strength and fulvic acid. Environ. Chem. 2013, 10, 306–312. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Ren, M.; Varela-Ramirez, A.; Li, C.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: Electron microprobe and confocal microscopy studies. Chem. Eng. J. 2012, 184, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, M.; Li, H.; Ren, Y.; Siddique, K.H.; Chen, Y.; Zhang, S. Effects of zinc fertilizer on maize yield and water-use efficiency under different soil water conditions. Field Crop Res. 2020, 248, 107718. [Google Scholar] [CrossRef]
- Akhtar, M.; Yousaf, S.; Sarwar, N.; Hussain, S. Zinc biofortification of cereals–role of phosphorus and other impediments in alkaline calcareous soils. Environ. Geochem. Health 2019, 41, 2365–2379. [Google Scholar] [CrossRef]
- Itroutwar, P.D.; Kasivelu, G.; Raguraman, V.; Malaichamy, K.; Sevathapandian, S.K. Effects of biogenic zinc oxide nanoparticles on seed germination and seedling vigor of maize (Zea mays). Biocatal. Agric. Biotechnol. 2020, 29, 101778. [Google Scholar] [CrossRef]
- Atar, B.; Uygur, V.; Sukuşu, E. Effects of priming with copper, zinc and phosphorus on seed and seedling composition in Wheat and Barley. Turk. J. Agric. Nat. Sci. 2020, 7, 104–111. [Google Scholar] [CrossRef]
- Rameshraddy, G.; Pavithra, J.; Mahesh, S.; Geetha, K.N.; Shankar, A.G. Seed priming and foliar spray with nano zinc improves stress adaptability and seed zinc content without compromising seed yield in ragi (Finger millet). Int. J. Pure Appl. Biosci. 2017, 5, 251–258. [Google Scholar] [CrossRef]
- Kumar, G.D.; Raja, K.; Natarajan, N.; Govindaraju, K.; Subramanian, K.S. Invigouration treatment of metal and metal oxide nanoparticles for improving the seed quality of aged chilli seeds (Capsicum annum L.). Mater. Chem. Phys. 2020, 242, 122492. [Google Scholar] [CrossRef]
- Afrayeem, S.M.; Chaurasia, A.K. Effect of zinc oxide nanoparticles on seed germination and seed vigour in chilli (Capsicum annuum L.). J. Pharmacogn. Phytochem. 2017, 6, 1564–1566. [Google Scholar]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. (Ed.) Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; 672p. [Google Scholar]
- Doğaroğlu, Z.G.; Eren, A.; Baran, M.F. Effects of ZnO nanoparticles and ethylenediamine-N, N′-disuccinic acid on seed germination of four different plants. Glob. Chall. 2019, 3, 1800111. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Bansal, S.; Jangir, L.K.; Awasthi, G.; Awasthi, K.K.; Awasthi, K. Effect of ZnO nanoparticles on germination of Triticum aestivum seeds. Macromol. Symp. 2017, 376, 1700043. [Google Scholar] [CrossRef]
- Solanki, P.; Laura, J.S. Effect of ZnO nanoparticles on seed germination and seedling growth in wheat (Triticum aestivum). J. Pharmacogn. Phytochem. 2018, 7, 2048–2052. [Google Scholar]
- Chanda, S.; Donga, S.; Pande, J. Green synthesized zinc oxide nanoparticles: A review of antimicrobial, antioxidant, cytotoxic and photocatalytic properties. In Zinc Oxide: Production, Properties and Applications; Chapter 1; Galvan, C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2020; pp. 1–45. [Google Scholar]
- Prasad, T.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant. Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Tejaswini, K.S.; Kurnalliker, V.J.; Shakuntala, N.M.; Macha, S.I.; Khan, H.; Hiregoudar, S. Studies on efficacy of nanoparticles in improving seed physiological parameters in groundnut (Arachis hypogaea L.). Int. J. Chem. Stud. 2019, 7, 1786–1791. [Google Scholar]
- Subbaiah, L.V.; Prasad, T.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agric. Food. Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef] [PubMed]
- Alhammad, B.A.; Ahmad, A.; Seleiman, M.F.; Tola, E. Seed priming with nanoparticles and 24-epibrassinolide improved seed germination and enzymatic performance of Zea mays L. in salt-stressed soil. Plants 2023, 12, 690. [Google Scholar] [CrossRef]
- Boonyanitipong, P.; Kositsup, B.; Kumar, P.; Baruah, S.; Dutta, J. Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinforma. 2011, 1, 282. [Google Scholar] [CrossRef]
- Afzal, S.; Singh, N.K. Effect of zinc and iron oxide nanoparticles on plant physiology, seed quality and microbial community structure in a rice-soil-microbial ecosystem. Environ. Pollut. 2022, 314, 120224. [Google Scholar] [CrossRef]
- Pandey, A.C.; Sanjay, S.; Yadav, R. Application of ZnO nanoparticles in influencing the growth rate of Cicer arietinum. J. Exp. Nanosci. 2010, 5, 488–497. [Google Scholar] [CrossRef]
