Spatial Heterogeneity Analysis and Risk Assessment of Potentially Toxic Elements in Soils of Typical Green Tea Plantations
Abstract
1. Introduction
2. Materials and Methods
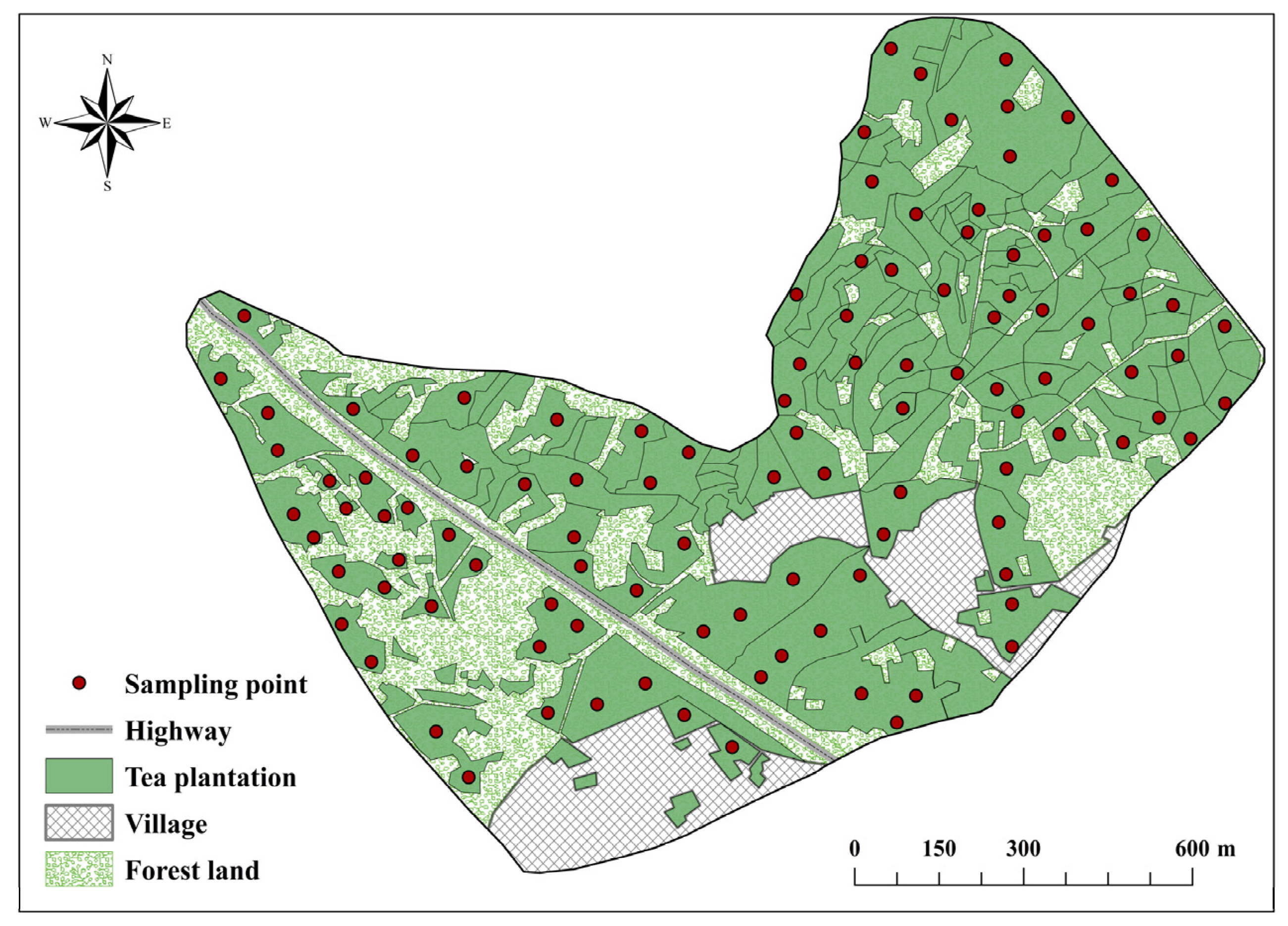
2.1. Study Area, Soil Collection, and Chemical Analysis
2.2. Spatial Analysis Methods
2.3. Potentially Toxic Elements Source Apportionment Models
2.4. Potentially Toxic Elements Risk Assessment Models
2.5. Statistical Analysis
3. Results and Discussion
3.1. Soil Properties and Accumulation of Potentially Toxic Elements in Soils and Tea Leaves
3.2. Spatial Characteristics of Potentially Toxic Elements in Tea Garden Soil
3.2.1. Spatial Structure Characteristics of Potentially Toxic Elements in Tea Garden Soil
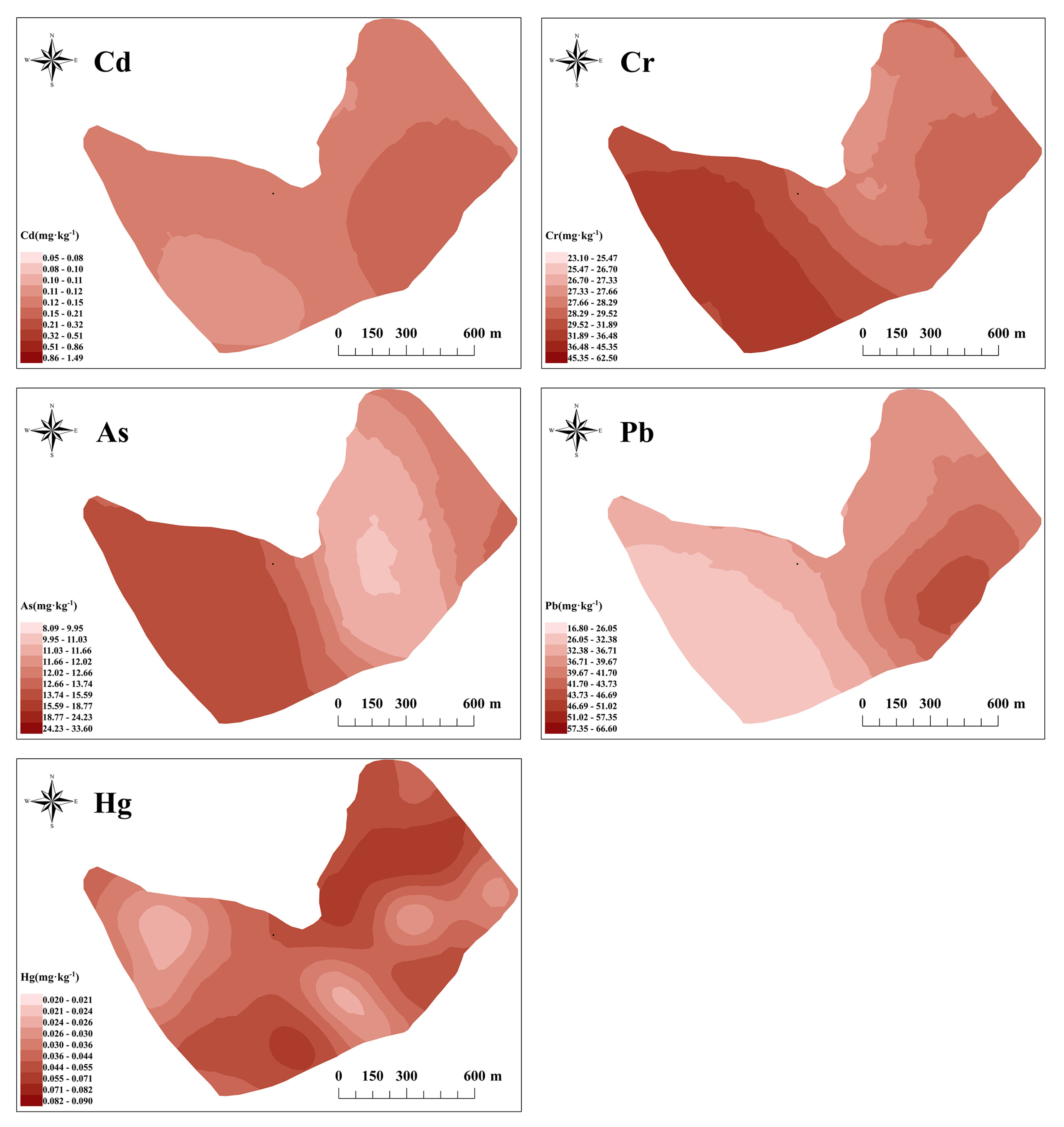
3.2.2. Spatial Distribution Characteristics of Potentially Toxic Elements in Tea Garden Soil
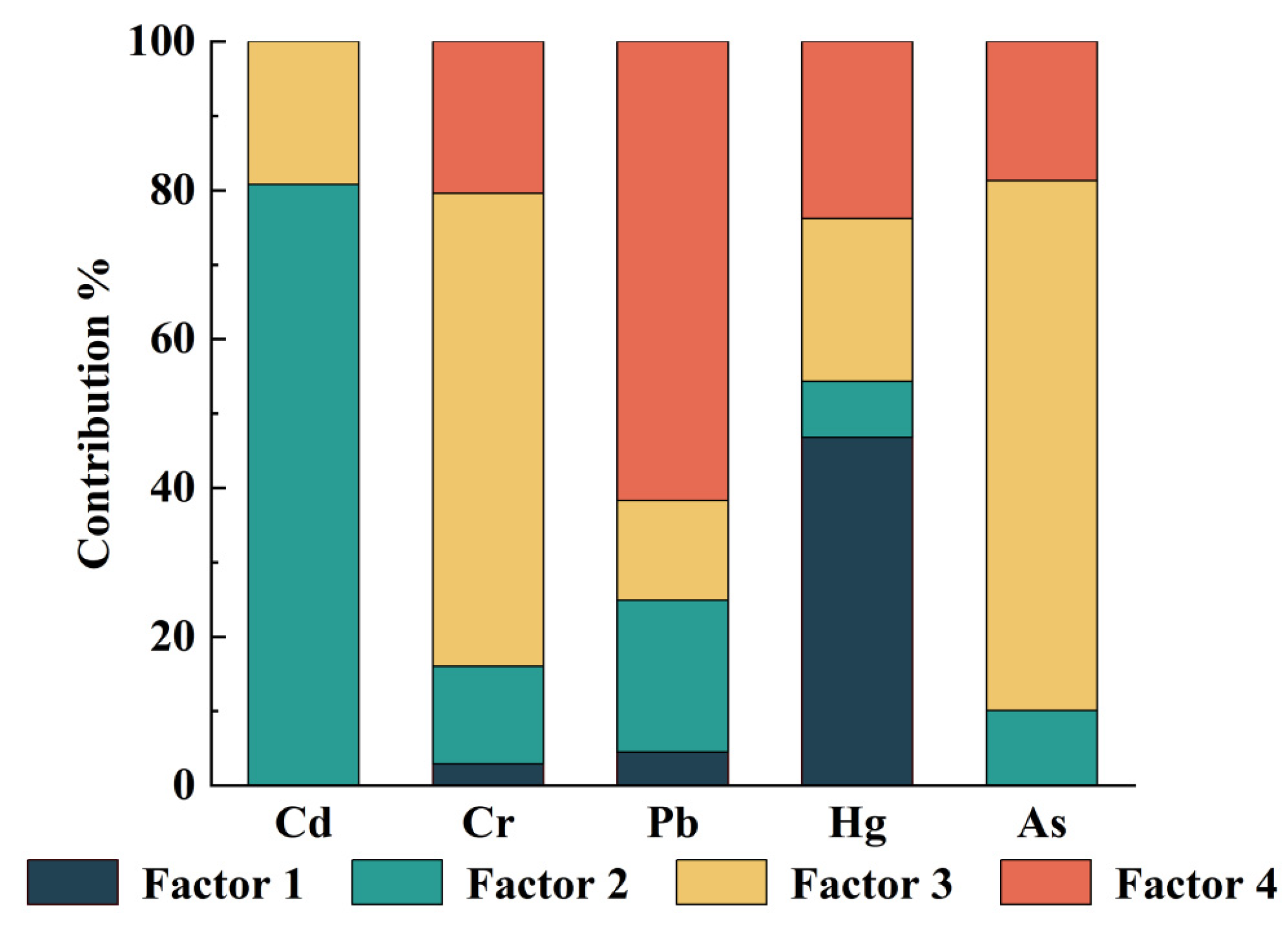
3.3. Source of Potentially Toxic Elements in Tea Garden Soil
3.4. Assessment of Potentially Toxic Elements Risks in Tea Garden Soil
3.4.1. Potential Ecological Risk Assessment
3.4.2. Human Health Risk Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mao, C.; Song, Y.; Chen, L.; Ji, J.; Li, J.; Yuan, X.; Yang, Z.; Ayoko, G.A.; Frost, R.L.; Theiss, F. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena 2019, 175, 339–348. [Google Scholar] [CrossRef]
- Xu, W.; Hou, S.; AmanKhan, M.; Chao, Y.; Xiao, L.; Ruan, Z.; Hong, L.; Chen, Z.; Ceng, S.; Ye, Z.; et al. Effect of water and fertilization management on Cd immobilization and bioavailability in Cd-polluted paddy soil. Chemosphere 2021, 276, 13016. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.; Gai, X.; Cai, J.; Zhang, X.; Chen, X.; Zhu, Y.; Zhang, Z. Astilbin attenuates apoptosis induced by cadmium through oxidative stress in carp (Cyprinus carpio L.) head kidney lymphocyte. Fish Shellfish Immunol. 2022, 125, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Zhang, H.; Mao, K.; Shafeeque, M.; Aslam, M.W.; Yang, X.; Zhong, L.; Feng, X.; Podgorski, J. Chromium contamination in paddy soil-rice systems and associated human health risks in Pakistan. Sci. Total Environ. 2022, 826, 153910. [Google Scholar] [CrossRef] [PubMed]
- Tête, N.; Afonso, E.; Bouguerra, G.; Scheifler, R. Blood parameters as biomarkers of cadmium and lead exposure and effects in wild wood mice (Apodemus sylvaticus) living along a pollution gradient. Chemosphere 2015, 138, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.; Woo, H.D.; Kim, D.W.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Dietary mercury intake and colorectal cancer risk: A case-control study. Clin. Nutr. 2020, 39, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H. Pathophysiology of arsenic-induced adverse health effects. Hong Kong J. Dermatol. 2017, 25, 171–177. [Google Scholar]