- Farhana Munis, M.F.H.; Alamer, K.H.; Althobaiti, A.T.; Kamal, A.; Liaquat, F.; Attia, H. ZnO nanoparticle-mediated seed priming induces biochemical and antioxidant changes in chickpea to alleviate Fusarium wilt. J. Fungi 2022, 8, 753. [Google Scholar] [CrossRef]
- Iziy, E.; Majd, A.; Vaezi-Kakhki, M.R.; Nejadsattari, T.; Noureini, S.K. Effects of zinc oxide nanoparticles on enzymatic and nonenzymatic antioxidant content, germination, and biochemical and ultrastructural cell characteristics of Portulaca oleracea L. Acta Soc. Bot. Pol. 2019, 88, 3639. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Wojnarowicz, J. Zinc oxide and zinc oxide nanoparticles impact on in vitro germination and seedling growth in Allium cepa L. Materials 2020, 13, 2784. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Peralta-Videa, J.R.; Plascencia-Villa, G.; José-Yacamán, M.; Gardea-Torresdey, J.L. Comparative toxicity assessment of CeO2 and ZnO nanoparticles towards Sinorhizobium meliloti, a symbiotic alfalfa associated bacterium: Use of advanced microscopic and spectroscopic techniques. J. Hazard. Mater. 2012, 241, 379–386. [Google Scholar] [CrossRef]
- Wang, P.; Menzies, N.W.; Lombi, E.; McKenna, B.A.; Johannessen, B.; Glover, C.J.; Kopittke, P.M. Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ. Sci. Technol. 2013, 47, 13822–13830. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, Y.; Hernandez-Viezcas, J.A.; Servin, A.D.; Hong, J.; Niu, G.; Gardea-Torresdey, J.L. Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: A life cycle study. J. Agric. Food. Chem. 2013, 61, 11945–11951. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Kouh, S.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative phytotoxicity of ZnO nanoparticles, ZnO microparticles, and Zn2+ on rapeseed (Brassica napus L.): Investigating a wide range of concentrations. Toxicol. Environ. Chem. 2014, 96, 861–868. [Google Scholar] [CrossRef]
- Donia, D.T.; Carbone, M. Seed priming with zinc oxide nanoparticles to enhance crop tolerance to environmental stresses. Int. J. Mol. Sci. 2023, 24, 17612. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomat. 2021, 2011, 6677616. [Google Scholar] [CrossRef]
- Molina, L.; Wittich, R.M.; van Dillewijn, P.; Segura, A. Plant-bacteria interactions for the elimination of atmospheric contaminants in cities. Agronomy 2021, 11, 493. [Google Scholar] [CrossRef]
- Tredenick, E.C.; Farrell, T.W.; Forster, W.A.; Psaltis, S.T. Nonlinear porous diffusion modeling of hydrophilic Ionic agrochemicals in astomatous plant cuticle aqueous pores: A mechanistic approach. Front. Plant Sci. 2017, 8, 746. [Google Scholar] [CrossRef]
- Bala, R.; Kalia, A.; Dhaliwal, S.S. Evaluation of efficacy of ZnO nanoparticles as remedial zinc nanofertilizer for rice. J. Soil Sci. Plant Nutr. 2019, 19, 379–389. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef]
- Mahajan, P.; Dhoke, S.K.; Khanna, A.S. Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnol. 2011, 2011, 696535. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Zeidan, M.S.; Mohamed, M.F.; Hamouda, H.A. Effect of foliar fertilization of Fe, Mn and Zn on wheat yield and quality in low sandy soils fertility. World J. Agric. Sci. 2010, 6, 696–699. [Google Scholar]
- Ghoneim, A.M. Effect of different methods of Zn application on rice growth, yield and nutrients dynamics in plant and soil. J. Agric. Ecol. Res. Int. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wang, W.; Hou, C.; Wang, S. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. Plant. Sci. 2016, 6, 1243. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, Z.; Akhtar, J.; Alhodaib, A.; Naz, T.; Zafar, M.I.; Iqbal, M.M.; Naz, I. Efficacy of ZnO nanoparticles in Zn fortification and partitioning of wheat and rice grains under salt stress. Sci. Rep. 2023, 13, 2022. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.; Kumar, S.; Patil, G.; Mankad, M.; Shah, Z. Impact of ZnO nanopriming on physiological and biochemical traits of wheat (Triticum aestivum L.) seedling. CABI Agric. Biosci. 2024, 5, 27. [Google Scholar] [CrossRef]
- Nazir, M.A.; Hasan, M.; Mustafa, G.; Tariq, T.; Ahmed, M.M.; Golzari Dehno, R.; Ghorbanpour, M. Zinc oxide nano-fertilizer differentially effect on morphological and physiological identity of redox-enzymes and biochemical attributes in wheat (Triticum aestivum L.). Sci. Rep. 2024, 14, 13091. [Google Scholar] [CrossRef]