- Yang, S.; Yang, D.; Taylor, D.; He, M.; Liu, X.; Xu, J. Tracking cadmium pollution from source to receptor: A health-risk focused transfer continuum approach. Sci. Total Environ. 2023, 867, 161574. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shangguan, Y.; Zhou, Z.; Guo, S.; Yu, H.; Chen, K.; Zeng, X.; Qin, Y. Status assessment and probabilistic health risk modeling of polycyclic aromatic hydrocarbons (PAHs) in surface soil across China. Front. Environ. Sci. 2023, 11, 1114027. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Zhang, Y.; Zhong, J.; Li, J.; Yang, S.; Ta, W.; Zhang, Y. Characteristics, source analysis, and health risk assessment of potentially toxic elements pollution in soil of dense molybdenum tailing ponds area in central China. Environ. Geochem. Health 2024, 46, 129. [Google Scholar] [CrossRef]
- Huang, C.C.; Cai, L.M.; Xu, Y.H.; Wen, H.H.; Jie, L.; Hu, G.C.; Chen, L.G.; Wang, H.Z.; Xu, X.B.; Mei, J.X. Quantitative analysis of ecological risk and human health risk of potentially toxic elements in farmland soil using the PMF model. Land Degrad. Dev. 2022, 33, 1954–1967. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Xie, M.; Yang, B. Ecological risk and health risk analysis of soil potentially toxic elements from oil production plants in central China. Sci. Rep. 2022, 12, 17077. [Google Scholar] [CrossRef]
- Liu, W.; Tang, Y.; Ma, J.; Zhang, W.; Liao, S.; Cui, S.; Ye, Z.; Liu, D. Effects of biochar and inorganic amendments on soil fertility, tea yield, and quality in both Pb-Cd-contaminated and acidified tea plantations. J. Soils Sediments 2023, 23, 3275–3284. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Wu, Q.; Zhang, Z.; Yang, H.; Wen, X. Effects of multiple-metal-compound contamination on the soil microbial community in typical Karst tea plantations. Forests 2023, 14, 1840. [Google Scholar] [CrossRef]
- Pourramezani, F.; Mohajeri, F.A.; Salmani, M.H.; Tafti, A.D.; Sadrabad, E.K. Evaluation of heavy metal concentration in imported black tea in Iran and consumer risk assessments. Food Sci. Nutr. 2019, 7, 4021–4026. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Wu, Y.; Zhang, W.; Yang, X.; Lv, H.; Xia, S.; Zhao, S.; Tian, J.; Cui, P.; et al. Selection of flagship species and their use as umbrellas in bird conservation: A case study in Lishui, Zhejiang Province, China. Animals 2023, 13, 1825. [Google Scholar] [CrossRef]