- Wei, X.; Cao, P.; Wang, G.; Liu, Y.; Song, J.; Han, J. CuO, ZnO, and γ-Fe2O3 nanoparticles modified the underground biomass and rhizosphere microbial community of Salvia miltiorrhiza (Bge.) after 165-day exposure. Ecotoxicol. Environ. Saf. 2021, 217, 112232. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dou, R.; Yang, Z.; You, T.; Gao, X.; Wang, L. Phytotoxicity and bioaccumulation of zinc oxide nanoparticles in rice (Oryza sativa L.). Plant Physiol. Biochem. 2018, 130, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lv, L.; Ahmed, T.; Jin, S.; Shahid, M.; Noman, M.; Li, B. Effect of the nanoparticle exposures on the tomato bacterial wilt disease control by modulating the rhizosphere bacterial community. Int. J. Mol. Sci. 2021, 23, 414. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Hasnain, A.; Wang, J.; Zheng, H.; Naqvi, S.; Prasad, R.; Moustafa, M. Current progress and open challenges for combined toxic effects of manufactured nano-sized objects (MNO’s) on soil biota and microbial community. Coatings 2023, 13, 212. [Google Scholar] [CrossRef]
- Giovannetti, M.; Salvioli di Fossalunga, A.; Stringlis, I.A.; Proietti, S.; Fiorilli, V. Unearthing soil-plant-microbiota crosstalk: Looking back to move forward. Front. Plant Sci. 2023, 13, 1082752. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Shang, Y.; Liu, D.; Liesack, W.; Cui, Z.; Zhang, F. Peat-vermiculite alters microbiota composition towards increased soil fertility and crop productivity. Plant Soil 2021, 470, 21–34. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Yadav, A.N.; Verma, P.; Singh, B.; Chauhan, V.S.; Suman, A.; Saxena, A.K. Plant growth promoting bacteria: Biodiversity and multifunctional attributes for sustainable agriculture. Adv. Biotechnol. Microbiol. 2017, 5, 555671. [Google Scholar] [CrossRef]
- Qin, S.; Li, W.J.; Dastager, S.G.; Hozzein, W.N. Actinobacteria in special and extreme habitats: Diversity, function roles, and environmental adaptations. Front. Microbiol. 2016, 7, 1415. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef]
- Chavan, S.; Nadanathangam, V. Effects of nanoparticles on plant growth-promoting bacteria in Indian agricultural soil. Agronomy 2019, 9, 140. [Google Scholar] [CrossRef]
- Kumar, G.; Kanaujia, N.; Bafana, A. Functional and phylogenetic diversity of root-associated bacteria of Ajuga bracteosa in Kangra valley. Microbiol. Res. 2012, 167, 220–225. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Pan, B.; Zhang, X.; Zhang, H.; Steinberg, C.E.; Peijnenburg, W.J. Application of low dosage of copper oxide and zinc oxide nanoparticles boosts bacterial and fungal communities in soil. Sci. Total Environ. 2021, 757, 143807. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, Z.; Peijnenburg, W.; Liu, W.; Lu, T.; Hu, B.; Qian, H. Rhizosphere microbiome assembly and its impact on plant growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef]
- Fan, W.; Deng, J.; Shao, L.; Jiang, S.; Xiao, T.; Sun, W.; Xiao, E. The rhizosphere microbiome improves the adaptive capabilities of plants under high soil cadmium conditions. Front. Plant Sci. 2022, 13, 914103. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Z.; Liu, Y.; Wang, F.; Liu, Y.; Zhao, J.; Zhu, N. Roles of plant-associated microorganisms in regulating the fate of Hg in croplands: A perspective on potential pathways in maintaining sustainable agriculture. Sci. Total Environ. 2022, 834, 155204. [Google Scholar] [CrossRef] [PubMed]
- Umar, W.; Czinkota, I.; Gulyás, M.; Aziz, T.; Hameed, M.K. Development and characterization of slow release N and Zn fertilizer by coating urea with Zn fortified nano-bentonite and ZnO NPs using various binders. Environ. Technol. Innov. 2022, 26, 102250. [Google Scholar] [CrossRef]
- Santaella, C.; Plancot, B. Interactions of nanoenabled agrochemicals with soil microbiome. In Nanopesticides: From Research and Development to Mechanisms of Action and Sustainable Use in Agriculture; Fraceto, L.F., de Castro, V.L.S.S., Grillo, R., Ávila, D., Caixeta Oliveira, H., Lima, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 137–163. [Google Scholar] [CrossRef]
- Liu, L.; Nian, H.; Lian, T. Plants and rhizospheric environment: Affected by zinc oxide nanoparticles (ZnO NPs). A review. Plant Physiol. Biochem. 2022, 185, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Schimel, J.P.; Holden, P.A. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 2011, 45, 1659–1664. [Google Scholar] [CrossRef]