- Li, C. Research on the Development of Tea Culture Tourism in Lishui, Zhejiang. Master’s Thesis, Northwest Normal University, Lanzhou, China, 2020. [Google Scholar] [CrossRef]
- Xu, W.; Xie, X.; Li, Q.; Yang, X.; Ren, J.; Shi, Y.; Liu, D.; Shaheen, S.M.; Rinklebe, J. Biochar co-pyrolyzed from peanut shells and maize straw improved soil biochemical properties, rice yield, and reduced cadmium mobilization and accumulation by rice: Biogeochemical investigations. J. Hazard. Mater. 2024, 466, 133486. [Google Scholar] [CrossRef]
- Seyedmohammadi, J.; Navidi, M.N.; Esmaeelnejad, L. Geospatial modeling of surface soil texture of agricultural land using fuzzy logic, geostatistics and GIS techniques. Commun. Soil Sci. Plant Anal. 2019, 50, 1452–1464. [Google Scholar] [CrossRef]
- Wang, J.; Ouyang, J.; Zhang, M. Spatial distribution characteristics of soil and vegetation in a reclaimed area in an opencast coalmine. Catena 2020, 195, 104773. [Google Scholar] [CrossRef]
- Jiang, Y.; Chao, S.; Liu, J.; Yang, Y.; Chen, Y.; Zhang, A.; Cao, H. Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 2017, 168, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, Y.; Hua, Y. Source analysis based on the positive matrix factorization models and risk assessment of heavy metals in agricultural soil. Sustainability 2023, 15, 13225. [Google Scholar] [CrossRef]
- Jiang, H.H.; Cai, L.M.; Wen, H.H.; Hu, G.C.; Chen, L.G.; Luo, J. An integrated approach to quantifying ecological and human health risks from different sources of soil heavy metals. Sci. Total Environ. 2020, 701, 134466. [Google Scholar] [CrossRef]
- Tan, J.; Duan, J.; Ma, Y.; He, K.; Cheng, Y.; Deng, S.-X.; Huang, Y.-L.; Si-Tu, S.-P. Long-term trends of chemical characteristics and sources of fine particle in Foshan City, Pearl River Delta: 2008–2014. Sci. Total Environ. 2016, 565, 519–528. [Google Scholar] [CrossRef]
- Min, X.B.; Xie, X.D.; Chai, L.Y.; Liang, Y.J.; Li, M.; Ke, Y. Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue. Trans. Nonferr. Metal. Soc. 2013, 23, 208–218. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, S.; Duan, M.; Fu, J.; Wang, Q.; Liu, Z.; Song, F.; Tang, B.; Li, C.; Qin, G.; et al. Assessing heavy metal pollution and potential ecological risk of tea plantation soils. Int. J. Agric. Biol. Eng. 2019, 12, 185–191. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Fan, L.; Chen, T.; Bai, Y.; Yu, Q.; Liu, Y. Assessment of multiple exposure to chemical elements and health risks among residents near Huodehong lead-zinc mining area in Yunnan, southwest China. Chemosphere 2017, 174, 613–627. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, W.; Wang, H.; Liu, P.; Wang, X.; Huang, B. Spatial distribution, ecological risk and sources of heavy metals in soils from a typical economic development area, Southeastern China. Sci. Total Environ. 2021, 780, 146557. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, W.; Li, W.; Ma, T.; Xu, L.; Xia, M. Risk assessment of heavy metals in farmland soils in an agricultural region in the Yangtze River Delta. J. Agro-Environ. Sci. 2021, 40, 1441–1450. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Zhou, Q.; Liu, M.; Zhan, S.; Pan, X.; Zeng, Y. Response of soil fertility and Cu and Cd availability to biochar application on paddy soils with different acidification levels. Biomass Convers. Biorefin. 2022, 12, 1493–1502. [Google Scholar] [CrossRef]
- He, L.; Meng, J.; Wang, Y.; Tang, X.; Liu, X.; Tang, C.; Ma, L.Q.; Xu, J. Attapulgite and processed oyster shell powder effectively reduce cadmium accumulation in grains of rice growing in a contaminated acidic paddy field. Ecotox. Environ. Saf. 2021, 209, 111840. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Shi, B.; Yang, X.; Liang, T.; Liu, S.; Yan, X.; Li, J.; Liu, Z. Source apportionment of soil PTE in a northern industrial county using PMF model: Partitioning strategies and uncertainty analysis. Environ. Res. 2024, 252, 118855. [Google Scholar] [CrossRef]
- Alharbi, T.; El-Sorogy, A.S. Risk assessment of potentially toxic elements in agricultural soils of Al-Ahsa Oasis, Saudi Arabia. Sustainability 2023, 15, 659. [Google Scholar] [CrossRef]
- Wang, J.; Gao, B.; Yin, S.; Liu, L.; Xu, D.; Li, Y. Comprehensive evaluation and source apportionment of potential toxic Elements in Soils and Sediments of Guishui River, Beijing. Water 2019, 11, 1847. [Google Scholar] [CrossRef]
- Al Mamun, S.; Sultana, N.; Hasan, M.; Latif, M.B.; Islam, A.; Tusher, T.R. Phytoaccumulation of Cadmium in Leafy Vegetables Grown in Contaminated Soil under Varying Rates of Compost and Phosphate Fertilizer Application. Commun. Soil Sci. Plant Anal. 2021, 52, 2161–2176. [Google Scholar] [CrossRef]
- Wang, W.; Lu, N.; Pan, H.; Wang, Z.; Han, X.; Zhu, Z.; Guan, J. Heavy metal pollution and its prior pollution source identification in agricultural soil: A case study in the Qianguo Irrigation District, northeast China. Sustainability 2022, 14, 4494. [Google Scholar] [CrossRef]