- Chai, H.; Yao, J.; Sun, J.; Zhang, C.; Liu, W.; Zhu, M.; Ceccanti, B. The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull. Environ. Contam. Toxicol. 2015, 94, 490–495. [Google Scholar] [CrossRef]
- Meli, K.; Kamika, I.; Keshri, J.; Momba, M. The impact of zinc oxide nanoparticles on the bacterial microbiome of activated sludge systems. Sci. Rep. 2016, 6, 39176. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.H.; Lin, K.S.; Ke, W.J.; Hsieh, C.T.; Chiang, C.L.; Tzou, D.Y.; Liu, S.T. The antimicrobial properties of silver nanoparticles in Bacillus subtilis are mediated by released Ag+ ions. PLoS ONE 2015, 10, e0144306. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.; Jain, K.; Madamwar, D. Evaluation of in vitro Cr (VI) reduction potential in cytosolic extracts of three indigenous Bacillus sp. isolated from Cr (VI) polluted industrial landfill. Biores. Technol. 2008, 99, 6059–6069. [Google Scholar] [CrossRef] [PubMed]
- Mandic-Mulec, I.; Prosser, J.I. Diversity endospore—Forming bacteria in soil: Characterization and driving mechanisms. In Endospore-Forming Soil Bacteria. Soil Biology; Logan, N., Vos, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 27, pp. 31–59. [Google Scholar] [CrossRef]
- Preston, G.M. Plant perceptions of plant growth-promoting Pseudomonas. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, F.; Huang, Y.; Zhou, M.; Gao, J.; Yan, T.; Sheng, H.; An, L. Sphingomonas sp. Cra20 increases plant growth rate and alters rhizosphere microbial community structure of Arabidopsis thaliana under drought stress. Front. Microbiol. 2019, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.; Luxton, T.; Kumar, N.; Shah, S.; Walker, V.K.; Shah, V. Assessing the impact of copper and zinc oxide nanoparticles on soil: A field study. PLoS ONE 2012, 7, e42663. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Schimel, J.P.; Holden, P.A. Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Appl. Environ. Microbiol. 2012, 78, 6749–6758. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Xu, M.; Yin, Y.; Sun, Y.; Wu, J.; Zhu, J.; Guo, H. Elevated CO2 levels alleviated toxicity of ZnO nanoparticles to rice and soil bacteria. Sci. Total Environ. 2022, 804, 149822. [Google Scholar] [CrossRef]
- Verma, Y.; Singh, S.K.; Jatav, H.S.; Rajput, V.D.; Minkina, T. Interaction of zinc oxide nanoparticles with soil: Insights into the chemical and biological properties. Environ. Geochem. Health 2022, 44, 221–234. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase activity in the soil environment. Dehydrogenases 2012, 10, 183–210. [Google Scholar] [CrossRef]
- Zavyalova, N.E.; Vasbieva, M.T.; Fomin, D.S. Microbial biomass, respiratory activity and nitrogen fixation in soddy-podzolic soils of the pre-urals area under various agricultural uses. Eurasian Soil Sci. 2020, 53, 383–388. [Google Scholar] [CrossRef]
- Shi, Y.; Xiao, Y.; Li, Z.; Zhang, X.; Liu, T.; Li, Y.; Yan, W. Microorganism structure variation in urban soil microenvironment upon ZnO nanoparticles contamination. Chemosphere 2021, 273, 128565. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rui, J.; Mao, Y.; Yannarell, A.; Mackie, R. Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar. Soil Biol. Biochem. 2014, 68, 392–401. [Google Scholar] [CrossRef]
- Ma, Q.; Qian, Y.; Yu, Q.; Cao, Y.; Tao, R.; Zhu, M.; Zhu, X. Controlled-release nitrogen fertilizer application mitigated N losses and modified microbial community while improving wheat yield and N use efficiency. Agric. Ecosyst. Environ. 2023, 349, 108445. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Y.Y.; Wang, D.; Ren, Y.H.; Liu, H.L.; Tian, Y.; Chen, X.F. Effects of nanoscale zinc oxide treatment on growth, rhizosphere microbiota, and metabolism of Aconitum carmichaelii. PeerJ 2023, 11, e16177. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- Luo, J.; Song, Y.; Liang, J.; Li, J.; Islam, E.; Li, T. Elevated CO2 mitigates the negative effect of CeO2 and Cr2O3 nanoparticles on soil bacterial communities by alteration of microbial carbon use. Environ. Pollut. 2020, 263, 114456. [Google Scholar] [CrossRef]
- Saleem, S.; Malik, A.; Khan, S.T. ZnO nanoparticles in combination with Zn biofertilizer improve wheat plant growth and grain Zn content without significantly changing the rhizospheric microbiome. Environ. Exp. Bot. 2023, 213, 105446. [Google Scholar] [CrossRef]
- Gómez Expósito, R.; Postma, J.; Raaijmakers, J.M.; De Bruijn, I. Diversity and activity of Lysobacter species from disease suppressive soils. Front. Microbiol. 2015, 6, 1243. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wan, W. Phosphate-solubilizing bacterium Acinetobacter pittii gp-1 affects rhizosphere bacterial community to alleviate soil phosphorus limitation for growth of soybean (Glycine max). Front. Microbiol. 2021, 12, 737116. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, X.; Wang, Y.; Feng, Y. Evaluation of zinc oxide nanoparticles on lettuce (Lactuca sativa L.) growth and soil bacterial community. Environ. Sci. Pollut. Res. Int. 2018, 25, 6026–6035. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhao, L.; Jiang, W.; Wang, M.; Chen, X.; Shen, X.; Mao, Z. Effect of zinc oxide nanoparticles on the growth of Malus hupehensis Rehd. seedlings. Front. Environ. Sci. 2022, 10, 82. [Google Scholar] [CrossRef]
- Palmieri, V.; Bugli, F.; Lauriola, M.C.; Cacaci, M.; Torelli, R.; Ciasca, G.; Conti, C.; Sanguinetti, M.; Papi, M.; De Spirito, M. Bacteria meet graphene: Modulation of graphene oxide nanosheet interaction with human pathogens for effective antimicrobial therapy. ACS Biomater. Sci. Eng. 2017, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Nagar, V.; Singh, T.; Tiwari, Y.; Aseri, V.; Pandit, P.P.; Chopade, R.L.; Awasthi, G. ZnO Nanoparticles: Exposure, toxicity mechanism and assessment. Mater. Today Proc. 2022, 69, 56–63. [Google Scholar] [CrossRef]
- Lahive, E.; Matzke, M.; Svendsen, C.; Spurgeon, D.J.; Pouran, H.; Zhang, H.; Lofts, S. Soil properties influence the toxicity and availability of Zn from ZnO nanoparticles to earthworms. Environ. Pollut. 2023, 319, 120907. [Google Scholar] [CrossRef]
- Wang, H.; Wick, R.L.; Xing, B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ. Pollut. 2009, 157, 1171–1177. [Google Scholar] [CrossRef]
- Hu, C.W.; Li, M.; Cui, Y.B.; Li, D.S.; Chen, J.; Yang, L.Y. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil. Biol. Biochem. 2010, 42, 586–591. [Google Scholar] [CrossRef]
- Fatima, N.; Singh, P.K.; Singh, A.; Singh, K. Impact of nanoparticles on the environment and earthworms: A comprehensive review. Munis Entomol. Zool. 2024, 19, 711–726. [Google Scholar]
- Alves, M.L.; Oliveira Filho, L.C.I.D.; Nogueira, P.; Ogliari, A.J.; Fiori, M.A.; Baretta, D.; Baretta, C.R.D.M. Influence of ZnO nanoparticles and a non-nano ZnO on survival and reproduction of earthworm and springtail in tropical natural soil. Rev. Bras. Ciênc. 2019, 43, e0180133. [Google Scholar] [CrossRef]
- Samarasinghe, S.V.A.C.; Krishnan, K.; Aitken, R.J.; Naidu, R.; Megharaj, M. Chronic effects of TiO2 and ZnO nanoparticles to earthworm Eisenia fetida. Environ. Chem. Toxicol. 2023, 5, 129–134. [Google Scholar] [CrossRef]
- Valerio-Rodríguez, M.F.; Trejo-Téllez, L.I.; Aguilar-González, M.Á.; Medina-Pérez, G.; Zúñiga-Enríquez, J.C.; Ortegón-Pérez, A.; Fernández-Luqueño, F. Effects of ZnO, TiO2 or Fe2O3 Nanoparticles on the Body Mass, Reproduction, and Survival of Eisenia fetida. Pol. J. Environ. Stud. 2020, 29, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Zöngür, A.; Er Zeybekler, S. Evaluation of the effects of zinc oxide (ZnO NPs) nanoparticles synthesized by green synthesis on Caenorhabditis elegans. Biol. Futura. 2024; online first. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, L.; Yang, B.; Ding, Y.; Zhao, Y.; Wu, Y.; Nie, Y.; Liu, Y.; Xu, A. Zinc oxide/graphene oxide nanocomposites specifically remediated Cd-contaminated soil via reduction of bioavailability and ecotoxicity of Cd. Sci. Total Environ. 2024, 940, 173641. [Google Scholar] [CrossRef]
- Al-Azzazy, M.M.; Ghani, S.B.A.; Alhewairini, S.S. Acaricidal activity of zinc oxide nanoparticles against mites associated with date palm trees. Pak. J. Agric. Sci. 2024, 61, 351–357. [Google Scholar]
- Oleszczuk, P.; Czech, B.; Kończak, M.; Bogusz, A.; Siatecka, A.; Godlewska, P.; Wiesner, M. Impact of ZnO and ZnS nanoparticles in sewage sludge-amended soil on bacteria, plant and invertebrates. Chemosphere. 2019, 237, 124359. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Int. J. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Inter-actions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Kang, M.; Liu, Y.; Weng, Y.; Wang, H.; Bai, X. A critical review on the toxicity regulation and ecological risks of zinc oxide nanoparticles to plants. Environ. Sci. Nano. 2024, 11, 14–35. [Google Scholar] [CrossRef]