- Moghtaderi, T.; Shakeri, A.; Rodríguez-Seijo, A. Potentially toxic element content in arid agricultural soils in south Iran. Agronomy 2020, 10, 564. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [CrossRef]
- Xu, C.; Lu, X.; Huang, C.; Sun, R.; Gu, A.; Pan, W.; He, L.; Bao, J.; Zou, R.; Fu, C.; et al. Positive matrix factorization as source apportionment of paddy soil heavy metals in black shale areas in western Zhejiang Province, China. Sustainability 2023, 15, 4547. [Google Scholar] [CrossRef]
- Guo, G.; Wang, Y.; Zhang, D.; Lei, M. Source-specific ecological and health risks of potentially toxic elements in agricultural soils in Southern Yunnan Province and associated uncertainty analysis. J. Hazard. Mater. 2021, 417, 126144. [Google Scholar] [CrossRef]
- Yuan, B.; Cao, H.; Du, P.; Ren, J.; Chen, J.; Zhang, H.; Zhang, Y.; Luo, H. Source-oriented probabilistic health risk assessment of soil potentially toxic elements in a typical mining city. J. Hazard. Mater. 2023, 443, 130222. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, D.; Liu, K.; Meng, H.; Zheng, Y.; Yuan, F.; Zhu, G. Levels, sources, and spatial distribution of heavy metals in soils from a typical coal industrial city of Tangshan, China. Catena 2019, 175, 101–109. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Y.; Qin, J.; Zhao, S.; Cao, D.; Zhu, M.; Jiang, Y. Potential risk, spatial distribution, and soil identification of potentially toxic elements in Lycium barbarum L. (Wolfberry) fruits and soil system in Ningxia, China. Int. J. Env. Res. Public Health 2022, 19, 16186. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Franco, A.-M.; Elio, P. Health risk assessment via ingestion and inhalation of soil PTE of an urban area. Chemosphere 2021, 281, 130964. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Cuevas, J.; Fuentes, B.; Fernández, E.; Arce, W.; Guerrero, M.; Letelier, M.V. Distribution of potentially toxic elements in soils surrounding abandoned mining waste located in Taltal, Northern Chile. J. Geochem. Explor. 2021, 220, 106653. [Google Scholar] [CrossRef]
- Rinklebe, J.; Antoniadis, V.; Shaheen, S.M.; Rosche, O.; Altermann, M. Health risk assessment of potentially toxic elements in soils along the Central Elbe River, Germany. Environ. Int. 2019, 126, 76–88. [Google Scholar] [CrossRef]
- Ma, W.; Ding, M.; Bian, Z. Comprehensive assessment of exposure and environmental risk of potentially toxic elements in surface water and sediment across China: A synthesis study. Sci. Total Environ. 2024, 926, 172061. [Google Scholar] [CrossRef]