- Song, U.; Jun, H.; Waldman, B.; Roh, J.; Kim, Y.; Yi, J.; Lee, E.J. Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 2013, 93, 60–67. [Google Scholar] [CrossRef]
- Amde, M.; Liu, J.F.; Tan, Z.Q.; Bekana, D. Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review. Environ. Pollut. 2017, 230, 250–267. [Google Scholar] [CrossRef]
- Sauvé, S.; Hendershot, W.; Allen, H.E. Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ. Sci. Technol. 2000, 34, 1125–1131. [Google Scholar] [CrossRef]
- Gao, X.; Spielman-Sun, E.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. Time and nanoparticle concentration affect the extractability of Cu from CuO NP-amended soil. Environ. Sci. Technol. 2017, 51, 2226–2234. [Google Scholar] [CrossRef]
- Jośko, I.; Dobrzyńska, J.; Dobrowolski, R.; Kusiak, M.; Terpiłowski, K. The effect of pH and ageing on the fate of CuO and ZnO nanoparticles in soils. Sci. Total Environ. 2020, 721, 137771. [Google Scholar] [CrossRef]
- García-Gómez, C.; Fernández, M.D.; García, S.; Obrador, A.F.; Letón, M.; Babín, M. Soil pH effects on the toxicity of zinc oxide nanoparticles to soil microbial community. Environ. Sci. Pollut. Res. 2018, 25, 28140–28152. [Google Scholar] [CrossRef] [PubMed]
- Tipping, E.; Rieuwerts, J.; Pan, G.; Ashmore, M.R.; Lofts, S.; Hill, M.T.R.; Thornton, I. The solid–solution partitioning of heavy metals (Cu, Zn, Cd, Pb) in upland soils of England and Wales. Environ. Pollut. 2003, 125, 213–225. [Google Scholar] [CrossRef]
- Tourinho, P.S.; van Gestel, C.A.; Lofts, S.; Soares, A.M.; Loureiro, S. Influence of soil pH on the toxicity of zinc oxide nanoparticles to the terrestrial isopod Porcellionides pruinosus. Environ. Toxicol. Chem. 2013, 32, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Waalewijn-Kool, P.L.; Ortiz, M.D.; Lofts, S.; van Gestel, C.A. The effect of pH on the toxicity of zinc oxide nanoparticles to Folsomia candida in amended field soil. Environ. Toxicol. Chem. 2013, 32, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Lee, I. Effects of Zn and ZnO nanoparticles and Zn2+ on soil enzyme activity and bioaccumulation of Zn in Cucumis sativus. Chem. Ecol. 2011, 27, 49–55. [Google Scholar] [CrossRef]
- Heggelund, L.R.; Diez-Ortiz, M.; Lofts, S.; Lahive, E.; Jurkschat, K.; Wojnarowicz, J.; Svendsen, C. Soil pH effects on the comparative toxicity of dissolved zinc, non-nano and nano ZnO to the earthworm Eisenia fetida. Nanotoxicology 2014, 8, 559–572. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, B.; Wang, H.; Chen, Q.; Cao, X.; Gao, Y.; Yin, K. Insights on the effects of ZnO nanoparticle exposure on soil heterotrophic respiration as revealed by soil microbial communities and activities. J. Soils Sediments 2021, 21, 2315–2326. [Google Scholar] [CrossRef]
- Shirvani, M.; Ghalandari, Y. Effects of zinc oxide nanoparticles on carbon mineralization kinetics and microbial attributes in plant residue-amended soils. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100939. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Soil organic matter priming: The pH effects. Glob. Chang. Biol. 2024, 30, e17349. [Google Scholar] [CrossRef]
- Watson, J.L.; Fang, T.; Dimkpa, C.O.; Britt, D.W.; McLean, J.E.; Jacobson, A.; Anderson, A.J. The phytotoxicity of ZnO nanoparticles on wheat varies with soil properties. Biometals 2015, 28, 101–112. [Google Scholar] [CrossRef]
- Wu, P.; Cui, P.; Du, H.; Alves, M.E.; Zhou, D.; Wang, Y. Long-term dissolution and transformation of ZnO in soils: The roles of soil pH and ZnO particle size. J. Hazard. Mater. 2021, 415, 125604. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Świątek, Z.M.; Woźnicka, O.; Bednarska, A.J. Unravelling the ZnO-NPs mechanistic pathway: Cellular changes and altered morphology in the gastrointestinal tract of the earthworm Eisenia andrei. Ecotoxicol. Environ. Saf. 2020, 196, 110532. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, Z.; Hou, Z.; Li, T.; Lu, X. Ecotoxicological effect of zinc oxide nanoparticles on soil microorganisms. Front. Environ. Sci. Eng. 2015, 9, 912–918. [Google Scholar] [CrossRef]