| Parameter | Parameter Meaning and Unit | Adult | Children |
|---|---|---|---|
| OSIR | daily soil intake (mg d−1) | 100 | 200 |
| ED | exposure duration (a) | 24 | 6 |
| BW | body weight (kg) | 61.8 | 19.2 |
| SAE | exposed skin area (cm2) | 5374 | 2848 |
| SSAR | soil adherence factor to skin (mg cm−2 d−1) | 0.07 | 0.2 |
| EV | daily skin contact event frequency (d a−1) | 1 | 1 |
| EF | exposure frequency (d a−1) | 350 | 350 |
| PM10 | particulate matter concentration in air (mg m−3) | 0.119 | 0.119 |
| DAIR | daily inhalation volume of air (m3 d−1) | 14.5 | 7.5 |
| AF | proportion of inhaled soil particles retained in the body | 0.75 | 0.75 |
| fspo | proportion of soil particles in outdoor air | 0.8 | 0.8 |
| fs | proportion of soil particles in indoor air | 0.5 | 0.5 |
| EFO | outdoor exposure frequency (d a−1) | 87.5 | 87.5 |
| EFI | indoor exposure frequency (d a−1) | 262.5 | 262.5 |
| AT | non-carcinogenic effect average time (d) | 8760 | 2190 |
| AT | carcinogenic effect average time (d) | 27,740 | 27,740 |
| ABSo | oral intake absorption efficiency factor | 1 | 1 |
| ABSd | dermal contact absorption efficiency factor | As: 0.03, others: 0.001 | |
| PTEs | RfD/(mg kg−1·d−1) | SF/(mg kg−1·d−1) | ||||
|---|---|---|---|---|---|---|
| Oral Ingestion | Inhalation | Dermal Contact | Oral Ingestion | Inhalation | Dermal Contact | |
| Cd | 1.00 × 10−3 | 1.00 × 10−3 | 2.50 × 10−5 | 0.38 | 6.1 | 0.38 |
| As | 3.00 × 10−4 | 3.00 × 10−4 | 3.50 × 10−4 | 1.5 | 18.3 | 1.5 |
| Cr | 3.00 × 10−3 | 2.86 × 10−5 | 7.50 × 10−5 | 42 | ||
| Pb | 3.50 × 10−3 | 3.50 × 10−3 | 5.30 × 10−5 | |||
| Hg | 3.00 × 10−4 | 8.57 × 10−5 | 2.10 × 10−5 | |||
| Soil Index | Min | Max | Mean | SD | CV | Background Value a | Screening Value a | Exceedance Rate | K-S |
|---|---|---|---|---|---|---|---|---|---|
| pH | 4.06 | 4.78 | 4.39 | 0.19 | 4.31 | 0.85 | |||
| SOM | 6.45 | 46.60 | 32.06 | 8.20 | 25.58 | 0.99 | |||
| Cd | 0.05 | 1.49 | 0.19 | 0.25 | 132.43 | 0.20 | 0.3 | 6.48 | 0.52 * |
| Cr | 23.10 | 62.50 | 32.70 | 9.07 | 27.74 | 90 | 150 | 0 | 0.28 ** |
| Pb | 16.80 | 66.60 | 36.03 | 9.74 | 27.04 | 35 | 70 | 0 | 0.53 |
| Hg | 0.02 | 0.09 | 0.04 | 0.02 | 49.08 | 0.15 | 1.3 | 0 | 0.26 |
| As | 8.09 | 33.60 | 12.50 | 4.64 | 34.43 | 15 | 40 | 0 | 0.18 ** |
| Plant Index | Min | Max | Mean | SD | CV | Tea Safety Standard * |
|---|---|---|---|---|---|---|
| Cd | 0.003 | 0.058 | 0.019 | 0.015 | 77.72 | 1.0 |
| Cr | 0.037 | 0.550 | 0.293 | 0.106 | 35.99 | 5.0 |