- Quik, J.T.; Lynch, I.; Van Hoecke, K.; Miermans, C.J.; De Schamphelaere, K.A.; Janssen, C.R.; Van De Meent, D. Effect of natural organic matter on cerium dioxide nanoparticles settling in model fresh water. Chemosphere 2010, 81, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Lavallee, J.M. Soil organic matter formation, persistence, and functioning: A synthesis of cur-rent understanding to inform its conservation and regeneration. Adv. Agron. 2022, 172, 1–66. [Google Scholar] [CrossRef]
- Waalewijn-Kool, P.L.; Rupp, S.; Lofts, S.; Svendsen, C.; van Gestel, C.A. Effect of soil organic matter content and pH on the toxicity of ZnO nanoparticles to Folsomia candida. Ecotoxicol. Environ. Saf. 2014, 108, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lead, J.R.; Wilkinson, K.J. Aquatic colloids and nanoparticles: Current knowledge and future trends. Environ. Chem. 2006, 3, 159–171. [Google Scholar] [CrossRef]
- Diegoli, S.; Manciulea, A.L.; Begum, S.; Jones, I.P.; Lead, J.R.; Preece, J.A. Interaction between manufactured gold nanoparticles and naturally occurring organic macromolecules. Sci. Total Environ. 2008, 402, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, J.; Fawcett, S.R.; Renshaw, J.C.; Lead, J.R. Silver nanoparticle impact on bacterial growth: Effect of pH, concentration, and organic matter. Environ. Sci. Technol. 2009, 43, 7285–7290. [Google Scholar] [CrossRef]
- Moghaddasi, S.; Fotovat, A.; Khoshgoftarmanesh, A.H.; Karimzadeh, F.; Khazaei, H.R.; Khorassani, R. Bioavailability of coated and uncoated ZnO nanoparticles to cucumber in soil with or without organic matter. Ecotoxicol. Environ. Saf. 2017, 144, 543–551. [Google Scholar] [CrossRef]
- Kirschling, T.L.; Golas, P.L.; Unrine, J.M.; Matyjaszewski, K.; Gregory, K.B.; Lowry, G.V.; Tilton, R.D. Microbial bioavailability of covalently bound polymer coatings on model engineered nanomaterials. Environ. Sci. Technol. 2011, 45, 5253–5259. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Shahzad, T.; Shahid, M.; Ismail, I.M.; Shah, G.M.; Almeelbi, T. Zinc oxide nanoparticles affect carbon and nitrogen mineralization of Phoenix dactylifera leaf litter in a sandy soil. J. Hazard. Mater. 2017, 324, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Hashmi, S.S.; Palma, J.M.; Corpas, F.J. Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere 2022, 290, 133329. [Google Scholar] [CrossRef] [PubMed]
- García-Ovando, A.E.; Piña, J.E.R.; Naranjo, E.U.E.; Chávez, J.A.C.; Esquivel, K. Biosynthesized nanoparticles and implications by their use in crops: Effects over physiology, action mechanisms, plant stress responses and toxicity. Plant Stress 2022, 6, 100109. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticles on crop plants: A perspective analysis. In Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development; Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 83–99. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A.; Mukherjee, A. Effects of ZnO nanoparticles in plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 807, 25–32. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Beers, E.P.; Dangl, J.L.; Franklin-Tong, V.E.; Gallois, P.; Hara-Nishimura, I.; Bozhkov, P. Morphological classification of plant cell deaths. Cell Death Differ. 2011, 18, 1241–1246. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Ahmed, B.; Rizvi, A.; Syed, A.; Elgorban, A.M.; Khan, M.S.; Al-Shwaiman, H.A.; Lee, J. Differential responses of maize (Zea mays) at the physiological, biomolecular, and nutrient levels when cultivated in the presence of nano or bulk ZnO or CuO or Zn2+ or Cu2+ ions. J. Hazard. Mater. 2021, 419, 126493. [Google Scholar] [CrossRef]
- Baskar, V.; Safia, N.; Sree Preethy, K.; Dhivya, S.; Thiruvengadam, M.; Sathishkumar, R. A comparative study of phytotoxic effects of metal oxide (CuO, ZnO and NiO) nanoparticles on in-vitro grown Abelmoschus esculentus. Plant Biosyst. 2021, 155, 374–383. [Google Scholar] [CrossRef]
- Azarin, K.; Usatov, A.; Minkina, T.; Plotnikov, A.; Kasyanova, A.; Fedorenko, A.; Alamri, S. Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in Hordeum vulgare L. Chemosphere 2022, 287, 132167. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Applerot, G.; Lellouche, J.; Perkas, N.; Nitzan, Y.; Gedanken, A.; Banin, E. ZnO nanoparticle-coated surfaces inhibit bacterial biofilm formation and increase antibiotic susceptibility. RSC Adv. 2012, 2, 2314–2321. [Google Scholar] [CrossRef]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 1998, 86, 521–522. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.L.; Han, P.; Guo, L.C.; Cao, Y.Q.; Li, A.D.; Kong, J.Z.; Wu, D. The antibacterial activity of Ta-doped ZnO nanoparticles. Nanoscale Res. Lett. 2015, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin, N.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Mao, Z.; Yu, D.; Gao, C. Toxicity of ZnO nanoparticles to macrophages due to cell uptake and intracel-lular release of zinc ions. J. Nanosci. Nanotechnol. 2014, 14, 5688–5696. [Google Scholar] [CrossRef]