| Pb | 0.083 | 0.400 | 0.220 | 0.066 | 29.85 | 5.0 |
| Hg | 0.010 | 0.015 | 0.0104 | 0.001 | 10.09 | 0.3 |
| As | 0.079 | 0.210 | 0.139 | 0.034 | 24.64 | 2.0 |
| Soil Index | Model | Nugget | Sill | Range (m) | Nugget/Sill (%) | R2 |
|---|---|---|---|---|---|---|
| Cd | Exponential | 37.7 | 92.4 | 657.00 | 40.8 | 0.574 |
| Cr | Gaussian | 0.0055 | 0.0749 | 95.26 | 7.3 | 0.612 |
| Pb | Linear | 29.6031 | 94.9838 | 719.56 | 31.2 | 0.615 |
| Hg | Gaussian | 0.00011 | 0.00052 | 400.10 | 21.2 | 0.944 |
| As | Gaussian | 6.4 | 26.42 | 398.37 | 24.2 | 0.577 |
| Soil Index | pH | SOM | Cd | Cr | Pb | Hg | As |
|---|---|---|---|---|---|---|---|
| pH | 1.000 | ||||||
| SOM | 0.189 | 1.000 | |||||
| Cd | 0.471 ** | 0.324 * | 1.000 | ||||
| Cr | 0.020 | −0.555 ** | 0.063 | 1.000 | |||
| Pb | 0.388 ** | 0.714 ** | 0.650 ** | −0.477 ** | 1.000 | ||
| Hg | −0.022 | 0.177 | 0.007 | −0.046 | 0.081 | 1.000 | |
| As | 0.049 | −0.625 ** | −0.096 | 0.895 ** | −0.536 ** | −0.144 | 1.000 |
| Soil Index | Min | Max | Mean | Ecological Risk |
|---|---|---|---|---|
| Cd | 7.05 | 223.5 | 28.28 | Low potential risk |
| Cr | 0.51 | 1.39 | 0.73 | Low potential risk |
| Pb | 0.03 | 0.10 | 0.07 | Low potential risk |
| Hg | 4.00 | 18.02 | 8.52 | Low potential risk |
| As | 5.39 | 22.10 | 8.99 | Low potential risk |
| RI | 26.24 | 246.59 | 46.58 | Mild ecological hazard |
| Index | HQois | HQdcs | HQpis | HQ | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adult | Child | Adult | Child | Adult | Child | Adult | Child | ||
| Cd | Mean | 5.85 × 10−4 | 3.77 × 10−3 | 8.80 × 10−5 | 4.29 × 10−4 | 4.35 × 10−6 | 7.25 × 10−6 | 6.77 × 10−4 | 4.20 × 10−3 |
| Max | 4.62 × 10−3 | 2.98 × 10−2 | 6.96 × 10−4 | 3.39 × 10−3 | 3.44 × 10−5 | 5.73 × 10−5 | 5.35 × 10−3 | 3.32 × 10−2 | |
| Cr | Mean | 3.38 × 10−2 | 2.18 × 10−1 | 5.09 × 10−1 | 2.48 × 10−2 | 2.64 × 10−2 | 4.40 × 10−2 | 6.53 × 10−2 | 2.86 × 10−1 |
| Max | 6.47 × 10−2 | 4.16 × 10−1 | 9.73 × 10−1 | 4.74 × 10−2 | 5.05 × 10−2 | 8.40 × 10−2 | 1.25 × 10−1 | 5.48 × 10−1 | |
| Pb | Mean | 3.19 × 10−2 | 2.06 × 10−1 | 7.94 × 10−1 | 3.87 × 10−2 | 2.38 × 10−4 | 3.96 × 10−4 | 4.01 × 10−2 | 2.45 × 10−1 |
| Max | 5.91 × 10−2 | 3.80 × 10−1 | 1.47 × 10−2 | 7.15 × 10−2 | 4.39 × 10−4 | 7.32 × 10−4 | 7.42 × 10−2 | 4.52 × 10−1 | |
| Hg | Mean | 4.41 × 10−4 | 2.84 × 10−3 | 2.37 × 10−5 | 1.15 × 10−4 | 1.15 × 10−5 | 1.91 × 10−5 | 4.76 × 104 | 2.97 × 10−3 |
| Max | 9.32 × 10−4 | 6.00 × 10−3 | 5.01 × 10−5 | 2.44 × 10−4 | 2.43 × 10−5 | 4.04 × 10−5 | 1.01 × 10−3 | 6.28 × 10−3 | |
| As | Mean | 1.39 × 10−1 | 8.98 × 10−1 | 1.57 × 10−2 | 7.67 × 10−2 | 1.04 × 10−3 | 1.73 × 10−3 | 1.56 × 10−1 | 9.76 × 10−1 |
| Max | 3.48 × 10−1 | 2.24 | 3.92 × 10−2 | 1.91 × 10−1 | 2.59 × 10−3 | 4.31 × 10−3 | 3.89 × 10−1 | 2.43 | |
| THI | Mean | 2.06 × 10−1 | 1.33 | 2.89 × 10−2 | 1.41 × 10−1 | 2.77 × 10−2 | 4.61 × 10−2 | 2.63 × 10−1 | 1.51 |
| Max | 4.30 × 10−1 | 2.77 | 5.33 × 10−2 | 2.60 × 10−1 | 5.32 × 10−2 | 8.86 × 10−2 | 5.37 × 10−1 | 3.12 | |
| Index | CRois | CRdcs | CRpis | CR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adult | Child | Adult | Child | Adult | Child | Adult | Child | ||
| Cd | Mean | 3.51 × 10−8 | 5.65 × 10−8 | 1.32 × 10−10 | 1.61 × 10−10 | 4.19 × 10−9 | 1.74 × 10−9 | 3.94 × 10−8 | 5.84 × 10−8 |
| Max | 2.77 × 10−7 | 4.46 × 10−7 | 1.04 × 10−9 | 1.27 × 10−9 | 3.31 × 10−8 | 1.38 × 10−8 | 3.11 × 10−7 | 4.61 × 10−7 | |
| As | Mean | 9.91 × 10−6 | 1.59 × 10−5 | 1.12 × 10−6 | 1.36 × 10−6 | 8.99 × 10−7 | 3.74 × 10−7 | 1.19 × 10−5 | 1.76 × 10−5 |
| Max | 2.47 × 10−5 | 3.97 × 10−5 | 2.79 × 10−6 | 3.40 × 10−6 | 2.24 × 10−6 | 9.33 × 10−7 | 2.97 × 10−5 | 4.40 × 10−5 | |
| Cr | Mean | 5.01 × 10−6 | 2.08 × 10−6 | 5.01 × 10−6 | 2.08 × 10−6 | ||||
| Max | 9.57 × 10−6 | 3.98 × 10−6 | 9.57 × 10−6 | 3.98 × 10−6 | |||||
| TCR | Mean | 9.95 × 10−6 | 1.60 × 10−5 | 1.12 × 10−6 | 1.36 × 10−6 | 5.91 × 10−6 | 2.46 × 10−6 | 1.70 × 10−5 | 1.98 × 10−5 |
| Max | 2.50 × 10−5 | 4.01 × 10−5 | 2.79 × 10−6 | 3.40 × 10−6 | 1.18 × 10−5 | 4.93 × 10−6 | 3.94 × 10−5 | 4.81 × 10−5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, Y.; Shafi, A.; He, M.; He, L.; Liu, D. Spatial Heterogeneity Analysis and Risk Assessment of Potentially Toxic Elements in Soils of Typical Green Tea Plantations. Agronomy 2024, 14, 1599. https://doi.org/10.3390/agronomy14081599
Xu Y, Wang Y, Shafi A, He M, He L, Liu D. Spatial Heterogeneity Analysis and Risk Assessment of Potentially Toxic Elements in Soils of Typical Green Tea Plantations. Agronomy. 2024; 14(8):1599. https://doi.org/10.3390/agronomy14081599
Chicago/Turabian StyleXu, Yaonan, Ying Wang, Abbas Shafi, Mingjiang He, Lizhi He, and Dan Liu. 2024. "Spatial Heterogeneity Analysis and Risk Assessment of Potentially Toxic Elements in Soils of Typical Green Tea Plantations" Agronomy 14, no. 8: 1599. https://doi.org/10.3390/agronomy14081599
APA StyleXu, Y., Wang, Y., Shafi, A., He, M., He, L., & Liu, D. (2024). Spatial Heterogeneity Analysis and Risk Assessment of Potentially Toxic Elements in Soils of Typical Green Tea Plantations. Agronomy, 14(8), 1599. https://doi.org/10.3390/agronomy14081599