- Hou, J.; Wu, Y.; Li, X.; Wei, B.; Li, S.; Wang, X. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere 2018, 193, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Maedler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huo, L.L.; Shi, X.F.; Bai, R.; Zhang, Z.J.; Zhao, Y.L.; Chang, Y.Z.; Chen, C.Y. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano 2014, 8, 2562–2574. [Google Scholar] [CrossRef] [PubMed]

| Soil pH | Size of ZnO NPs, nm | Concentration of ZnO NPs, mg/kg | Representatives of Soil Microbiota and Enzymes | Reference | |
|---|---|---|---|---|---|
| Stimulates (Increases) | Inhibits (Reduces) | ||||
| 6.0 | 20–30 | 500 | - | Microbial biomass and diversity | [107] |
| 7.5 | 15 | 1000 | - | Azotobacter, phosphate and potassium solubilizing bacteria, urease, CAT, hydrolysis of fluorescein diacetate | [108] |
| 6.9 | n.d. 1 | 500 | Firmicutes, Bacteroidetes, Bacillales, Burkholderiales, Lactobacillales, Pseudomonadales, Pseudomonas, Streptococcus, Dialister | Proteobacteria, Actinobacteria, Actinomycetales, Desulfivibrionales, Alteromonadales, Oceanospirillales, Halomonas | [98] |
| 7.5 | 20–50 | 550 | Rhizobiales | Sphingomonadales | [116] |
| 6.0 | 20–30 | 50–500 | Sphingomonadaceae, Streptomycetaceae, Streptomyces | Rhizobiales, Bradyrhizobiaceae, Bradyrhizobium, Methylobacteriaceae | [117] |
| 7.9–8.2 | 25 | 100, 500 | Proteobacteria, Nitrospirae, Ascomycota, Zygomycota, Basidiomycota | Bacteroidetes, Acidobacteria, Actinobacteria, Firmicutes | [12] |
| 8.58 | 65.8 | 0.5, 1.25, 2.5 | - | The total content of bacteria, fungi, actinomycetes, dehydrogenase and carbon fraction of microbial biomass | [119] |
| 7.24 | 50 | <250 | Proteobacteria, Ascomycota, Sphingomonadales | Solirubrobacterales, Catenulisporales, Armatimonadetes | [100] |
| 7.56 | 50 | 200, 500, 1000 | Proteobacteria, Actinobacteria, Piscinibacter, Streptomyces, Burkholderiales, Altererythrobacter, Massilia | Bacteroidetes, Terrimonas, Flavitalea, Ohtaekwangia, Pseudomonas, phenoloxidase activity | [122] |
| 6.4 | 50 | 30 | Bacteroidetes, Actinobacteria, Streptomycetaceae, Rhizobiaceae, Oxalobacteraceae, Chitinophagaceae, Solibacteraceae | Gemmatimonadaceae, Sphingomonadaceae, Haliangiaceae | [124,125] |
| 7.4 | 35 | 25 | diversity and abundance of microflora | - | [62] |
| 7.7–7.8 | 20 | 5 kg/ha | Bacillus, Acinetobacter, Pedobacter, Massilia, Lysobacter, Pseudomonas | - | [129] |
| 7.12 | 20 | 100–700 | Proteobacteria, Methylobacillus, Humicola, Aminobacter, Arenimonas, Thiobacillus, Metarhizium | - | [88] |
| 7.2 | 90 | 0.1, 10, 100 | Cyanobacteria, Nostoc, Scenedesmus, Xanthomonas, Galbibacter, Burkholderia | - | [133] |
| n.d. | 16–31 | 500 | Chloroflexi, Sphingomonadaceae, Rhizobiaceae, Rhodanobacteraceae, Xanthomonadaceae, Nitrosomonadaceae, Methylophilaceae, Microscillaceae, Gemmatimonadaceae | Proteobacteria, Patescibacteria, Actinobacteria | [90] |
| 5.59 | 30 | 250–1000 | Proteobacteria, Chloroflexi, Gemmatimonadota, Bacteroidota, Myxococcota, Cyanobacteria, Tausonia, Chaetomium, Mrakia | Firmicutes, Acidobacteriota, Nitrospirota, Verrucomicrobiota, Neocosmospora, Gibberella, Fusarium | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strekalovskaya, E.I.; Perfileva, A.I.; Krutovsky, K.V. Zinc Oxide Nanoparticles in the “Soil–Bacterial Community–Plant” System: Impact on the Stability of Soil Ecosystems. Agronomy 2024, 14, 1588. https://doi.org/10.3390/agronomy14071588
Strekalovskaya EI, Perfileva AI, Krutovsky KV. Zinc Oxide Nanoparticles in the “Soil–Bacterial Community–Plant” System: Impact on the Stability of Soil Ecosystems. Agronomy. 2024; 14(7):1588. https://doi.org/10.3390/agronomy14071588
Chicago/Turabian StyleStrekalovskaya, Elena I., Alla I. Perfileva, and Konstantin V. Krutovsky. 2024. "Zinc Oxide Nanoparticles in the “Soil–Bacterial Community–Plant” System: Impact on the Stability of Soil Ecosystems" Agronomy 14, no. 7: 1588. https://doi.org/10.3390/agronomy14071588





